Abstract
Anthropogenic environmental changes, or ‘stressors’, increasingly threaten biodiversity and ecosystem functioning worldwide. Multiple-stressor research is a rapidly expanding field of science that seeks to understand and ultimately predict the interactions between stressors. Reviews and meta-analyses of the primary scientific literature have largely been specific to either freshwater, marine or terrestrial ecology, or ecotoxicology. In this cross-disciplinary study, we review the state of knowledge within and among these disciplines to highlight commonality and division in multiple-stressor research. Our review goes beyond a description of previous research by using quantitative bibliometric analysis to identify the division between disciplines and link previously disconnected research communities. Towards a unified research framework, we discuss the shared goal of increased realism through both ecological and temporal complexity, with the overarching aim of improving predictive power. In a rapidly changing world, advancing our understanding of the cumulative ecological impacts of multiple stressors is critical for biodiversity conservation and ecosystem management. Identifying and overcoming the barriers to interdisciplinary knowledge exchange is necessary in rising to this challenge. Division between ecosystem types and disciplines is largely a human creation. Species and stressors cross these borders and so should the scientists who study them.
Keywords: multiple stressors, global change factors, multiple drivers, synergism, antagonism, combined effects
1. Introduction
The most severe threats to global biodiversity and ecosystem functioning are anthropogenic environmental changes, or ‘stressors,’ such as habitat loss, climate change, pollution and invasive species [1,2]. These stressors often interact in complex and unexpected ways [3–6]. Multiple-stressor research seeks to understand and predict interactions between stressors. Importantly, owing to these interactions, the combined effect of two or more stressors is frequently more than (synergistic) or less than (antagonistic) expected based on their individual effects [7,8]. The study of multiple stressors is not a novel pursuit in science; toxicologists, and later ecotoxicologists, have been identifying the combined impact of multiple chemical stressors on individual organisms or populations for almost a century [9,10]. Multiple-stressor research has now expanded to more diverse stressor combinations and has become a prominent feature of global change biology. Consequently, the concepts and terms used in the multiple-stressor literature have become common in mainstream biology.
Aquatic, terrestrial and ecotoxicological investigations into multiple stressors differ greatly in their approach. In the freshwater and marine ecology literature, numerous studies have measured biological responses to specific stressor combinations [3,5]. Such work has been conducted across the globe, from the Arctic [11] to the Antarctic [12], and has focused on virtually all taxonomic groups, including bacteria [13], algae [14], invertebrates [15], amphibians [16] and fishes [17]. Parallel to this research, and with almost no lateral exchange, the effects of multiple stressors on ecosystems have been the focus of many terrestrial experiments [18–20]. Contrary to the freshwater and marine literature, the response variables of interest in terrestrial studies are mostly the fluxes and pools of matter such as water, carbon, nitrogen or other nutrients. Another discipline that has dealt with impacts of multiple stressors is ecotoxicology, which focuses on the effects of chemical pollutants and their interactions with other stressors [6,21,22]. Although freshwater, marine and terrestrial subdisciplines exist within ecotoxicology, they share a basic scientific foundation (e.g. methods, journals and conferences), which merits their aggregation as one discipline in this review.
Regardless of differing approaches, the underpinning concepts of multiple-stressor research are similar across the different disciplines. Despite this, exchange and cross-fertilization of ideas and conceptual models have been limited. For example, the co-tolerance concept [23], a number of stressor interaction classification systems (e.g. [7]) and various null models predicting the combined effect of stressors (e.g. [24,25]) have virtually escaped the terrestrial ecology community [4,18,26]. Moreover, models and methods developed in the context of ecotoxicology have largely been ignored in aquatic and terrestrial ecology [27]. Even reviews and meta-analyses of the multiple-stressor literature have primarily been specific to either freshwater [5], marine [3] or terrestrial systems [18], or to ecotoxicology [6] (but see [28,29]).
Differences in terminology attest to the disconnection of freshwater, marine and terrestrial ecologists, as well as ecotoxicologists, from each other. For example, while the terms ‘stressors’, ‘antagonism’ and ‘synergism’ are common within the freshwater, marine and ecotoxicology literature [5,24,30], many terrestrial and some marine ecologists often use the terms ‘drivers/factors’, ‘dampening’ and ‘amplification’, respectively [18,26,31,32]. Other terms such as ‘cumulative effects’, ‘combined effects’, ‘net effects’ or ‘interactive effects’ are used across all disciplines, but without consistent definitions [3,33,34]. The pre-existing separation among scientific disciplines further contributes to this division in multiple-stressor research, exemplified by how ecologists tend not to cite work carried out in systems different from their own [35,36].
A better exchange between the different disciplines studying multiple stressors would be highly desirable. The separation of disciplines, including inconsistency in the terminology, hampers progress in multiple-stressor research because scarce resources are wasted owing to the parallel development of similar methods and tools in different disciplines. Equally, incomplete literature searches and meta-analyses create an ignorance of the complete evidence, which can mislead research directions, impede the spread of ideas and slow down the development of overarching theoretical concepts. In this cross-disciplinary review, we use quantitative bibliometric analysis to identify and illustrate the division between multiple-stressor researchers from different disciplines, we discuss qualitative differences in methods and terminology between the disciplines and we provide a common glossary to harmonize concepts and terminology. Towards a unified research framework, we identify and discuss three common research goals that all multiple-stressor researchers share, specifically (i) increased ecological complexity, (ii) increased temporal scale and realism, with the overarching aim of (iii) improving predictive power.
2. Bibliometric analysis
(a). Methods
Using terms identified during our cross-discipline review, we performed a search of the ISI Web of Knowledge database (https://apps.webofknowledge.com) to collect publications from the multiple-stressor literature (electronic supplementary material, S1). Next, we constructed citation networks where nodes represent specific publications and links indicate a citation between connected publications. Clustering algorithms and citation analysis were used to group publications that cite each other more than they cite other publications in the same network [37]. To enhance visibility, only the most influential publications (top 300 most cited) were used to construct the citation networks. Given that this was biased towards marine and freshwater publications, the 25 next most highly cited terrestrial and ecotoxicological publications were added to ensure a similar number of publications across disciplines. The largest connected network (150 publications: electronic supplementary material, S2) from this pool of 350 publications was selected, ignoring publications outside the multiple-stressor literature. We also created term networks, based on the 150 multiple-stressor publications, using text-mining techniques to identify different clusters of terminology. The publications and terms were manually assigned to one of the disciplines. For details on the bibliometric analysis, see the electronic supplementary material, S2.
(b). Results
A citation network of 150 publications from the multiple-stressor literature with colours representing clusters emerged from our analysis (electronic supplementary material, S3). The size of the nodes was based on the number of citations normalized by the age of publication. When the size of the nodes was based on the number of links in the network, emphasis was put on different nodes (electronic supplementary material, S4). Supplementing our networks with additional publications reduced a bias in terms of nodes but may not have reduced a bias in terms of links (citations); on average, the freshwater and marine publications had more citations than publications from the other disciplines. Consequently, we constructed larger networks using a lower common threshold of citations resulting in networks based on the 500, 1000, 1500 and 2000 most highly cited publications (electronic supplementary material, S5). Although these larger networks are much more difficult to read, clustering patterns similar to electronic supplementary material, S3 are conserved.
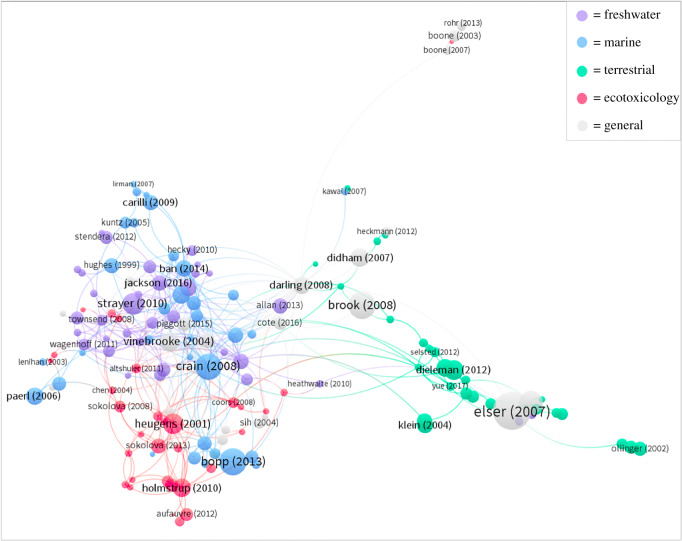
Customizing the colours of the nodes and links to represent the different disciplines reveals the division between disciplines (figure 1). Some of the key papers in the multiple-stressor literature are cited across disciplines and are found towards the centre of the networks [7,8,23,28,29]. Although the freshwater, marine and ecotoxicology literature clearly have their own clusters, these disciplines substantially overlap (particularly freshwater and marine). By contrast, the terrestrial publications form a distinct cluster that is only connected to the rest of the network via five key nodes, which are mostly meta-analyses or reviews [18,28,29,34,38].
Figure 1.
Citation network where the nodes represent publications and the links indicate the presence of a citation between connected publications. The size of the nodes represents the number of citations normalized by age. The distance between nodes is calculated using a citation analysis algorithm which determines the relatedness of items based on the number of times they cite each other. The colours of the nodes and their links represent the disciplines they belong to. (Online version in colour.)
A heat map was produced to quantify the division between disciplines in the citation networks (electronic supplementary material, S6). The terrestrial publications are found almost exclusively in cluster 1 (82.8%) of the citation network (electronic supplementary material, S3). The ecotoxicological publications are found primarily in cluster 4 (54.8%). The freshwater publications are found primarily in clusters 2 (44.1%) and 6 (23.5%). The marine publications are well represented in all clusters in the network, except for clusters 1 and 4.
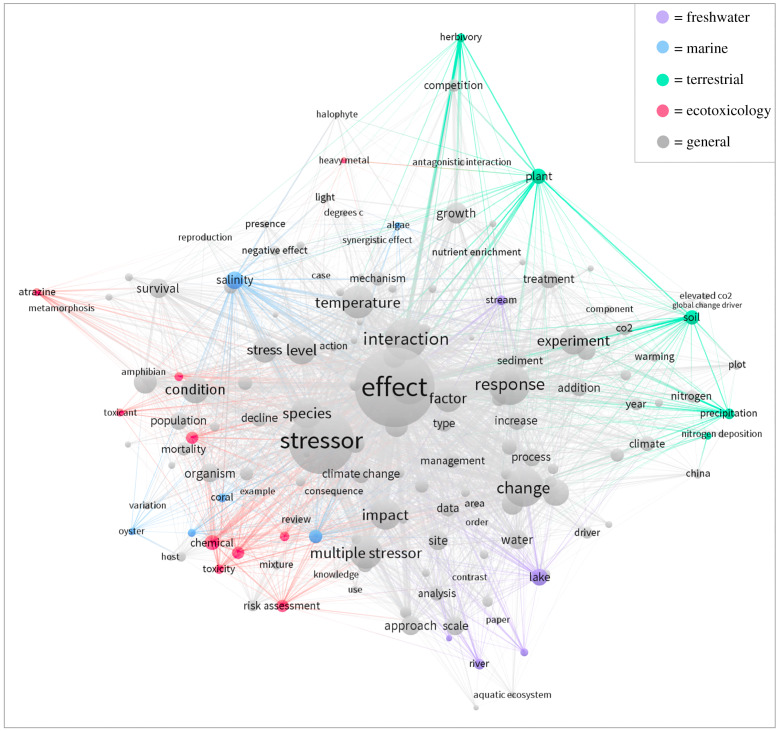
In the term network, nodes towards the centre of the network (e.g. effect, interaction and response) are used by all multiple-stressor researchers, whereas some nodes at the edges of the network are discipline-specific (figure 2). The coloured nodes have been assigned to specific disciplines to outline the approximate location of disciplines in the network (full list of terms in the electronic supplementary material, S7). These coloured terms act as markers against which the location of general terms of interest can be compared. For example, the term ‘multiple stressor’ is found towards the edge of the network near freshwater, marine and ecotoxicological terms; it is on the opposite side of the network from where the terrestrial terms are. Similarly, the term ‘global change driver’ is found among the terrestrial terms and away from the terms specific to the other disciplines.
Figure 2.
Term network constructed using text-mining techniques with the publications from the citation networks (figure 1) as source documents. Terms that occurred at least 10 times were included. The size of the nodes represents the frequency of a term and the links represent co-occurrence. The colours of the nodes and their links represent the disciplines they are associated with. (Online version in colour.)
3. Synthesis
As well as bibliometric analysis, a review of the literature was carried out to investigate how disciplines differ in their study of multiple stressors (summarized in the electronic supplementary material, S8). Our aim was to compare the predictor and response variables, methods and key findings from meta-analyses of multiple-stressor research across disciplines. One of the key findings from our review was that multiple-stressor researchers from different disciplines, despite studying fundamentally the same phenomena, are using different terminology for predictor variables and interactions. Equally, the most common predictor and response variables studied differ among disciplines (table 1), which probably reflect alternative perspectives on which stressors are most important [36].
Table 1.
Comparison of multiple-stressor research across freshwater, marine and terrestrial ecology and ecotoxicology.
| discipline | terminology for predictor variables | terminology for interactions | common predictor variables | common response variables | key references |
|---|---|---|---|---|---|
| freshwater | stressor | additive synergistic antagonistic reversal |
increased temperature altered flow nutrients toxicants habitat modification invasive species |
population metrics functional traits biodiversity |
[5,39,40] |
| marine | stressor driver |
additive synergistic antagonistic |
increased temperature acidification pollutants nutrients high/low salinity hypoxia habitat modification |
physiology population metrics functional traits biodiversity |
[3,30,41] |
| terrestrial | factor driver |
additive synergistic antagonistic dampening amplifying counteracting |
increased temperature increased CO2 land use change nutrient modification altered precipitation invasive species |
fluxes and pools of elements, compounds and nutrients productivity biodiversity |
[18,34,42] |
| ecotoxicology | stressor toxicant toxic chemical |
additive synergistic antagonistic |
toxicants increased temperature salinization drought pathogens or predators |
physiology population metrics biodiversity |
[6,22,24] |
Another difference between and within disciplines is how researchers define a stressor. Many researchers associate stress with a negative biological response [23,43], but others argue that the effect of any stressor is context-dependent and can be positive or negative [7,29,44]. For example, all common stressors (predictor variables) listed in table 1 can cause positive or negative effects depending on the study species or the response variable. Another element to consider is whether a stressor can be natural, or only anthropogenic. Some researchers keep the definition as broad as possible [29,45], whereas others state that what separates a stressor from a ‘driver’, ‘factor’ or ‘disturbance’ is that it is anthropogenic [7,46]. For the latter definition, it is important to note that natural factors such as predation or herbivory can become stressors under human modification.
There is a clear division between terrestrial researchers, who tend not to use the term ‘stressor’ and the rest of the multiple-stressor community. Terrestrial ecology has provided crucial evidence of the combined effect of stressors, but the language used leads to multiple-stressor meta-analyses missing these studies. That is because rather than using the common terminology of multiple-stressor research (e.g. stressor, antagonism or synergism), some studies only refer to the specific factors examined and describe effects as ‘dampening’, ‘amplifying’ or ‘counteracting forces' [26,42,47]. For example, in Darling & Côté's [28] meta-analysis of factorial experiments examining the effects of multiple stressors on animal mortality in freshwater, marine and terrestrial communities, the keywords used in their search included ‘synergy’, ‘antagonism’ and ‘stress’ but lacked ‘amplifying’, ‘dampening’ or ‘factor/driver’. Potentially as a result of this, only four of the 112 experiments in the meta-analysis were conducted with terrestrial organisms (excluding amphibians) [28]. Hence, meta-analyses are useful in that they can identify knowledge gaps and pose new questions, but they reinforce division between disciplines when restricted to certain search terms. Another potential issue is that the same word can have different meanings or connotations in different disciplines, although this is difficult to quantify. For example, the word ‘stressor’ is often associated with negative effects, whereas some researchers, particularly from aquatic disciplines, employ a more neutral interpretation [7,29,44]. This highlights the potential importance of metaphors in creating barriers between disciplines.
As a result of the division between these research communities, certain ideas or approaches can become confined to different disciplines. For example, the terminology and concept of global versus local stressors is often mentioned in the marine literature [14,48,49] but is rarely discussed elsewhere. Similarly, it seems that only freshwater ecologists use the term ‘reversals’ when one stressor reverses the effect of another [5]. For instance, Christensen et al. [38] found that a positive effect of acidification on phytoplankton became negative when warming was introduced. Ecotoxicologists have developed considerable theory on null model selection [24,50], which is only now being introduced to other communities of multiple-stressor research [27]. Novel concepts and approaches do not need to be (re-)discovered multiple times and all disciplines would benefit from a mutual exchange of ideas. We provide a glossary of terms (table 2), with synonyms grouped, as a step towards the unification of multiple-stressor research.
Table 2.
Glossary of widely used terms and concepts in multiple-stressor research; when multiple terms are grouped together we consider them synonyms.
| terms/concepts | our definition | source |
|---|---|---|
| stressor factor driver |
any natural or anthropogenic variable that causes a quantifiable change, irrespective of its direction (increase or decrease), in a biological response. However, many researchers associate the term ‘stressor’ with an anthropogenic variable that has a negative impact | [29] |
| multiple stressors | two or more co-occurring or sequential stressors | n.a. |
| combined effect cumulative effect net effect |
the aggregate effect of multiple stressors and their interactions | n.a. |
| stressor interaction | modification of a stressor's intensity or the sensitivity of an organism or ecosystem towards this stressor by another stressor or multiple other stressors. Thus, the term refers to the interaction between stressors in the real world. By contrast, concepts such as the multiplicative null model rely on mathematical interactions that do not necessarily imply interactions in the real world. Not to be confused with biotic interactions among organisms | [27] |
| additive | when the combined effect of multiple stressors is equal to the sum of their individual effects, i.e. no interaction effect | [8] |
| antagonistic dampening counteracting |
interactions between stressors that result in a lesser combined effect than that predicted by a null model (i.e. an interaction between stressors making their observed net effect less than expected) | [27] |
| synergistic amplifying |
interactions between stressors that result in a greater combined effect than that predicted by a null model (i.e. an interaction between stressors making their observed net effect more than expected) | [27] |
| reversal | interactions that result in the combined effect of two stressors being opposite in the direction (negative or positive) from that of the sum of their single effects | [5] |
| null models | a model that predicts the combined effect of multiple stressors assuming the absence of interactions among stressors as defined above. However, some null models contain mathematical interactions to capture stochastic aspects in the action of two stressors, for example the multiplicative null model | [27] |
| ecological surprises | scenarios where the mechanisms of stressor interactions are not understood and predictions based on null models fail | [51] |
| discipline | a field of science that is represented by specific journals and conferences and consequently establishes a community of scientists. Disciplines are typically taught and researched separately as part of higher education | n.a. |
4. Towards a unified research framework
Despite the division between disciplines described above, all multiple-stressor researchers share the same goals. Elements of these common goals have been identified before but are scattered across the literature in both primary research and reviews. Here, we integrate and develop on these shared research goals of increased (i) ecological complexity, (ii) temporal scale and realism, and (iii) prediction. Our conceptual framework offers a future direction for multiple-stressor research (figure 3). Greater interdisciplinary knowledge exchange, facilitated by this review, is a key component of this framework.
Figure 3.
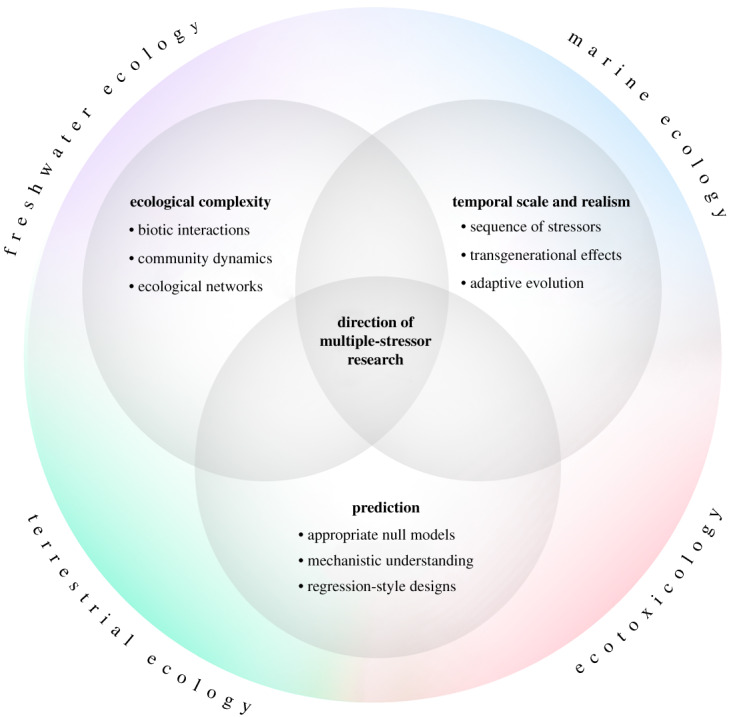
An integrative conceptual framework of research goals shared by all disciplines, highlighting the future direction of multiple-stressor research. (Online version in colour.)
(a). Ecological complexity
Multiple-stressor research needs to shift its focus towards higher levels of biological organization as ecosystem managers are primarily interested in the effect of stressors on communities and ecosystems [25,51]. Researchers have called for this increase in ecological complexity in freshwater [52,53], marine [31,54] and terrestrial [26] ecology as well as in ecotoxicology [55]. A key question is to what extent species interactions explain statistical interactions between stressors themselves at the community and ecosystem level.
Several different approaches have been taken to evaluate the roles of species interactions and the level of organization in responses to multiple stressors. For example, in their review of 171 multiple-stressor studies in marine and coastal ecosystems, Crain et al. [3] found that synergism was most common in population-level studies, but antagonism was most common in community-level studies. Similarly, Côté et al. [29] found that synergism became less common as a biological scale increased in their quantitative review across disciplines. However, Jackson et al. [5] found no significant difference in the frequencies of interaction types at the different biological levels in their review of freshwater studies. Moving beyond this ‘vote-counting’ approach, researchers have conducted specific experimental [56,57] and modelling [58,59] research on this topic. For example, Galic et al. [58] used population models to show that hypothetical stressors with different modes of action primarily interacted antagonistically at the individual level but synergistically at the population level.
Some theory has been developed to predict the impacts of multiple stressors at higher levels of organization [25,51]. De Laender [51] showed how competition for common resources can lead to both synergistic and antagonistic effects of multiple stressors on species richness. In general, the combined effect of multiple stressors can be amplified at the community level when stressors act on influential groups such as keystone species or ecosystem engineers [45,60]. Likewise, biotic interactions can mitigate the effect of stressors (e.g. [61,62]). For example, a modelling study showed that negative interactions among species (e.g. competition) increased the net negative effects of external stressors on community-level properties, while positive species interactions (e.g. mutualism) lessened negative impacts [44].
Interspecific interactions may themselves change after exposure to stressors. For example, stressors may influence resource competition [63] and may change the susceptibility of hosts to pathogens and parasites [64,65]. Equally, stressors can alter the trophic relationships of species [56,66]. Schrama et al. [67] applied multiple pesticides to pond mesocosms and used stable isotope analysis to show that these stressors and their interactions modified the flow of energy through the food web by inducing shifts in trophic links. Furthermore, biotic interactions can themselves act as stressors and consequently interact with other stressors. For instance, the interactions between climate change and ungulate herbivory modulate effects on forest ecosystems (e.g. [68]).
The importance of biotic interactions in understanding the effects of stressors highlights the need for an ecological network approach towards multiple-stressor studies [69]. Developments in technologies such as DNA metabarcoding and stable isotope analysis are improving our ability to detect and quantify biotic interactions [70,71]. With these technologies, multiple-stressor researchers will be able to clarify to what degree biotic interactions mediate the statistical interactions between stressors and to ultimately determine how we understand and predict the effects of multiple stressors.
(b). Temporal scale and realism
The combined effects of stressors depend on various, largely overlooked, factors related to different time scales [29,30]. At the time scale within one generation, several temporal factors have been identified that may determine responses to multiple stressors. First, the sequence of exposure to stressors may be crucial. For example, the order of exposure of two toxicants determined their combined effect on Gammarus pulex [72]. Here, if species' responses to stressors are negatively correlated, the sequence of exposure may be more important than if their responses are positively correlated [23]. Specifically, if paired stressors each exert a different effect on species, the order of exposure may be more important than if their effects are redundant. Second, the time interval between stressors may influence their combined impact. Gunderson et al. [30] developed a conceptual framework that predicts the interaction type between sequential exposure to two stressors to be additive when the time interval between exposure is long, but synergistic when time interval is short. Notably, there may also be a time lag between the simultaneous exposure to two stressors and the synergistic effect. For example, combined exposure to both warming and a pollutant in the larval stage of a damselfly generated a strong synergistic effect across metamorphosis by reducing adult lifespan [73]. Interactions between stressors can also depend on the developmental stage of an organism. Indeed, interactive effects may change, and even reverse, throughout ontogeny. Przeslawski et al. [41] showed in a meta-analysis of marine organisms that the combination of thermal and salinity stress was more likely to be synergistic for embryonic than for larval life stages, yet the opposite pattern occurred between thermal and pH stress. Few studies, however, have tested variation in interactions across developmental stages within the same species (but see [74]).
At the time scale of a few generations, little is known about how the interaction type between stressors in offspring depends on the exposure of the parents to those stressors. As a rare example, a synergistic interaction between warming and a pollutant was detected in the mosquito Culex pipiens both in the parents and in the offspring of parents exposed to none or a single stressor. By contrast, an additive effect was present in the offspring of parents exposed to both stressors simultaneously, because in this condition the pesticide was already more lethal at the lower temperature [75]. At the time scale of tens of generations, the evolution of adaptation to a stressor may shape tolerance to subsequent stressors because of pleiotropic effects where the same set of genes contributes to tolerance against different stressors. This may cause co-tolerance where the acquisition of genetic adaptation to one stressor increases tolerance to another [76], which is likely as genetic mechanisms of tolerance to stressors are often conserved [77]. However, pleiotropic effects may also be antagonistic, resulting in adaptive evolution to one stressor actually reducing tolerance to a second [78]. It is important to note that adaptation [79] and acclimatization [80] to a stressor may come at a fitness cost. Finally, at a time scale of hundreds of generations, the evolution of thermal tolerance of a damselfly most likely resulted in the synergistic interaction between warming and a pollutant in high-latitude populations to become additive in low-latitude populations [81].
Experiments should attempt to use realistic timing of stressors over meaningful time scales (e.g. [82]), but this can be impractical, and observational studies may need to fill this gap [83]. Furthermore, certain stressors, for example nitrogen deposition [84], accumulate over time, which can delay ecological effects and further complicate multiple-stressor predictions. Importantly, the background variation under ambient conditions needs to be considered: a recent example from plant communities showed that ambient changes may actually outweigh the impact of stressors over time [85]. Understanding if and how interactions between stressors can change over time is a goal shared by all disciplines.
(c). Prediction
The ultimate goal of multiple-stressor research is the prediction of the combined effect of stressors. This would allow for the incorporation of multiple-stressor research into a risk assessment framework [86]. Over the past 20 years, a vast amount of research has been conducted to test the effects of specific combinations of stressors on specific response variables. However, very few, if any, general patterns have emerged from meta-analyses [3–6,17,18]. This approach to studying multiple stressors, calculating proportions of interaction types across different environments, conditions and responses, does not improve our predictive capacity of multiple stressors for a variety of reasons, including the existence of a publication bias towards synergism [29]. Furthermore, the results are often context-dependent [45] and prevent generalization, apart from the fact that non-additivity between stressors is common.
To advance research of multiple stressors, there is a need to move beyond comparing proportions of interaction types and shift focus towards improving our mechanistic understanding of stressor interactions. A shift towards regression-style experimental designs would enhance our understanding of stressor–response relationships, thus increasing our ability to predict threshold responses [87,88]. When predicting the combined effects of multiple stressors, it is important to consider both the modes of action of stressors and their interactions. For example, the similarity or dissimilarity of stressors’ modes of action may reveal important information about how they may interact [8,23]. Equally, according to Boyd & Brown [31], there are multiple modes of interaction between stressors at the physico-chemical, organismal and ecosystem levels. This concept, of statistical interactions between stressors occurring as a result of interactions between stressors at different scales, is gaining more attention (e.g. [45,54]).
A major issue that needs to be resolved is the use of null models. The additive null model has been widely used, but also widely criticized for being inappropriate in many scenarios [29]. For example, it is biased towards antagonism when metrics with a fixed boundary, such as mortality, are used as response variables [8,17]. Many null models can be useful for multiple-stressor researchers, including both established models from the ecotoxicological literature and new developments such as the Stress Addition Model [24] and the Compositional Null Model [25]. Researchers need to be aware of the different null models available and their association with statistical tests [55]. A recent framework for a mechanistic basis to null model selection aims to facilitate a shift towards a more predictive approach [27]. The objective is to use null models that accurately predict the combined effects of stressors. ‘Ecological surprises' arise when our null models are wrong, and researchers are unable to explain why. Debate over null models and the emerging publications have almost entirely bypassed the terrestrial global change research community, even though such considerations could influence the interpretation of some of their findings considerably. Predicting the impacts of multiple stressors is a common goal shared by all disciplines, and achieving this goal is vital for the sustainable management of resources and for the conservation of biodiversity and ecosystem services.
5. Conclusion
Multiple-stressor researchers from different disciplines are clearly separated. This was identified during our cross-disciplinary review and was confirmed using bibliometric analysis. The use of different terminology for predictor variables and for interactions between those variables has reinforced this separation. Common terminology, or at least awareness of the different terms in online searches and meta-analyses, would greatly enhance cross-disciplinary collaboration and would encourage the integration of multiple-stressor research into mainstream ecology. In fact, our conclusion that researchers should be aware of terminology from different disciplines applies to all ecological research.
In future work, researchers should consider multiple-stressor literature from other disciplines for guidance on methods and analyses. Authors of primary research should include multiple terms in their keyword section to enhance the visibility of their research. However, limits on the number of keywords in journals may incentivize authors to only use keywords relevant to their own discipline. Meta-analyses of the multiple-stressor literature should consider the broader range of terminology identified in this review (see common glossary in table 2) and, where possible, be repeated to include relevant but previously missed studies. Multiple-stressor research is moving forward with all disciplines converging towards the same common goals, and the time is ripe for a unified approach. Division between ecosystem types and disciplines is largely a human creation. Species and stressors cross these borders, and so should the scientists who study them.
Supplementary Material
Acknowledgements
This manuscript is the product of a cross-disciplinary workshop, StressNet, intended to bridge the gaps among the different disciplines studying the impacts of multiple stressors.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
J.A.O. was funded by an Irish Research Council Laureate Award (IRCLA/2017/112) and TCD Provost's PhD Award held by J.J.P. during the writing of this review. R.B.S. received funding for the StressNet workshop from the DFG and the University of Koblenz-Landau.
References
- 1.Urban MC. 2015. Accelerating extinction risk from climate change. Science 348, 571–573. ( 10.1126/science.aaa4984) [DOI] [PubMed] [Google Scholar]
- 2.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 3.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 4.Dieleman WI, et al. 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Change Biol. 18, 2681–2693. ( 10.1111/j.1365-2486.2012.02745.x) [DOI] [PubMed] [Google Scholar]
- 5.Jackson MC, Loewen CJ, Vinebrooke RD, Chimimba CT. 2016. Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob. Change Biol. 22, 180–189. ( 10.1111/gcb.13028) [DOI] [PubMed] [Google Scholar]
- 6.Holmstrup M, et al. 2010. Interactions between effects of environmental chemicals and natural stressors: a review. Sci. Total Environ. 408, 3746–3762. ( 10.1016/j.scitotenv.2009.10.067) [DOI] [PubMed] [Google Scholar]
- 7.Piggott JJ, Townsend CR, Matthaei CD. 2015. Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547. ( 10.1002/ece3.1465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folt C, Chen C, Moore M, Burnaford J. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3_part_2.0864) [DOI] [Google Scholar]
- 9.Bliss C. 1939. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26, 585–615. ( 10.1111/j.1744-7348.1939.tb06990.x) [DOI] [Google Scholar]
- 10.Loewe S, Muischnek H. 1926. Über Kombinationswirkungen. Naunyn-Schmiedebergs Archiv. Exp. Pathol. Pharmakol. 114, 313–326. ( 10.1007/BF01952257) [DOI] [Google Scholar]
- 11.Andersen JH, Berzaghi F, Christensen T, Geertz-Hansen O, Mosbech A, Stock A, Zinglersen KB, Wisz MS. 2017. Potential for cumulative effects of human stressors on fish, sea birds and marine mammals in Arctic waters. Estuarine Coastal Shelf Sci. 184, 202–206. ( 10.1016/j.ecss.2016.10.047) [DOI] [Google Scholar]
- 12.Lenihan HS, Peterson CH, Miller RJ, Kayal M, Potoski M. 2018. Biotic disturbance mitigates effects of multiple stressors in a marine benthic community. Ecosphere 9, e02314 ( 10.1002/ecs2.2314) [DOI] [Google Scholar]
- 13.Salis R, Bruder A, Piggott J, Summerfield T, Matthaei C. 2017. High-throughput amplicon sequencing and stream benthic bacteria: identifying the best taxonomic level for multiple-stressor research. Sci. Rep. 7, 44657 ( 10.1038/srep44657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strain EM, van Belzen J, van Dalen J, Bouma TJ, Airoldi L. 2015. Management of local stressors can improve the resilience of marine canopy algae to global stressors. PLoS ONE 10, e0120837 ( 10.1371/journal.pone.0120837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaunisto S, Ferguson LV, Sinclair BJ. 2016. Can we predict the effects of multiple stressors on insects in a changing climate? Curr. Opin. Insect Sci. 17, 55–61. ( 10.1016/j.cois.2016.07.001) [DOI] [PubMed] [Google Scholar]
- 16.Boone MD, Semlitsch RD, Little EE, Doyle MC. 2007. Multiple stressors in amphibian communities: effects of chemical contamination, bullfrogs, and fish. Ecol. Appl. 17, 291–301. ( 10.1890/1051-0761(2007)017[0291:MSIACE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 17.Lange K, Bruder A, Matthaei CD, Brodersen J, Paterson RA. 2018. Multiple-stressor effects on freshwater fish: importance of taxonomy and life stage. Fish Fisheries 19, 974–983. ( 10.1111/faf.12305) [DOI] [Google Scholar]
- 18.Yue K, Fornara DA, Yang W, Peng Y, Li Z, Wu F, Peng C. 2017. Effects of three global change drivers on terrestrial C: N: P stoichiometry: a global synthesis. Glob. Change Biol. 23, 2450–2463. ( 10.1111/gcb.13569) [DOI] [PubMed] [Google Scholar]
- 19.Larsen KS, et al. 2011. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments. Glob. Change Biol. 17, 1884–1899. ( 10.1111/j.1365-2486.2010.02351.x) [DOI] [Google Scholar]
- 20.Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang G. 2019. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366, 886–890. ( 10.1126/science.aay2832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laskowski R, Bednarska AJ, Kramarz PE, Loureiro S, Scheil V, Kudłek J, Holmstrup M. 2010. Interactions between toxic chemicals and natural environmental factors: a meta-analysis and case studies. Sci. Total Environ. 408, 3763–3774. ( 10.1016/j.scitotenv.2010.01.043) [DOI] [PubMed] [Google Scholar]
- 22.Moe SJ, De Schamphelaere K, Clements WH, Sorensen MT, Van den Brink PJ, Liess M. 2013. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ. Toxicol. Chem. 32, 49–61. ( 10.1002/etc.2045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinebrooke RD, Cottingham KL, Norberg MS, Dodson SI, Maberly SC, Sommer U. 2004. Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104, 451–457. ( 10.1111/j.0030-1299.2004.13255.x) [DOI] [Google Scholar]
- 24.Liess M, Foit K, Knillmann S, Schäfer RB, Liess H-D. 2016. Predicting the synergy of multiple stress effects. Sci. Rep. 6, 32965 ( 10.1038/srep32965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson PL, MacLennan MM, Vinebrooke RD. 2018. An improved null model for assessing the net effects of multiple stressors on communities. Glob. Change Biol. 24, 517–525. ( 10.1111/gcb.13852) [DOI] [PubMed] [Google Scholar]
- 26.Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol. Evol. 26, 236–241. ( 10.1016/j.tree.2011.02.011) [DOI] [PubMed] [Google Scholar]
- 27.Schäfer RB, Piggott JJ. 2018. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Glob. Change Biol. 24, 1817–1826. ( 10.1111/gcb.14073) [DOI] [PubMed] [Google Scholar]
- 28.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. ( 10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 29.Côté IM, Darling ES, Brown CJ. 2016. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 283, 20152592 ( 10.1098/rspb.2015.2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunderson AR, Armstrong EJ, Stillman JH. 2016. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annu. Rev. Marine Sci. 8, 357–378. ( 10.1146/annurev-marine-122414-033953) [DOI] [PubMed] [Google Scholar]
- 31.Boyd PW, Brown CJ. 2015. Modes of interactions between environmental drivers and marine biota. Front. Marine Sci. 2, 9 ( 10.3389/fmars.2015.00009) [DOI] [Google Scholar]
- 32.Sirami C, Caplat P, Popy S, Clamens A, Arlettaz R, Jiguet F, Brotons L, Martin J-L. 2017. Impacts of global change on species distributions: obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 26, 385–394. ( 10.1111/geb.12555) [DOI] [Google Scholar]
- 33.Harvey BP, Gwynn-Jones D, Moore PJ. 2013. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3, 1016–1030. ( 10.1002/ece3.516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Zhou X, Shao J, Nie Y, He Y, Jiang L, Wu Z, Hosseini Bai S. 2016. Interactive effects of global change factors on soil respiration and its components: a meta-analysis. Glob. Change Biol. 22, 3157–3169. ( 10.1111/gcb.13253) [DOI] [PubMed] [Google Scholar]
- 35.Menge BA, et al. 2009. Terrestrial ecologists ignore aquatic literature: asymmetry in citation breadth in ecological publications and implications for generality and progress in ecology. J. Exp. Mar. Biol. Ecol. 377, 93–100. ( 10.1016/j.jembe.2009.06.024) [DOI] [Google Scholar]
- 36.Knapp S, et al. 2017. Do drivers of biodiversity change differ in importance across marine and terrestrial systems: or is it just different research communities' perspectives? Sci. Total Environ. 574, 191–203. ( 10.1016/j.scitotenv.2016.09.002) [DOI] [PubMed] [Google Scholar]
- 37.Van Eck N, Waltman L. 2009. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. ( 10.1007/s11192-009-0146-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen MR, Graham MD, Vinebrooke RD, Findlay DL, Paterson MJ, Turner MA. 2006. Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob. Change Biol. 12, 2316–2322. ( 10.1111/j.1365-2486.2006.01257.x) [DOI] [Google Scholar]
- 39.Ormerod SJ, Dobson M, Hildrew AG, Townsend C. 2010. Multiple stressors in freshwater ecosystems. Freshw. Biol. 55, 1–4. ( 10.1111/j.1365-2427.2009.02395.x) [DOI] [Google Scholar]
- 40.Hering D, et al. 2015. Managing aquatic ecosystems and water resources under multiple stress: an introduction to the MARS project. Sci. Total Environ. 503, 10–21. ( 10.1016/j.scitotenv.2014.06.106) [DOI] [PubMed] [Google Scholar]
- 41.Przeslawski R, Byrne M, Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21, 2122–2140. ( 10.1111/gcb.12833) [DOI] [PubMed] [Google Scholar]
- 42.Borer ET, et al. 2014. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508, 517–520. ( 10.1038/nature13144) [DOI] [PubMed] [Google Scholar]
- 43.Boyd PW, Hutchins DA. 2012. Understanding the responses of ocean biota to a complex matrix of cumulative anthropogenic change. Mar. Ecol. Progr. Ser. 470, 125–135. ( 10.3354/meps10121) [DOI] [Google Scholar]
- 44.Thompson PL, MacLennan MM, Vinebrooke RD. 2018. Species interactions cause non-additive effects of multiple environmental stressors on communities. Ecosphere 9, e02518 ( 10.1002/ecs2.2518) [DOI] [Google Scholar]
- 45.Kroeker KJ, Kordas RL, Harley CD. 2017. Embracing interactions in ocean acidification research: confronting multiple stressor scenarios and context dependence. Biol. Lett. 13, 20160802 ( 10.1098/rsbl.2016.0802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend CR, Uhlmann SS, Matthaei CD. 2008. Individual and combined responses of stream ecosystems to multiple stressors. J. Appl. Ecol. 45, 1810–1819. ( 10.1111/j.1365-2664.2008.01548.x) [DOI] [Google Scholar]
- 47.Gruner DS, et al. 2008. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 11, 740–755. ( 10.1111/j.1461-0248.2008.01192.x) [DOI] [PubMed] [Google Scholar]
- 48.Russell BD, Connell SD. 2012. Origins and consequences of global and local stressors: incorporating climatic and non-climatic phenomena that buffer or accelerate ecological change. Mar. Biol. 159, 2633–2639. ( 10.1007/s00227-011-1863-8) [DOI] [Google Scholar]
- 49.Brown CJ, Saunders MI, Possingham HP, Richardson AJ. 2013. Managing for interactions between local and global stressors of ecosystems. PLoS One 8, e65765 ( 10.1371/journal.pone.0065765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backhaus T, Faust M. 2012. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ. Sci. Technol. 46, 2564–2573. ( 10.1021/es2034125) [DOI] [PubMed] [Google Scholar]
- 51.De Laender F. 2018. Community- and ecosystem-level effects of multiple environmental change drivers: beyond null model testing. Glob. Change Biol. 24, 5021–5030. ( 10.1111/gcb.14382) [DOI] [PubMed] [Google Scholar]
- 52.Bray J, Reich J, Nichols S, Kon Kam King G, Mac Nally R, Thompson R, O'Reilly-Nugent A, Kefford BJ. 2018. Biological interactions mediate context and species-specific sensitivities to salinity. Phil. Trans. R. Soc. B 374, 20180020 ( 10.1098/rstb.2018.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuwirth N, Dietzel A, Reichert P. 2016. The importance of biotic interactions for the prediction of macroinvertebrate communities under multiple stressors. Funct. Ecol. 30, 974–984. ( 10.1111/1365-2435.12605) [DOI] [Google Scholar]
- 54.Griffen BD, Belgrad BA, Cannizzo ZJ, Knotts ER, Hancock ER. 2016. Rethinking our approach to multiple stressor studies in marine environments. Mar. Ecol. Progr. Ser. 543, 273–281. ( 10.3354/meps11595) [DOI] [Google Scholar]
- 55.Van den Brink PJ, et al. 2018. Toward sustainable environmental quality: priority research questions for Europe. Environ. Toxicol. Chem. 37, 2281–2295. ( 10.1002/etc.4205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruder A, Salis RK, Jones PE, Matthaei CD. 2017. Biotic interactions modify multiple-stressor effects on juvenile brown trout in an experimental stream food web. Glob. Change Biol. 23, 3882–3894. ( 10.1111/gcb.13696) [DOI] [PubMed] [Google Scholar]
- 57.O'Gorman EJ, Fitch JE, Crowe TP. 2012. Multiple anthropogenic stressors and the structural properties of food webs. Ecology 93, 441–448. ( 10.1890/11-0982.1) [DOI] [PubMed] [Google Scholar]
- 58.Galic N, Sullivan LL, Grimm V, Forbes VE. 2018. When things don't add up: quantifying impacts of multiple stressors from individual metabolism to ecosystem processing. Ecol. Lett. 21, 568–577. ( 10.1111/ele.12923) [DOI] [PubMed] [Google Scholar]
- 59.Griffith GP, Strutton PG, Semmens JM, Fulton EA. 2019. Identifying important species that amplify or mitigate the interactive effects of human impacts on marine food webs. Conserv. Biol. 33, 403–412. ( 10.1111/cobi.13202) [DOI] [PubMed] [Google Scholar]
- 60.Gooding RA, Harley CD, Tang E. 2009. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc. Natl Acad. Sci. USA 106, 9316–9321. ( 10.1073/pnas.0811143106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bulleri F, et al. 2018. Harnessing positive species interactions as a tool against climate-driven loss of coastal biodiversity. PLoS Biol. 16, e2006852 ( 10.1371/journal.pbio.2006852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piscart C, Webb D, Beisel JN. 2007. An acanthocephalan parasite increases the salinity tolerance of the freshwater amphipod Gammarus roeseli (Crustacea: Gammaridae). Sci. Nat. 94, 741–747. ( 10.1007/s00114-007-0252-0) [DOI] [PubMed] [Google Scholar]
- 63.Kroeker KJ, Micheli F, Gambi MC. 2013. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Change 3, 156 ( 10.1038/nclimate1680) [DOI] [Google Scholar]
- 64.Lafferty KD, Holt RD. 2003. How should environmental stress affect the population dynamics of disease? Ecol. Lett. 6, 654–664. ( 10.1046/j.1461-0248.2003.00480.x) [DOI] [Google Scholar]
- 65.Lenihan HS, Micheli F, Shelton SW, Peterson CH. 1999. The influence of multiple environmental stressors on susceptibility to parasites: an experimental determination with oysters. Limnol. Oceanogr. 44, 910–924. ( 10.4319/lo.1999.44.3_part_2.0910) [DOI] [Google Scholar]
- 66.Arnold T, Mealey C, Leahey H, Miller AW, Hall-Spencer JM, Milazzo M, Maers K. 2012. Ocean acidification and the loss of phenolic substances in marine plants. PLoS ONE 7, e35107 ( 10.1371/journal.pone.0035107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schrama M, Barmentlo SH, Hunting ER, van Logtestijn RS, Vijver MG, van Bodegom PM. 2017. Pressure-induced shifts in trophic linkages in a simplified aquatic food web. Front. Environ. Sci. 5, 75 ( 10.3389/fenvs.2017.00075) [DOI] [Google Scholar]
- 68.Didion M, Kupferschmid A, Wolf A, Bugmann H. 2011. Ungulate herbivory modifies the effects of climate change on mountain forests. Clim. Change 109, 647–669. ( 10.1007/s10584-011-0054-4) [DOI] [Google Scholar]
- 69.Bruder A, Frainer A, Rota T, Primicerio R. 2019. The importance of ecological networks in multiple-stressor research and management. Front. Environ. Sci. 7, 59 ( 10.3389/fenvs.2019.00059) [DOI] [Google Scholar]
- 70.Layman CA, et al. 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol. Rev. 87, 545–562. ( 10.1111/j.1469-185X.2011.00208.x) [DOI] [PubMed] [Google Scholar]
- 71.Roslin T, Majaneva S. 2016. The use of DNA barcodes in food web construction: terrestrial and aquatic ecologists unite! Genome 59, 603–628. ( 10.1139/gen-2015-0229) [DOI] [PubMed] [Google Scholar]
- 72.Ashauer R, O'Connor I, Escher BI. 2017. Toxic mixtures in time: the sequence makes the poison. Environ. Sci. Technol. 51, 3084–3092. ( 10.1021/acs.est.6b06163) [DOI] [PubMed] [Google Scholar]
- 73.Debecker S, Dinh KV, Stoks R. 2017. Strong delayed interactive effects of metal exposure and warming: latitude-dependent synergisms persist across metamorphosis. Environ. Sci. Technol. 51, 2409–2417. ( 10.1021/acs.est.6b04989) [DOI] [PubMed] [Google Scholar]
- 74.Fitzgerald JA, Katsiadaki I, Santos EM. 2017. Contrasting effects of hypoxia on copper toxicity during development in the three-spined stickleback (Gasterosteus aculeatus). Environ. Pollut. 222, 433–443. ( 10.1016/j.envpol.2016.12.008) [DOI] [PubMed] [Google Scholar]
- 75.Tran TT, Janssens L, Dinh KV, Stoks R. 2018. Transgenerational interactions between pesticide exposure and warming in a vector mosquito. Evolution. Appl. 11, 906–917. (do 10.1111/eva.12605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bubliy O, Loeschcke V. 2005. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 18, 789–803. ( 10.1111/j.1420-9101.2005.00928.x) [DOI] [PubMed] [Google Scholar]
- 77.Sikkink KL, Reynolds RM, Cresko WA, Phillips PC. 2015. Environmentally induced changes in correlated responses to selection reveal variable pleiotropy across a complex genetic network. Evolution 69, 1128–1142. ( 10.1111/evo.12651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hua J, Wuerthner VP, Jones DK, Mattes B, Cothran RD, Relyea RA, Hoverman JT. 2017. Evolved pesticide tolerance influences susceptibility to parasites in amphibians. Evolution. Appl. 10, 802–812. ( 10.1111/eva.12500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffmann AA, Sørensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. ( 10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 80.Goussen B, Price OR, Rendal C, Ashauer R. 2016. Integrated presentation of ecological risk from multiple stressors. Sci. Rep. 6, 36004 ( 10.1038/srep36004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van K D, Janssens L, Debecker S, De Jonge M, Lambret P, Nilsson-Örtman V, Bervoets L, Stoks R. 2013. Susceptibility to a metal under global warming is shaped by thermal adaptation along a latitudinal gradient. Glob. Change Biol. 19, 2625–2633. ( 10.1111/gcb.12243) [DOI] [PubMed] [Google Scholar]
- 82.Cheng BS, et al. 2015. Testing local and global stressor impacts on a coastal foundation species using an ecologically realistic framework. Glob. Change Biol. 21, 2488–2499. ( 10.1111/gcb.12895) [DOI] [PubMed] [Google Scholar]
- 83.Hättenschwiler S, Miglietta F, Raschi A, Körner C. 1997. Thirty years of in situ tree growth under elevated CO2: a model for future forest responses? Glob. Change Biol. 3, 463–471. ( 10.1046/j.1365-2486.1997.00105.x) [DOI] [Google Scholar]
- 84.Payne RJ, et al. 2019. What is the most ecologically-meaningful metric of nitrogen deposition? Environ. Pollut. 247, 319–331. ( 10.1016/j.envpol.2019.01.059) [DOI] [PubMed] [Google Scholar]
- 85.Langley JA, et al. 2018. Ambient changes exceed treatment effects on plant species abundance in global change experiments. Glob. Change Biol. 24, 5668–5679. ( 10.1111/gcb.14442) [DOI] [PubMed] [Google Scholar]
- 86.Van den Brink PJ, Choung CB, Landis W, Mayer-Pinto M, Pettigrove V, Scanes P, Smith R, Stauber J. 2016. New approaches to the ecological risk assessment of multiple stressors. Mar. Freshw. Res. 67, 429–439. ( 10.1071/MF15111) [DOI] [Google Scholar]
- 87.Kreyling J, Schweiger AH, Bahn M, Ineson P, Migliavacca M, Morel-Journel T, Christiansen JR, Schtickzelle N, Larsen KS. 2018. To replicate, or not to replicate—that is the question: how to tackle nonlinear responses in ecological experiments. Ecol. Lett. 21, 1629–1638. ( 10.1111/ele.13134) [DOI] [PubMed] [Google Scholar]
- 88.Boyd PW, et al. 2018. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change: a review. Glob. Change Biol. 24, 2239–2261. ( 10.1111/gcb.14102) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.