Letter to the Editor
The most common symptoms of COVID-19 are related to systemic and respiratory involvement. (Mao et al., 2019) We report two cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) infection with acute onset of altered mental status and delirium with normal respiration and metabolic balance in the first 48 h. Informed consent for publication was obtained from both patients.
1. Case 1
A 46-year-old male presented with two days history of headache followed by acute hypoactive delirium, disinhibition, confusion, but no fever, cough, or systemic manifestations. He had no previous medical or psychiatric illness, smoking, alcohol or illicit drug use. He was apyrexic but encephalopathic, nonverbal, and unable to follow commands. There was no weakness, but he had ankle clonus, brisk reflexes and Babinski’s sign. Baseline brain CT and blood tests were unremarkable (Table 1 ). Nasopharyngeal swab real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) test was positive for SARS-CoV2.
Table 1.
Patient data at the time of presentation to hospital and ≥48 h after the onset of altered mental status.
| Value (normal range) | Case 1 |
Case 2 |
||
|---|---|---|---|---|
| at presentation | 48 h latera | at presentation | 60 h latera | |
| Body temperature, °C | 36.7 | 38.3 | 36.9 | 38.4 |
| Pulse rate, beats/minute | 86 | 90 | 91 | 82 |
| Blood pressure, mmHg | 116/74 | 142/75 | 185/93 | 147/81 |
| Oxygen saturation on air | 97% | 100% | 99% | 88% |
| Hemoglobin, g/L (130–180) | 147 | 137 | 132 | 97 |
| White cell count, × 109/L (4–11) | 7.12 | 9.2 | 6.36 | 9.65 |
| Neutrophil count, × 109/L (2–7) | 3.6 | 7.97 | 5.83 | 8.68 |
| Lymphocyte count, × 109/L (1–4) | 2.82 | 0.56 | 0.39 | 0.38 |
| Platelet count, × 109/L (150–450) | 133 | 132 | 313 | 232 |
| C-Reactive Protein, mg/L (0–10) | <5 | <5 | <5 | 12 |
| D-Dimer, ug/L (0–500) | – | Up to 1659b | – | Up to 3748b |
| Alanine transaminase, U/L (0–35) | 15 | Up to 165b | 12 | 145 |
| Sodium, mmol/L (134–145) | 133 | 136 | 134 | 115 |
| Urea, mmol/L (2–6.5) | 3.2 | 8.8 | 5.8 | 5.1 |
| Creatinine, umol/L (59–104) | 88 | 99 | 65 | 53 |
| Serum osmolality, mosmol/kg (275–295) | – | – | – | 257 |
| Urine osmolality, mosmol/kg (50–1200) | – | – | – | 664 |
| Serum glucose mmol/L (3–7.8) | 8.7 | 5.8 | 8.1 | 5.4 |
| CSF glucose, mmol/L (~60 to 80% of serum value) | – | 4.5 | – | 4.1 |
| CSF opening pressure, cmH2O | – | 18 | – | 13 |
| CSF white cell × 109/L (0) | – | 0 | – | 0 |
| CSF protein, mg/L (150–450) | – | 987 | – | 340 |
| CSF oligoclonal bands | – | Positive | – | Not found |
| Serum oligoclonal bands | – | Positive | – | Not found |
| CSF PCR for SARS-CoV2 | Negative | Negative | ||
| CSF PCR for Herpes Simplex 1 and 2, Varicella zoster, enteroviruses | – | Negative | – | Negative |
| Antibodies against NMDAR, LGi1, CASPR2, GABA-B, AMPA in serum and CSF | – | Not detected | – | Not detected |
| Paraneoplastic anti-neuronal antibodies in serum and CSFc | – | Not detected | – | Not detected |
| Antiphospholipid antibodies | – | Negative | – | – |
| Thrombophilia screen | – | Normal | – | – |
| Thrombin Time, seconds (14–19) | – | 16.6 | 33.9 | – |
| APTT, seconds (21–29) | – | 20.8 | 54.2 | – |
| Serum alpha-galactosidase A, pmol/punch/h (6.3–47) | – | 26.1 | – | – |
| 12-lead electrocardiogram | sinus rhythm | sinus rhythm | sinus rhythm | sinus rhythm |
CSF, cerebrospinal fluid; NMDAR, N-methyl-D-aspartate receptor; LGi1, leucine-rich glioma inactivated 1; CASPR2, contactin-associated protein 2; GABA-B, γ-Aminobutyric acid-B receptor; AMPA, GluR1 and GluR2 subunits of the AMPA receptor; APTT: activated partial prothrombin time.
After the onset of altered mental state.
The highest level measured between day 6 and day 8.
Paraneoplastic antibodies included anti-Hu, anti-Yo, anti-Ri, and anti-amphiphysin.
After 2 days, he developed fever and status epilepticus requiring sedation, neuromuscular blockade and intubation. The fraction of inspired oxygen (FiO2) remained at 21% and no additional respiratory support was needed. Chest X-ray only showed patchy consolidation without significant progression on subsequent imaging. Cerebrospinal fluid (CSF) showed mildly raised protein and oligoclonal bands, and was negative for SARS-CoV2.
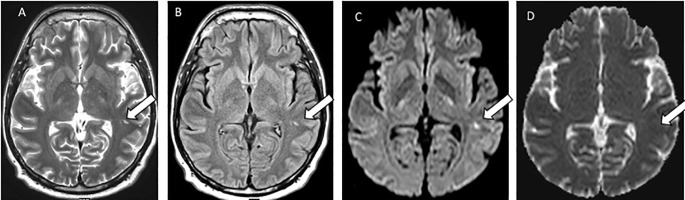
Neuromuscular blockade was withdrawn on day 5, when the new symptom of cough emerged. Despite normal brain CT at 48 h, MRI on day 6 showed three hyperintense foci on diffusion-weighted images, but no overt restriction, consistent with T2-shine-through suggesting cellular infiltration/inflammation or small infarcts (Fig. 1 ). The presistent diffusion-weighted hyperintensity on subsequent MRI supported neuroinflammation. Cranial arteries were normal on CT angiogram. Empirical treatment included Ceftriaxone, Aciclovir and Sodium Valproate.
Fig. 1.
Case 1, MRI head 6 days after the onset of delirium: white arrows show the signal hyperintensity changes in the subcortex of the left lateral temporal lobe on T2-Weighted (A), FLAIR (B), and Diffusion Weighted Image (C), and ADC map (D).
He noted hyposmia and dysgeusia during the convolescence. Despite improvement after a week, he had impairements in verbal fluecy, linguistic abstraction, phrase repetition, and delayed recall memory. He scored 20/30 on Montreal Cognitive Assessment on day 8.
2. Case 2
A 79-year-old female was admitted to hospital following a seizure resulting in injuries to her face. According to her relatives, the symptoms started a few hours earlier with confusion and verbal communication difficulties. There was no preceding illness. She had no fever, cough, or respiratory symptoms at presentation. Vital signs were normal. There were no sensory or motor deficits. She had dysphasia and impaired orientation, attention and memory. The first brain MRI only showed chronic small vessel ischemic changes. Chest CT scan only demonstrated bilateral pleural effusion.
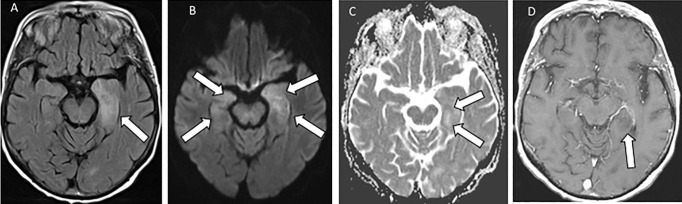
After 60 h, she had two generalized seizures, followed by fever and low oxygen saturation. Neurological examination confirmed poor mental state, with Glasgow Coma Scale scores between 8 and 13. Repeat blood tests showed hyponatremia. The second nasopharyngeal sample rRT-PCR was positive for SARS-CoV2. CSF revealed normal constituents (Table 1). CT scan and repeat brain MRI showed new changes in the limbic system with partial diffusion restriction, suggestive of limbic encephalitis (Fig. 2 ). Treatment included Levetiracetam and sodium correction.
Fig. 2.
Case 2, MRI head 12 days after the onset of altered mental state: white arrows show the signal hyperintensity changes in the limbic system, predominantly in the left amygdala and hippocampus on FLAIR (A), partial restricted diffusion (B, C) and T1 hypointensity on post-Gadolinium-enhanced T1-weighted image (D).
The patient’s persistent delirium improved over 10 days with mild respiratory symptoms, hyposmia and dysgeusia. On day 15, she scored 19/30 on Montreal Cognitive Assessment with impaired verbal fluency, repetition, abstraction, and delayed recall memory.
3. Discussion
There is emerging evidence that SARS-CoV2 can present with neurological features and concomitant encephalopathy (Mao et al., 2019, Moriguchi et al., 2020). However, there is currently no report of limbic encephalitis associated with COVID-19 that is presented with delirium in the absence of respiratory, metabolic or systemic features, while these patients may be hidden sources of spreading the virus in busy clinical settings.
The detection of SARS-CoV2 in the CSF in a patient with meningo-encephalitis supports neurotropic and neuroinvasive potential of the virus (Moriguchi et al., 2020) presumably through the blood vessel-rich meninges once the blood brain barrier is damaged (Baig et al., 2020). The evidence of SARS-CoV2 viral particles in brain capillary endothelial cells of an infected patient suggests hematogenous CNS entry (Paniz‐Mondolfi et al., 2020). The olfactory neuronal pathway could explain hyposmia (Desforges et al., 2019). Angiotensin-converting enzyme 2 (ACE2) receptors of vascular endothelium in respiratory system and meningeal capillaries can be the binding target for SARS-CoV2 proteins (Hamming et al., 2004).
In the absence of detectable virus in the CSF, it is plausible that SARS-CoV2 causes an immunologic response that results in parenchymal inflammatory injury, cerebral edema and clinical manifestations of encephalopathy (Wu et al., 2020). The presence of oligoclonal bands in one of our patient’s CSF and serum supports immune-mediated response that is not restricted to intrathecal production of immunoglobulins. Increased interleukin-6 in severe SARS-Cov2 disease highlights the occurrence of immunologic response and indicates intracranial cytokine storms (Mehta et al., 2020). Post-infection or non-infectious autoimmune encephalitis are known to be associated with antibodies against neuronal cell-surface or synaptic proteins (Leypoldt et al., 2015). The clinical phenotype similarly includes neurological and psychiatric presentations without CSF pleocytosis, and early immunotherapy improves outcome (Leypoldt et al., 2015). In patients with recent episodes of psychosis, higher prevalence of antibodies against four coronaviruses strains are reported (Severance et al., 2011), which supports the possibility of SARS-CoV2 encephalopathy and psychosis. It is unknown if immunotherapy is required to improve neurocognitive outcome in patients with SARS-CoV2 encephalopathy.
Acknowledgments
Acknowledgement
We acknowledge the help from Dr Rob Dineen, Dr Yasser Falah, Dr Martin Beed, Dr Ashan Gunarathne and the doctors, nurses and healthcare professionals within the Stroke Services, General Medicine, and Intensive Care Unit at the Nottingham University Hospitals NHS Trust, the City Hospital Campus. Thanks to the Neuroradiology, Neurology, Pathology and Virology departments at the Nottingham Queen’s Medical Centre that contributed to diagnostic investigations. We thank the patients for their participations in the study.
Funding
No specific grant from funding agencies.
Declaration of interest
None.
References
- Baig A., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v120100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F., Armangue T., Dalmau J. Autoimmune encephalopathies. Ann. N. Y. Acad. Sci. 2015;1338:94–114. doi: 10.1111/nyas.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz‐Mondolfi A., Bryce C., Grimes Z. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) J. Med. Virol. 2020 doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance E.G., Dickerson F.B., Viscidi R.P., Bossis I., Stallings C.R., Origoni A.E., Sullens A., Yolken R.H. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr. Bull. 2011;37:101–107. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]