Abstract
A shift from the traditional perspective that maternal stress is invariably costly has instigated recent interest into its adaptive role in offspring sex allocation. Stress generated by social instability has been linked to offspring sex ratio biases that favour the production of female offspring, which converges with the theoretical prediction that mothers in the poor condition are better off investing in daughters rather than sons. However, previous research has failed to disentangle two different processes: the passive consequence of maternal stress on sex-specific mortality and the adaptive effect of maternal stress at the time of conception. Here, I show that exposure to high male density social conditions leads to elevated stress hormone levels and female-biased in utero offspring sex ratios in house mice (Mus musculus domesticus), and identify that sex-specific offspring production—not sex-specific mortality—is the mechanism accounting for these sex ratio skews. This outcome reflects the optimal fitness scenario for mothers in a male-dominated environment: the production of daughters, who are guaranteed high mate availability, minimizes male–male competition for their sons. Overall, this study supports the idea that maternal stress has the potential to be adaptive and advances our understanding of how exposure to different social conditions can influence sex allocation in mammals.
Keywords: sex allocation, offspring sex ratios, male–male competition, glucocorticoids, social environment, mammal
1. Introduction
Theory predicts that parents will be advantaged by adjusting offspring sex ratios (nmales/ntotal offspring) in relation to their own ability to bear the costs of producing sons or daughters [1] and in relation to the future reproductive success of sons versus daughters [2–4]. Studies of sex allocation have traditionally addressed two central topics—the adaptive significance of producing offspring of either sex and the mechanism(s) that allow for facultative sex allocation—but integrating both topics has proved to be challenging. For example, in the absence of any evidential support of the underlying mechanism(s), correlations between social stressors and an elevation in female human births [5] are usually attributed to high rates of prenatal male death [6,7]. Human literature has debated whether differential embryonic mortality is an adaptive consequence of maternal manipulation (the ‘culled’ cohort hypothesis) or a by-product of maternal stress (the ‘damaged’ cohort hypothesis) [8], a debate that has also been addressed more generally in evolutionary biology [9]. From an evolutionary standpoint, the most cost-effective mechanisms are those that operate close to conception [10–12]. Surprisingly, sex-allocating mechanisms that occur early on in the reproductive sequence (e.g. sperm selection and differential implantation) are poorly studied, most likely owing to the requirement to prenatally quantify offspring sex which, in mammals, can be technically difficult. Because offspring sex ratio skews can compromise population viability by persisting in the adult cohort and distorting the ratio of males to females in the mating pool [13,14], understanding both the underlying mechanisms as well as the proximate causes of biased offspring sex ratios is critical to multiple facets of science, including biodiversity conservation [12,15,16].
The developmental gestation period that follows internal fertilization provides an inherent link between maternal condition and offspring sex allocation in mammals. The Trivers–Willard hypothesis (TWH) predicts that mothers in the good condition favour the production of male offspring, because sons—when competitive and successful—should generate more grandoffspring than daughters [4]. Typically, however, sons are also more ‘expensive’ to produce as they require greater maternal energy requirement during late gestation and lactation than do daughters [17,18]. Consequently, mothers in the poor condition are expected to have daughters [4] (note that this prediction also adheres to the cost of reproduction hypothesis; reviewed [1]). Despite its intuitive appeal, studies testing the TWH have produced equivocal results with inconsistencies being prevalent among and even within species [19]. One contributing factor to this ambiguity has been the use of a number of different measures of the maternal condition and variation in the level (i.e. population versus individual) and time point (e.g. birth versus conception) at which they are quantified [19,20]. The most commonly applied indices have included those that are associated with maternal age and reproductive experience, nutritional status (e.g. body mass and glucose level) and social rank (e.g. testosterone level) [11,21–25].
The degree of chronic stress that an individual has or is experiencing can be applied as an index of condition [26,27]. The TWH predicts that highly stressed, poor condition mothers should produce daughters [4]. In humans, exposure to distressing situations, such as severe life events, economic disruption and natural disasters, is often correlated with a reduction in male births [7]. A bias towards female offspring during times of stress is also often observed in non-human mammals [28], although patterns appear to vary based on the reproductive stage at which sex ratios are measured [29]. Stress hormones, which transduce stressful stimuli from the environment into internal physiological signals, have been identified to be likely candidates that initiate mechanisms of sex allocation by triggering physiological processes [29]. However, very few mammal studies have directly assessed the relationship between hormonal measures of maternal stress and variation in offspring sex ratios (reviewed [19,28,29]). Specifically, this relationship remains unexplored at the prenatal stage (i.e. the primary or implantation sex ratio) because studies, to date, have focused exclusively on birth sex ratios (i.e. the secondary sex ratio) [29].
The type and frequency of interactions that individuals share with conspecifics in the social environment can affect hormonal responses and strategies of sex allocation [29]. Sex-biased social interaction between individuals in the local environment, such as competition (local mate and resource competition; [2,30]) and cooperation (local resource enhancement; [31]) among relatives and other residents, can alter the costs and benefits associated with producing each sex. Accordingly, individuals are expected to produce female offspring under conditions of intensified local competition among males [2]. Female haplodiploid invertebrates, which have direct control over offspring sex by choosing whether (daughters) or not (sons) to release sperm and fertilize eggs, have been shown to maximize the fitness of their sons by mediating sex ratios based on local male density [32]. There is also convincing evidence that local conditions influence sex ratio variation in birds, where, here too, mothers govern the sex of ovulated eggs [28,33,34]. In mammals, sex allocation is more of a level playing field: the paternal gamete determines offspring sex, but further development is under maternal control [35]. Consequently, there are multiple reproductive stages at which the social environment can influence offspring sex, from mediating sperm sex ratios prior to mating [36] to differential postnatal maternal care [37]. Social factors, such as hierarchical rank, have been shown to shape stress levels and linked to adaptive sex allocation in mammals, albeit with inconsistencies in the direction of the response [29]. The key challenge lies in being able to disentangle multiple, interacting and potentially opposing sex allocation strategies that operate simultaneously within natural populations [1]. Experimental manipulation under controlled conditions is an effective way to address this complexity.
Here, I performed an experiment on wild-sourced individuals of a tractable mammal species to investigate the role of socially induced maternal stress in sex allocation during early development. Social instability has been identified to be an effective model of stress for many mammals, especially rodents [38]. Specifically, female house mice (Mus musculus domesticus) elicit different stress responses when exposed to male and female conspecifics [39]. Thus, I exposed female house mice to one of two different social environments during sexual development: low-stress, high female density conditions and high stress, high male density conditions [36]. I measured maternal blood corticosterone (CORT) (i.e. the predominant glucocorticoid and stress-associated hormone in rodents) and glucose levels, and genetically sexed both viable and non-viable 14-day developing embryos. In line with theory, I hypothesized that the male-dominated environment would favour the production of female offspring and that this sex ratio bias would be linked to elevated maternal stress [2,4]. I also measured embryo number, size and in utero position (i.e. in relation to the sex of neighbouring embryos) to further explore fitness implications of socially induced maternal stress.
2. Material and methods
(a). Experimental animals
Wild house mice (M. m. domesticus; n = 100) were sourced from Rat Island (28° 42′ S, 113° 47′ E), which is located off the coast of Western Australia, and maintained in the laboratory at the University of Western Australia. The animals were held in constant temperature rooms (24°C) on a reverse 10 L : 14 D cycle and provided with water and food ad libitum. Mice were outbred under common garden conditions for two generations. For this, male and female pairs were housed together for a maximum of 14 days, following which pregnant females were housed alone and left undisturbed to give birth and rear their litter. Female and male offspring were separated at three weeks of age; female offspring were housed in sister groups and male offspring were housed individually. A total of 24 experimental female sister pairs, 30 non-focal females and 30 non-focal males were used in the social manipulation. Another 24 sexually experienced males (i.e. those that mated with a sister pair) were used in the experimental matings (see below).
(b). Social environment manipulation
Social instability has been identified to be an effective model of stress in rodents [38]. For example, previous research has shown that perceiving the presence of a male, but not mating or being solicited to mate, generates a stress response in adult females [39]. It is believed that this result is owing to a mismatch between sexual anticipation and reward, with exposure to males and their scents generating an anticipatory increase in the CORT level, but the lack of mating meaning these levels remain consistently high [39]. Here, I manipulated social cues that had previously been shown to elicit responses in reproductive traits in house mice [36,40–42], with the expectation that exposure to sex-specific cues during development would result in varied levels of stress [39]. Thus, females were exposed to either high male (n = 24) or high female density (n = 24) conditions from the time of independence from their mother until sexual maturity (figure 1). The two social environments were created by housing females in standard cages (28 × 46 × 13 cm) on metal racks in two separate constant temperature rooms. In the high male density environment, females matured within close proximity to males and were exposed to males and their scents on a regular basis (for more detail, see the electronic supplementary material, figure S1a). By contrast, females in the high female density conditions matured within close proximity to females and were occasionally exposed to the scent of a single male (for more details, see the electronic supplementary material, figure S1b). Sisters were used across treatments to account for family-derived variation.
Figure 1.

The experimental design. Wild-sourced female mice were reared under standard conditions from birth to weaning and then exposed to social environments that were anticipated to induce variation in the maternal stress level: high male or high female density conditions. The different sized mice represent a family (mother and offspring) to demonstrate that sisters were used across treatments, which controlled for family-derived variation that might influence offspring sex allocation. This was reinforced by ensuring that sisters reproduced with the same male. CORT concentration, blood glucose level, prenatal offspring sex ratios and embryo traits were measured at 14 days gestation. (Online version in colour.)
(c). Experimental matings
Once sexually mature, females reproduced with males that had been reared under standard conditions (figure 1). The experimental matings were performed during the dark phase of the 10 L : 14 D cycle in a third constant temperature room (24°C) as described previously [43]. When oestrus was apparent [44], females were placed in a cage with a male. The cage was then placed inside a large, opaque plastic tub (49 × 74 × 41 cm) to eliminate female exposure to other conspecifics during the mating period. Females were checked every half an hour for the presence of a mating plug, which is indicative of a complete and successful mating [43]. After mating, females were weighed, placed in their own cage and provided with the nesting material. Mated females were returned to their designated social environment, but were no longer exposed to the scents of conspecifics. Each sister pair reproduced with the same sexually experienced male; the order of first and second females to mate was alternated between treatments. Pregnant females were housed alone and left undisturbed until 14 days after mating, at which time they were sacrificed.
(d). Serum corticosterone assays
Maternal CORT concentration—the predominant rodent glucocorticoid—and glucose level were measured in blood extracted via postmortem cardiac puncture. Females were sacrificed between 9.00 and 10.00 am on gestation day 14. Blood was collected via cardiac puncture, refrigerated overnight at 4°C and then centrifuged at 3250 rpm for 20 min. The serum was removed, aliquoted and stored at −80°C. CORT concentration in undiluted serum samples was assayed in duplicate using a commercially available enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Mouse Corticosterone ELISA Kit no. 80556, Crystal Chem, Elk Grove Village, IL, USA).
(e). Blood glucose measurement
Maternal blood glucose level, a major energy source that is indicative of nutritional status [45], has been linked to variation in offspring sex ratio (e.g. see [46]). Consequently, blood glucose concentration was quantified using a commercially available Accu-Chek Performa Nano glucometer from the serum samples described above. It was not possible to obtain glucose concentration for one sample (female treatment) owing to insufficient serum quantity.
(f). Embryo measurements
Embryo viability status was scored as appearing either normal in size and shape (viable) or as a large haematoma that was discernible in shape and being resorbed (non-viable) [47,48]. The uterine position of each embryo was recorded, following which they were resected and stored in 100% ethanol. The prenatal sex ratio was quantified by genetically sexing both viable and non-viable embryos [49]. Each embryo was sexed using a rapid polymerase chain reaction (PCR)-based method, which included a Y-chromosome (Sry)-specific primer and a myogenin (Myog) control primer [49,50]. The 10 µl reactions were performed with 1 ng of DNA template, 1× PCR buffer, 0.3 µl MgCl2 (50 mM), 2 µl dNTPs (200 mM), 0.1 µl Taq (0.5 units reaction−1) and 0.5 µl each primer (0.25 µM). PCR products were denatured at 94°C for 5 min, 30 cycles of 5 s at 94°C and 40 s at 67°C, and 1 min extension at 72°C. The PCR products were electrophoresed on a 1.5% agarose gel for 30 min at 100 V, visualized with gel red (Biotium, Inc.) under ultraviolet illumination and scored as either male (two bands) or female (single band). Two people blind to treatment independently scored the bands. Embryo length was measured to the nearest 0.01 mm using digital callipers. Of the total 339 viable embryos, seven were unable to be measured owing to failed storage and desiccation (nfemale treatment = 3; nmale treatment = 4).
(g). Statistical analyses
Linear mixed models (LMMs) and generalized linear mixed models (GLMMs) were conducted in R v.3.5.1 using the lmer or glmer functions implemented within the package lme4 [51]. LMMs were used to analyse treatment differences in (i) maternal and embryo anatomical traits, (ii) embryo number and (iii) concentrations of maternal blood constituents (glucose and CORT). GLMMs were used to analyse treatment differences in (i) embryo number (family = Poisson), (ii) the proportion of male offspring that mothers produced (i.e. the sex ratio; nmale embryos/ntotal embryo number; family = binomial), (iii) the proportion of viable embryos that mothers produced (nviable embryos/ntotal embryo number; family = binomial), and (iv) the proportion of 0M (embryos positioned between two female embryos) and 2M (embryos positioned between two male embryos) sons and daughters that mothers produced (npositioned embryos/ntotal embryo number−4; note that peripherally located embryos were not included in this analysis; family = binomial). A GLMM with the intercept removed was applied to test for embryo sex ratio parity within each treatment (i.e. against 0.5). Binomial GLMMs were performed using the cbind function except for (iii) the proportion of viable embryos that mothers produced, as this approach restricts testing for embryo-level differences (i.e. sex-specific variation). In the Poisson GLMM (embryo number), penalized quasi-likelihood was used by running the function glmmPQL from the MASS package to account for underdispersion [52]. In the embryo viability GLMM and the embryo length LMM, both female and family identities were included as random effects. In all other cases, family identity only was included as a random effect in the model.
A single female failed to become pregnant. Subsequently, this sister pair was excluded from the dataset, which resulted in a total sample size of 46 females (nfemale treatment = 23, nmale treatment = 23). An initial analysis confirmed that there was no relationship between the embryo number and the prenatal sex ratio (electronic supplementary material, figure S2). Female body mass was included as a covariate in the LMMs testing glucose and CORT concentration, as well as embryo number and length. Embryo sex was included as an additional covariate in the LMM testing embryo length. One glucose concentration was identified as an outlier (+2 s.d. from the mean) and therefore removed from further analysis (male treatment). The significance of the fixed effects was calculated using maximum-likelihood and Wald tests using the function Anova (car package). All non-significant two- and three-way interaction terms were removed from the models (full models: electronic supplementary material, tables S1 and S2; reduced models: electronic supplementary material, tables S3 and S4). The biological relevance of a borderline significant interaction term in the embryo viability GLMM (CORT × sex ratio bias; p = 0.047; electronic supplementary material, table S2) was unclear and therefore removed from the final model (electronic supplementary material, table S4).
Coefficients of variation (CVs) for embryo number and embryo length were calculated using the formula: CV = (standard deviation/mean) × 100, and then tested for differences among females reared in the different social environments using two-tailed z-tests compared with critical values from the t-distribution where the degrees of freedom are infinite [53].
3. Results and discussion
(a). Maternal stress and the prenatal sex ratio
The analyses revealed that there was no difference in maternal body weight or blood glucose level among females reared under different social conditions (electronic supplementary material, table S3 and figure S3). As expected, mothers exposed to the high male density conditions had elevated CORT concentrations compared to mothers subjected to high female density conditions (χ2 = 14.031, p = 0.001; figure 2). While mothers exposed to non-stressful social conditions produced sons and daughters in equal proportions, mothers exposed to stressful social conditions produced female-biased sex ratios (between treatments: χ2 = 4.711, p = 0.030; parity within treatments: high female density conditions: z-value = −0.666, p = 0.505, high male density conditions: z-value = −3.625, p = <0.001; electronic supplementary material, tables S4 and S5; figure 2). Although it cannot be determined unequivocally, the analyses do support a causal relationship between CORT concentration and offspring sex ratio. Overall, across both treatments, there was a significant negative relationship between CORT concentration and the proportion of male offspring produced (figure 2; electronic supplementary material, table S4). Further, when CORT concentration and treatment were included in the analysis together, CORT concentration accounted for a greater amount of the variation in offspring sex ratio than the social environment, which as a result dropped out of the analysis as non-significant (electronic supplementary material, table S4). This result makes it seem unlikely that exposure to high male conditions leads to sex allocation shifts via an alternative, non-stress-related pathway. An association between maternal CORT concentration and sex ratio could arise if the ‘cost’ of producing male versus female offspring differs during early development, for example if gestating female embryos impose greater maternal stress than male embryos. In turn, this could lead to different patterns of embryo viability among mothers carrying female- versus male-biased litters, with the expectation that a high proportion of female embryos leads to a general increase in reproductive failure owing to elevated maternal stress. Here, female embryo mortality was higher than male mortality (χ2 = 13.406, p = <0.001; electronic supplementary material, table S4; figure 3a); however, embryo viability was not linked to the direction of sex ratio bias nor maternal CORT concentration (electronic supplementary material, table S4). The idea that female offspring impose greater maternal stress during gestation actually contradicts what is known of differences in the energetic requirements of male and female embryos [54]. For example, a study on the bank vole (Myodes glareolus) found that maternal daily energy expenditure was greatest among females that were concurrently gestating and lactating male-biased litters, pointing to a higher cost of producing sons than daughters [55]. Elevated maternal stress therefore appears to be the cause, and not a consequence, of producing high numbers of female offspring.
Figure 2.
Social stress leads to high CORT levels and female-biased sex ratios. CORT levels and offspring sex ratios were higher among mothers reared in a high male density environment (a) compared to mothers reared in a high female density environment (b). The figure shows the observations (pink and blue circles), the prediction from the model in the scale of response variable (pink and blue solid lines) and the 95% confidence interval from the bootstrapped sample (shadowed area). The model prediction lines calculated with bootstrapping (n = 10 000) are just visible beneath the solid lines (pink and blue dashed lines). Sex ratios falling below the grey dashed line are female-biased. The inserts display the CORT concentration data as a boxplot for each treatment (ng ml−1; data points have been jittered). (Online version in colour.)
Figure 3.
Sex-biased offspring production, not mortality, as the explanatory mechanism (a). Boxplots displaying the proportion of viable son and daughter embryos for mothers reared in different social environments (n = 340 embryos from 46 females). Son viability was consistently high (electronic supplementary material, table S2). Extended fitness implications of the sex ratio skew for sons (b,c). Boxplots displaying the proportion of sons and daughters that were positioned in utero between two female (0M) or two male embryos (2M) for mothers reared in different social environments (n = 248 embryos from 46 females; asterisk (*) indicates significance at p < 0.05; electronic supplementary material, table S2). The in utero sex ratio skew did not influence the intrauterine position of daughter embryos (b). Higher proportions of 0M sons were produced in the high male density environment and higher proportions of 2M sons were produced in the high female density environment (c). Data points have been jittered. (Online version in colour.)
(b). Evidence of a low-cost sex allocation mechanism
Previous studies of mammals have produced qualitatively similar results to what was uncovered here—evidence which suggests that female-biased offspring production is a response to maternal stress that escalates around the time of conception [56–59] (although see [60,61])—but little is known about the underlying mechanism(s) responsible for stress-related offspring sex ratio manipulation. Mechanisms of sex ratio adjustment that operate late in development can be energetically costly and maladaptive for female mammals (e.g. differential embryo resorption; [62]). Instead, the most beneficial maternal strategy is to establish a bias in the primary or implantation sex ratio, which precedes any significant energetic investment in the offspring [63]. The current investigation provides novel insight into sex allocation mechanism(s) in operation, which can be isolated to operating either prior to or during implantation. Importantly, because both viable and non-viable in utero embryos were genetically sexed, it was established that skewed sex ratios produced under stressful social conditions were not attributable to sex-specific mortality during development (figure 3a). Further, there was no difference in overall offspring number between stressed and non-stressed mothers (electronic supplementary material, table S4), suggesting that there was no difference in egg/zygote death among mothers exposed to high male and high female density conditions during the earliest stages of reproduction. Rather, if mothers from both treatments ovulated an excess of eggs, which is typically a trait of female house mice (i.e. often up to 10 or more eggs are ovulated than what is implanted; see [64]), there would be opportunity for prenatal sex ratio biases to be established during implantation.
Maternal stress may influence the viability or competitiveness of X- and Y-chromosome-bearing sperm (CBS) within the reproductive tract [63]. For example, differences between the in utero survival of X- and Y-CBS subpopulations may be influenced by physical and/or biochemical changes in the female tract that are linked to maternal stress, and which result in sex-skewing during fertilization [65]. Alternatively, different immune responses to X- and Y-CBS elicited by specific regions of the female tract may lead to variation in the sex ratio of implanted embryos [10]. Sexual dimorphism and sex-specific signalling of conceptuses reinforce the possibility that offspring sex ratios can be facultatively adjusted during implantation [66]. The reproductive window during which the offspring sex ratio bias was laid down in this experiment (i.e. after insemination but no later than during implantation) supports the hypothesis for the evolution of prenatal sex-biasing mechanisms and builds on accumulating evidence that during challenging times mammalian mothers exert control over the sex of their offspring.
(c). Fitness implications of female-biased sex ratios in a male-dominated environment
The social conditions that parents experience at the time of reproduction may be reflective of the environment that their offspring will face. In order to offset the significant cost of reproduction and maximize their inclusive fitness, mothers especially have a vested interest in ensuring that offspring phenotype aligns with local conditions. It could be argued that offspring sex is the phenotypic trait that will have the greatest influence on the success of an individual in different social contexts. For example, the production of male offspring under high male density conditions would be maladaptive as sons will be forced to compete with their non-dispersing brothers, as well as unrelated local resident males, for access to females [2]. Male–male competition becomes more intense when females mate with multiple males and the sperm of those rivals are forced to compete for fertilizations [67]. Under such conditions, however, competition among female offspring is negligible. Rather, when male density is high and daughters are guaranteed of high mate availability, the production of female offspring will provide the best assurance for grand-parentage and consequently the most favourable fitness outcome [68]. The biased production of daughters under high male density conditions observed here in house mice aligns with theory which predicts that mothers will manipulate offspring sex ratios to avoid reproductive competition among their sons [2]. Although this result is seemingly adaptive, it must be noted that relationships between maternal condition and offspring sex ratios can change under different environmental conditions (e.g. see [69,70] on the red deer, Cervus elaphus). Further, changes in the demography of the local environment over time, for example owing to patterns of (sex-specific) dispersal or differences in the extent to which generations overlap, mean that the maternal environment would not necessarily reflect the environment that their sons experience and there is a high degree of complexity when it comes to the influence of prevailing local conditions (both social and ecological) on strategies of sex allocation [1]. Here, in this experiment, I focused on sex allocation in relation to maternal stress in isolation and discovered that social stress may have the potential to generate beneficial fitness outcomes by leading to an offspring sex bias that could offset future male–male competition. Of course, in nature, a suite of other factors may influence the direction and strength of this result.
The identification of a bias in the prenatal sex ratio can provide insights into subtle fitness implications that would otherwise go undetected if birth sex ratio was assessed. In litter-bearing mammals, the prenatal phase is a critical developmental period during which offspring are exposed to a milieu of hormones owing to (i) the close intrauterine proximity to neighbouring embryos and (ii) placental transfer from the mother [71]. In utero sex ratio skews will affect the probability that an embryo will neighbour embryos of the same- or opposite-sex. In this experiment, female embryos were positioned in equal proportions between two male (2M) and two female (0M) embryos across both treatments (electronic supplementary material, table S4 and figure 2b). However, the sons of stressed mothers (female-biased sex ratio) were more likely to develop between two female embryos (0M) (χ2 = 4.570, p = 0.033), while sons of non-stressed mothers (equal sex ratio) were more likely to be positioned between two male embryos (2M) (χ2 = 5.653, p = 0.017) (electronic supplementary material, table S4; figure 3c). The intrauterine position has been shown to affect the male adult phenotype in relation to traits that influence competitive ability (e.g. testosterone level, body size, testes size, scent marking frequency and territory size); 2M males ultimately becoming more masculinized, and 0M males more feminized, in adulthood (reviewed [72]). As a consequence, females prefer to mate with 2M males over 0M males [73]. Therefore, a prospective cost of producing female-biased offspring sex ratios is the making of non-competitive sons that have low reproductive potential. Following birth, 0M males receive less maternal attention compared to 2M males [74], suggesting that discriminative postnatal care may be an effective maternal strategy for minimizing expenditure on sons that are destined to be evolutionary dead ends.
(d). Further fitness implications of socially induced stress
In mice, different social environments experienced during postnatal development can affect the adult phenotype in terms of altered body size (e.g. high male social conditions lead to increased male body size; see [36,42]). In the current study, although there was no overall difference in body size among females maturing under high male (relatively stressful) and high female (relatively non-stressful) conditions, the relationship between maternal body weight and CORT concentration interacted in different ways: under high male density conditions, ‘more stressed’ females were heavier while under high female density conditions ‘less stressed’ females were heavier (χ2 = 5.591, p = 0.018; electronic supplementary material, table S3 and figure S4). These differences may reflect variation in activity levels under different social conditions, for example if generally high stress levels are associated with greater activity levels and lower body weights, but above some critical threshold the opposite is observed. In support, it has been shown that mice experiencing elevated baseline CORT levels exhibit high locomotor activity, but significant elevations lead to affective disorders (e.g. depression) characterized by reduced activity levels [75]. Further investigation is required to fully understand the difference in the direction of the relationship between body weight and stress level among female mice exposed to different social conditions.
In addition, prenatal maternal stress can affect in utero offspring development in terms of altered growth rates [76]. In many mammals, including humans, prenatal exposure to elevated maternal glucocorticoids have been shown to cause a wide range of stress effects, including both accelerated (adaptive developmental plasticity hypothesis) and deaccelerated (developmental constraint hypothesis) growth [77]. Here, the potential for flow-on fitness implications of maternal stress becomes evident in an analysis of offspring size, which revealed that the embryos of mothers exposed to a stressful environment are, on average, smaller than the embryos of mothers exposed to a non-stressful environment (χ2 = 5.908, p = 0.015; non-sex-specific effect; electronic supplementary material, table S3; figure 4). Stunted in utero growth owing to lowered maternal investment during stressful periods can result in delayed sexual maturation and/or small adult size, which in turn may reduce reproductive rate or lifespan [78]. Yet, a meta-analysis has demonstrated that stunted offspring growth trajectories imposed by maternal stress during early development are often accelerated during juvenility [77]. This suggests that small in utero size may not necessarily persist in the adult phenotype. Further, although there was no overall difference in offspring number (electronic supplementary material, table S4), variation in the embryo number was significantly reduced in mothers exposed to a stressful environment compared to mothers exposed to a non-stressful environment (CV: z-value = 2.813, p = 0.005; electronic supplementary material, table S6; figure 4). This result highlights the possibility that during periods of stress, mothers are strategically ‘selecting’ offspring number before or at the time of implantation. In line with offspring sex ratio adjustments being adaptive, a mechanism that selects for the optimal offspring number at the earliest reproductive stage represents the most cost-effective strategy for mothers in the poor condition. In this sense, prenatal maternal stress effects on both offspring number and in utero growth (i.e. when growth trajectories are accelerated later in life) allow both mothers and offspring to make the best of a bad situation [77].
Figure 4.
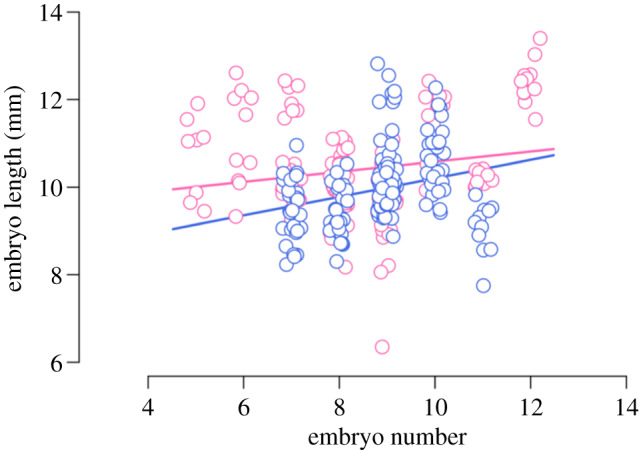
Reduced offspring size and optimized offspring number under stressful social conditions. There was no relationship between average embryo number and embryo size (r = 0.109, p = 0.223); however, there was less variation in the offspring number among mothers reared in the high male density environment (blue circles, solid line) compared to mothers reared in the high female density environment (pink circles, dashed line) (electronic supplementary material, table S4). These mothers also produced, on average, smaller embryos compared to mothers reared in the high female density environment (electronic supplementary material, table S1). Lines represent best fit. Data points have been jittered. (Online version in colour.)
4. Conclusion
The impact of maternal stress on juvenile development has intrigued scientists for many years. There has been a strong focus on the adverse effects of prenatal stress in terms of offspring cognitive ability, which relates to social behaviour [79]. In comparison, very little is known on the influence of different social conditions on maternal stress, and even less on how, in turn, socially induced maternal stress shapes offspring phenotype. The adaptive significance of maternal stress remains an ongoing core debate in diverse research fields, including animal ecology and human health. Despite the significance of this debate, many important offspring traits—including sex—have been ignored [29]. Here, using house mice, it was shown that exposure to high male density conditions during development leads to elevated maternal stress hormone levels and a bias in the prenatal sex ratio that aligns with theoretical predictions [2,4]. It was identified that the sex ratio bias was cost-effective (i.e. biased female production), and therefore seemingly an adaptive strategy, rather than a passive consequence (i.e. biased male mortality) of maternal stress. To unequivocally demonstrate adaptive significance, the next step is to show that the fitness of females that adjust offspring sex ratios is, in fact, greater than those that do not. In terms of evolutionary fitness, females will retain greater capacity for future reproductive events by mitigating wasted investment in the unwanted sex. Thus, a sex-biasing mechanism(s) that operates around the time of conception offers mothers the most cost-effective means of influencing offspring sex ratios. Finally, this investigation demonstrates that glucocorticoids are effective in translating information from the social environment into sex allocation responses that have the potential to be adaptive [29]. This outcome is relevant to a broad range of research areas, including species conservation and the management of threatened or fragmented populations [12,15] as well as captive breeding programmes [16].
Supplementary Material
Supplementary Material
Acknowledgements
I thank Jamie Tedeschi for molecular technical assistance (embryo sexing and scoring), Jessica Moran and Misha Lavoie for general technical assistance (social environment manipulation; embryo measurements), and Paco Garcia-Gonzalez for statistical advice. I also thank Mathieu Douhard and two anonymous reviewers for the insightful comments that they provided on the manuscript.
Ethics
The work reported in this article followed the guidelines for the ethical treatment of animals in research under UWA Ethics Committee approval (03/100/1456).
Data accessibility
The data file is provided in the online electronic supplementary material.
Authors' contributions
R.C.F. designed the study, performed the research, analysed the data and wrote the manuscript.
Competing interests
I declare I have no competing interests.
Funding
This research was funded by the Australian Research Council (FT180100625).
References
- 1.Cockburn A, Legge S, Double MC. 2002. Sex ratios in birds and mammals: can the hypotheses be disentangled? In Sex ratios: concepts and research methods (ed. Hardy I.), pp. 266–286. Cambridge: Cambridge University Press. [Google Scholar]
- 2.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 3.West SA. 2009. Sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. ( 10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 5.Navara KJ. 2018. Choosing sexes. Mechanisms and adaptive patterns of sex allocation in vertebrates. Cham, Switzerland: Springer. [Google Scholar]
- 6.Wells JCK. 2000. Natural selection and sex differences in morbidity and mortality in early life. J. Theor. Biol. 202, 65–76. ( 10.1006/jtbi.1999.1044) [DOI] [PubMed] [Google Scholar]
- 7.Navara KJ. 2010. Programming of offspring sex ratios by maternal stress in humans: assessment of physiological mechanisms using a comparative approach. J. Comp. Physiol. 180, 785–796. ( 10.1007/s00360-010-0483-9) [DOI] [PubMed] [Google Scholar]
- 8.Catalano R, Bruckner T. 2006. Secondary sex ratios and male lifespan: damaged or culled cohorts. Proc. Natl Acad. Sci. USA 103, 1639–1643. ( 10.1073/pnas.0510567103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clutton-Brock T. 1991. Sex ratios and differential mortality. In The evolution of parental care, pp. 229–253. Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Alminana C, et al. 2014. The battle of the sexes starts in the oviduct: modulation of the oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genomics 15, 293 ( 10.1186/1471-2164-15-293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron EZ. 2004. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. Lond B 271, 1723–1728. ( 10.1098/rspb.2004.2773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangel-Negrin A, Coyohua-Fuentes A, Canales-Espinosa D, Chavira-Ramirez DR, Dias PAD. 2018. Maternal glucocorticoid levels affect sex allocation in black howler monkeys. J. Zool. 304, 124–131. ( 10.1111/jzo.12503) [DOI] [Google Scholar]
- 13.West SA, Herre EA, Sheldon BC. 2000. The benefits of allocating sex. Science 290, 288–290. ( 10.1126/science.290.5490.288) [DOI] [PubMed] [Google Scholar]
- 14.Wedekind C. 2012. Managing population sex ratios in conservation practice: how and why? In Topics in conservation biology (ed. Povilitis T.), pp. 81–96. London, UK: InTech. [Google Scholar]
- 15.Robertson BC, Elliot GP, Eason DK, Clout MN, Gemmell NJ. 2006. Sex allocation theory aids species conservation. Biol. Lett. 2, 229–231. ( 10.1098/rsbl.2005.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faust LJ, Thompson SD. 2000. Birth sex ratio in captive mammals: patterns, biases, and the implications for management and conservation. Zoo Biol. 19, 11–25. () [DOI] [Google Scholar]
- 17.Parker KL, Barboza PS, Gillingham MP. 2009. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 23, 57–69. ( 10.1111/j.1365-2435.2009.01528.x) [DOI] [Google Scholar]
- 18.Rickard IJ, Russell AF, Lummaa V. 2007. Producing sons reduces lifetime reproductive success of subsequent offspring in pre-industrial Finns. Proc. R. Soc. B 274, 2981–2988. ( 10.1098/rspb.2007.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douhard M. 2017. Offspring sex ratio in mammals and the Trivers-Willard hypothesis: in the pursuit of unambiguous evidence. BioEssays 39, 1700043 ( 10.1002/bies.201700043) [DOI] [PubMed] [Google Scholar]
- 20.Schindler S, Gaillard M, Gruning A, Neuhaus P, Traill LW, Tuljapurkar S, Coulson T. 2015. Sex-specific demongraphy and generalization of the Trivers-Willard theory. Nature 526, 249–252. ( 10.1038/nature14968) [DOI] [PubMed] [Google Scholar]
- 21.Lindström J, Coulson T, Kruuk L, Forchhammer MC, Coltman DW, Clutton-Brock T. 2002. Sex-ratio variation in Soay sheep. Behav. Ecol. Sociobiol. 53, 25–30. ( 10.1007/s00265-002-0545-4) [DOI] [Google Scholar]
- 22.Brown GR, Silk JB. 2002. Reconsidering the null hypothesis: is maternal rank associated with birth sex ratios in primate groups? Proc. Natl Acad. Sci. USA 99, 11 252–11 255. ( 10.1073/pnas.162360599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishman R, Vortman Y, Shanas U, Koren L. 2018. Female-biased sex ratios are associated with higher maternal testosterone levels in nutria (Myocastor coypus). Behav. Ecol. Sociobiol. 72, 101 ( 10.1007/s00265-018-2517-3) [DOI] [Google Scholar]
- 24.Saltz D. 2001. Progeny sex ratio variation in ungulates: maternal age meets environmental perturbation of demography. Oikos 94, 377–384. ( 10.1034/j.1600-0706.2001.940220.x) [DOI] [Google Scholar]
- 25.Kojola I, Eloranta E. 1989. Influences of maternal body weight, age, and parity on sex ratio in semidomesticated reindeer (Rangifer t. tarandus). Evolution 43, 1331–1336. ( 10.1111/j.1558-5646.1989.tb02582.x) [DOI] [PubMed] [Google Scholar]
- 26.Romero LM. 2011. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. ( 10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 27.Shave JR, Derocher AE, Cherry SG, Thiemann GW. 2019. Chronic stress and body condition of wolf-killed prey in Prince Albert National Park, Saskatchewan. Conserv. Physiol. 7, coz037 ( 10.1093/conphys/coz037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navara KJ. 2018. Hormones rule the roost: hormonal influences on sex ratio adjustment in birds and mammals. In Choosing sexes mechanisms and adaptive patterns of sex allocation in vertebrates (ed. Navara KJ.), pp. 123–154. Cham, Switzerland: Springer. [Google Scholar]
- 29.Geffory B, Douhard M. 2019. The adaptive sex in stressful environments. Trends Ecol. Evol. 34, 628–640. ( 10.1016/j.tree.2019.02.012) [DOI] [PubMed] [Google Scholar]
- 30.Clark AB. 1978. Sex ratio and local resource competition in a prosimian primate. Science 201, 163–165. ( 10.1126/science.201.4351.163) [DOI] [PubMed] [Google Scholar]
- 31.Emlen ST, Emlen JM, Levin SA. 1986. Sex-ratio selection in species with helpers-at-the-nest. Am. Nat. 127, 1–8. ( 10.1086/284463) [DOI] [PubMed] [Google Scholar]
- 32.Macke E, Magalhaes S, Backh F, Olivieri I. 2011. Experimental evolution of reduced sex ratio adjustment under local mate competition. Science 334, 1127–1129. ( 10.1126/science.1212177) [DOI] [PubMed] [Google Scholar]
- 33.Komdeur J, Dann S, Tinbergen J, Mateman C. 1997. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs. Nature 385, 522–525. ( 10.1038/385522a0) [DOI] [Google Scholar]
- 34.Song Z, Lou Y, Hu Y, Deng Q, Gao W, Zhang K. 2016. Local resource competition affects sex allocation in a bird: experimental evidence. Anim. Behav. 121, 157–162. ( 10.1016/j.anbehav.2016.08.023) [DOI] [Google Scholar]
- 35.Edwards AM, Cameron EZ. 2014. Forgotten fathers: paternal influences on mammalian sex allocation. Trends Ecol. Evol. 29, 158–164. ( 10.1016/j.tree.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 36.Lavoie MD, Tedeschi JN, Garcia-Gonzalez F, Firman RC. 2019. Exposure to male-dominated environments during development influences sperm sex ratios at sexual maturity. Evol. Lett. 3-4, 392–402. ( 10.1002/evl3.123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskela E, Mappes T, Niskanen T, Rutkowska J. 2009. Maternal investment in relation to sex ratio and offspring number in a small mammal: a case for Trivers and Willard theory? J. Anim. Ecol. 78, 1007–1014. ( 10.1111/j.1365-2656.2009.01574.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haller J, Fuchs E, Halasz J, Makara GB. 1999. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res. Bull. 50, 33–39. ( 10.1016/S0361-9230(99)00087-8) [DOI] [PubMed] [Google Scholar]
- 39.Garratt M, Kee AJ, Palme R, Brooks RC. 2016. Male presence can increase body mass and induce a stress-response in female mice independent of costs of offspring production. Sci. Rep. 6, 23538 ( 10.1038/srep23538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firman RC, Simmons LW. 2013. Sperm competition risk generates phenotypic plasticity in ovum fertilizability. Proc. R. Soc. B 280, 20132097 ( 10.1098/rspb.2013.2097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firman RC, Garcia-Gonzalez F, Simmons LW, André GI. 2018. A competitive environment influences sperm production, but not testes tissue composition, in house mice. J. Evol. Biol. 31, 1647–1654. ( 10.1111/jeb.13360) [DOI] [PubMed] [Google Scholar]
- 42.André GI, Firman RC, Simmons LW. 2018. Phenotypic plasticity in genitalia: baculum shape responds to sperm competition risk in house mice. Proc. R. Soc. B 285, 20181086 ( 10.1098/rspb.2018.1086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Firman RC, Simmons LW. 2008. Polyandry, sperm competition, and reproductive success in mice. Behav. Ecol. 19, 695–702. ( 10.1093/beheco/arm158) [DOI] [Google Scholar]
- 44.Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS ONE 7, e35538 ( 10.1371/journal.pone.0035538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pambu-Gollah R, Cronjé PB, Casey NH. 2000. An evaluation of the use of blood metabolite concentrations as indicators of nutritional status in free-ranging indigenous goats. South African J. Anim. Sci. 30, 115–120. ( 10.4314/sajas.v30i2.3859) [DOI] [Google Scholar]
- 46.Cameron EZ, Lemons PR, Bateman PW, Bennett NC. 2008. Experimental alteration of litter sex ratios in a mammal. Proc. R. Soc. B 275, 323–327. ( 10.1098/rspb.2007.1401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firman RC, Simmons LW. 2012. Male house mice evolving with post-copulatory sexual selection sire embryos with increased viability. Ecol. Lett. 15, 42–46. ( 10.1111/j.1461-0248.2011.01706.x) [DOI] [PubMed] [Google Scholar]
- 48.Flores LE, Hildebrandt TB, Kuhl AA, Drews B. 2014. Early detection and staging of spontaneous embryo resorption by ultrasound biomicroscopy in murine pregnancy. Reprod. Biol. Endocrinol. 12, 38 ( 10.1186/1477-7827-12-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClive PJ, Sinclair AH. 2001. Rapid DNA extraction and PCR-sexing of mouse embryos. Mol. Reprod. Dev. 60, 225–226. ( 10.1002/mrd.1081) [DOI] [PubMed] [Google Scholar]
- 50.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121. ( 10.1038/351117a0) [DOI] [PubMed] [Google Scholar]
- 51.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 52.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 53.Zar JH. 1999. Biostatistical analysis, 4th edn Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 54.Tamimi RM, Lagiou P, Mucci LA, Hsieh C-C, Adami H-O, Trichopoulos D. 2003. Average energy intake among pregnant women carrying a boy compared with a girl. Br. Med. J. 326, 1245 ( 10.1136/bmj.326.7401.1245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rutkowska J, Koskela E, Mappes T, Speakman JR. 2011. A trade-off between current and future sex allocation revealed by maternal energy budget in a small mammal. Proc. R. Soc. B 278, 2962–2969. ( 10.1098/rspb.2010.2654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiringer E. 1961. Effect of ACTH on sex ratio of the albino rat. Proc. Soc. Exp. Biol. Med. 106, 752–754. ( 10.3181/00379727-106-26464) [DOI] [PubMed] [Google Scholar]
- 57.Lane EA, Hyde TS. 1973. Effect of maternal stress on fertility and sex ratio: a pilot study with rats. J. Abnorm. Psychol. 82, 78 ( 10.1037/h0034851) [DOI] [PubMed] [Google Scholar]
- 58.Mahmoodkhani M, Saboory E, Roshan-Milani S, Azizi N, Karimipour M, Sayyadi H, Rasmi Y. 2018. Pre-pregnancy stress suppressed the reproductive systems in parents and changed sex ratio in offspring. J. Appl. Biomed. 16, 370–377. ( 10.1016/j.jab.2018.08.002) [DOI] [Google Scholar]
- 59.Chason RJ, McLain AC, Sundaram R, Chen Z, Segars JH, Pyper C, Buck Louis GM. 2012. Preconception stress and the secondary sex ratio: a prospective cohort study. Fertil. Steril. 98, 937–941. ( 10.1016/j.fertnstert.2012.06.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helle S, Laaksonen T, Adamsson A, Paranko J, Huitu O. 2008. Female field voles with high testosterone and glucose levels produce male-biased litters. Anim. Behav. 75, 1031–1039. ( 10.1016/j.anbehav.2007.08.015) [DOI] [Google Scholar]
- 61.Bae J, Lynch CD, Kim S, Sundaram R, Sapra KJ, Buck Louis GM. 2017. Preconception stress and the secondary sex ratio in a population-based preconception cohort. Fertil. Steril. 107, 714–722. ( 10.1016/j.fertnstert.2016.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krackow S. 1992. Sex ratio manipulation in wild house mice: the effect of fetal resorption in relation to the mode of reproduction. Biol. Reprod. 47, 541–548. ( 10.1095/biolreprod47.4.541) [DOI] [PubMed] [Google Scholar]
- 63.Krackow S. 1995. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 70, 225–241. ( 10.1111/j.1469-185X.1995.tb01066.x) [DOI] [PubMed] [Google Scholar]
- 64.Firman RC, Simmons LW. 2010. Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution 64, 1245–1256. [DOI] [PubMed] [Google Scholar]
- 65.Holt WV, Fazeli A. 2016. Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: hypotheses, mechanisms, and new opportunities. Theriogenology 85, 105–112. ( 10.1016/j.theriogenology.2015.07.019) [DOI] [PubMed] [Google Scholar]
- 66.Cameron EZ, Edwards AM, Parsley LM. 2016. Developmental sexual dimorphism and the evolution of mechanisms for adjustment of sex ratios in mammals. Ann. N Y Acad. Sci. 1389, 147–163. ( 10.1111/nyas.13288) [DOI] [PubMed] [Google Scholar]
- 67.Parker GA. 2000. Sperm competition games between related males. Proc. R. Soc. Lond. B 267, 1027–1032. ( 10.1098/rspb.2000.1106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thogerson CM, Brady CM, Howard RD, Mason GJ, Pajor EA, Vicino GA, Garner JP. 2013. Winning the genetic lottery: biasing birth sex ratio results in more grandchildren. PLoS ONE 8, e67867 ( 10.1371/journal.pone.0067867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clutton-Brock T, Albon SD, Guinness FE. 1984. Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308, 358–360. ( 10.1038/308358a0) [DOI] [Google Scholar]
- 70.Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE. 1999. Population density affects sex ratio variation in red deer. Nature 399, 459–461. ( 10.1038/20917) [DOI] [PubMed] [Google Scholar]
- 71.Kaiser S, Sachser N. 2005. The effects of prenatal social stress on behaviour: mechanisms and function. Neurosci. Biobehav. Rev. 29, 283–294. ( 10.1016/j.neubiorev.2004.09.015) [DOI] [PubMed] [Google Scholar]
- 72.Ryan BC, Vandenbergh JG. 2002. Intrauterine position effects. Neurosci. Biobehav. Rev. 26, 665–678. ( 10.1016/S0149-7634(02)00038-6) [DOI] [PubMed] [Google Scholar]
- 73.Clark MM, Tucker L, Galef BG. 1992. Stud males and dud males: intra-uterine position effects on the reproductive success of male gerbils. Anim. Behav. 43, 215–221. ( 10.1016/S0003-3472(05)80217-9) [DOI] [Google Scholar]
- 74.Clark MM, Bone S, Galef BG. 1989. Uterine positions and schedules of urination: correlates of different maternal anogenital stimulation. Dev. Psychobiol. 22, 389–400. ( 10.1002/dev.420220406) [DOI] [PubMed] [Google Scholar]
- 75.Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T. 2009. Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav. Genet. 39, 192–201. ( 10.1007/s10519-008-9246-8) [DOI] [PubMed] [Google Scholar]
- 76.Dufty AM, Clobert J, Moller AP. 2002. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 17, 190–196. ( 10.1016/S0169-5347(02)02498-9) [DOI] [Google Scholar]
- 77.Berghanel A, Heistermann M, Schulke O, Ostner J. 2017. Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc. Natl Acad. Sci. USA 114, E10658–E10666. ( 10.1073/pnas.1707152114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheriff MJ, Love OP. 2013. Determining the adaptive potential of maternal stress. Ecol. Lett. 16, 271–280. ( 10.1111/ele.12042) [DOI] [PubMed] [Google Scholar]
- 79.Weinstock M. 2008. The longterm behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086. ( 10.1016/j.neubiorev.2008.03.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data file is provided in the online electronic supplementary material.




