Abstract
Head and neck cancers are a heterogeneous group of tumors that are highly aggressive and collectively represent the sixth most common cancer worldwide. 90% of head and neck cancers are squamous cell carcinomas (HNSCCs). The tumor microenvironment (TME) of HNSCCs consists of many different subsets of cells that infiltrate the tumors and interact with the tumor cells or with each other through various networks. Both innate and adaptive immune cells play a crucial role in mediating immune surveillance and controlling tumor growth. Here, we discuss the different subsets of immune cells and how they contribute to an immunosuppressive TME of HNSCCs. We also briefly summarize recent advances in immunotherapeutic approaches for HNSCC treatment. A better understanding of the multiple factors that play pivotal roles in HNSCC tumorigenesis and tumor progression may help define novel targets in order to develop more effective immunotherapies for HNSCC patients.
Keywords: cancer immunotherapy, head and neck cancers, tumor microenvironment, tumor infiltrating lymphocytes
Overview of head and neck squamous cell carcinoma (HNSCCs)
Head and neck cancers (HNC) are a heterogeneous group of tumors arising from the mucosal surfaces of the upper aerodigestive tract which includes the sinonasal and oral cavities, nasopharynx, oropharynx, hypopharynx, and larynx. Collectively, HNC is the sixth most prevalent cancer worldwide with over 880,000 new cases diagnosed and more than 450,000 patients die each year1. 90% of all HNCs are head and neck squamous cell carcinomas (HNSCCs) and roughly 75% of these cases are associated with alcohol and tobacco use. However, emergences of new studies have shown that oncogenic human papillomavirus (HPV) infection may be a risk factor associated with 22% of oropharyngeal squamous cell carcinoma (OSCC) and 47% of tonsillar squamous cell carcinomas (TSCC)2–5. HNSCCs can severely impact the quality of life of patients while have poor prognosis, low responsiveness to treatment and drug resistance. HNSCC malignancy has a high morbidity and mortality rate since only 50%–60% of patients have a survival rate of 5 years after diagnosis of HNSCC, and up to 30% of patients develop cancer relapse and treatment failure6. It has been found that one of the most imperative prognostic determinants of the survival rate from HNSCC is the presence of lymph node metastases7.
Immune cells in HNSCCs
A major deterrent of HNSCC treatment is the high rate of recurrence and/or metastases in patients, which not only stresses the difficulty in treating HNSCCs but also conveys the complex molecular conditions. The rationale behind the high rate of metastatic HNSCC and recurrence is likely due to the interactions of the surrounding tissue matrix and immune cells that make up the tumor microenvironment (TME). The host immune system is capable of recognizing and eliminating neoplastic cells; however, evasion of immunosurveillance generates an environment that accommodates the progression and survival of tumor cells6,8–10. Interestingly, HNSCCs have the ability to not only avoid recognition by immune cells, but they are also immune-suppressive6,10. This immune evasion is achieved by downregulating human leucocyte antigen (HLA) expression, which in turn impairs cancer cell recognition by T cells11. In addition, the HNSCC TME has also been shown to impair tumor-infiltrating lymphocyte (TIL) function10,12,13. TME is composed of different subsets of cells, such as cancer-associated stromal fibroblasts, T cells, B cells, neutrophils, macrophages, myeloid-derived suppressor cells (MDSC), natural killer (NK) cells and mast cells9,10,14–18. These different subsets of cells infiltrate the tumors and interact with the tumor cells as well as with each other through various networks (Figure 1). Both innate (e.g., NK cells) and adaptive (e.g., CD8+ T cells) immune cells play a crucial role in immune surveillance and controlling tumor growth; on the other hand, some subsets of immune cells (e.g., MDSCs and macrophages) can also promote tumor growth19. Thus, tumors progress if they can escape and/or suppress anti-tumor immune responses. In this regard, tumors often evade host’s immune surveillance by suppressing cytotoxic T cell function or by activating and expanding immunosuppressive cell populations. The mechanisms by which HNSCC TME may regulate and impair host’s immunity are discussed in the following sections.
Figure 1.
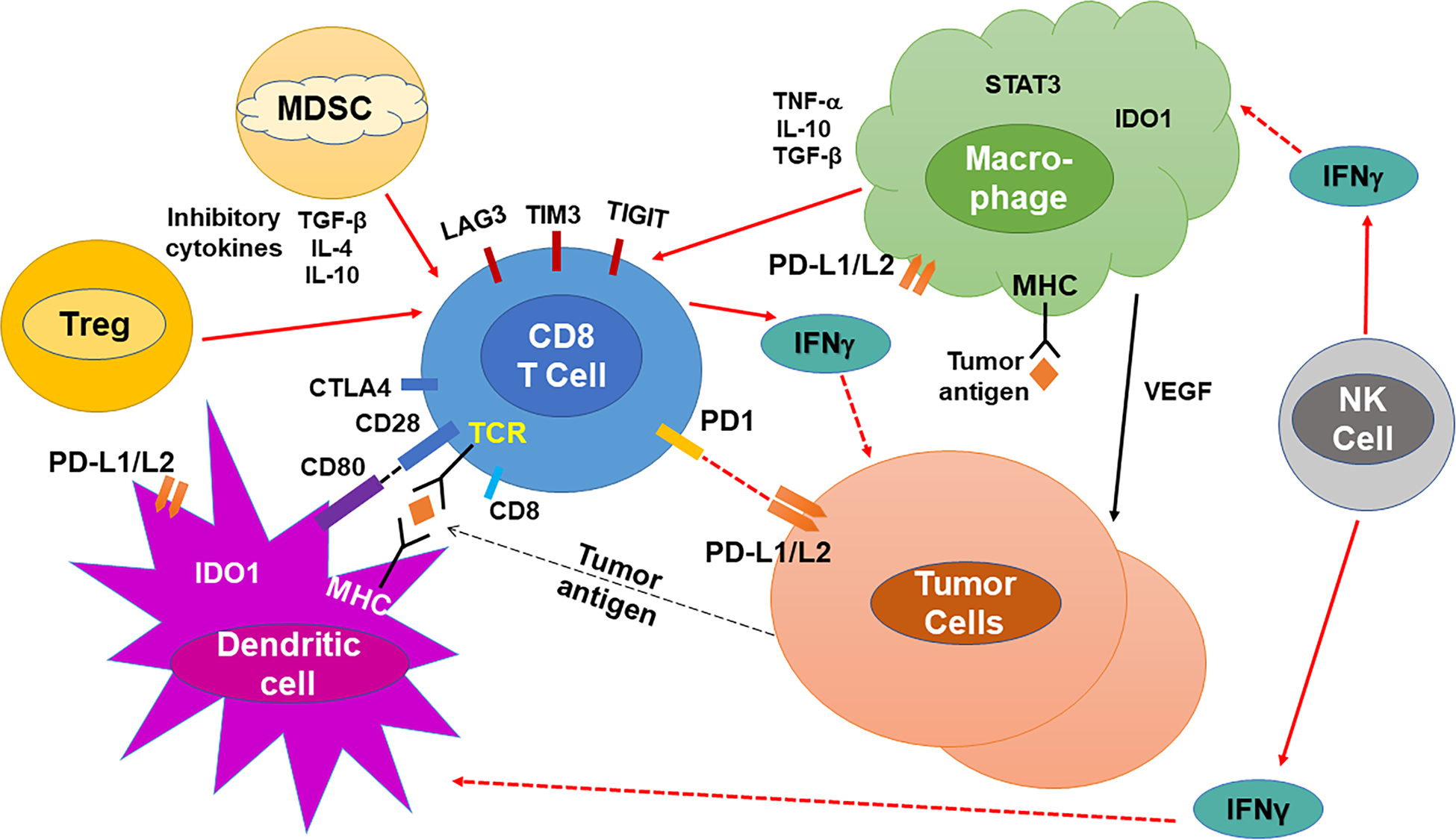
A schematic illustration of the tumor-associated immune cells and factors responsible for the immune-suppressive mechanisms in the tumor microenvironment (TME) of HNSCC.
T cells
T cells are lymphocytes that are a crucial component of the adaptive immune system and are categorized into CD4+ and CD8+ T cells. While CD8 TILs can directly kill tumor cells via producing perforin/granzymes, activated CD4 and CD8 TILs can also secrete cytokines (e.g., IFN-γ, TNFα) which have tumor cell killing activity or can recruit other immune cells that mediate cytotoxic anti-tumor immune responses12 (Figure 1).
In one study using OSCC patient samples, it was observed that the majority of intratumoral TILs were CD8+ T cells, while 67% of those patients had intratumoral CD4+ TILs20. They found that a higher frequency of surrounding peritumoral CD8+ TILs correlated with better clinical parameters in HNSCC patients (e.g. smaller tumor size and lower probability of lymph node metastasis); however, it did not correlate with patient survival20. The ratio of CD4:CD8 TILs was 0.745, and this ratio was found to be higher in large tumors and advanced stage tumors20. While the density of peritumoral CD8+ TILs in OSCCs was associated with some clinical parameters, neither the frequency of intratumoral CD8+ TILs nor the ratio of CD4:CD8 TILs was shown to have any relationship with clinical parameters20. In contrast, another study reported that higher CD4 and CD8 TIL levels were correlated with improved overall survival (OS), and relapse-free survival (RFS)21. Furthermore, after controlling for other prognostic factors, higher CD4 levels predicted improved OS and disease-specific survival21. Consistently, another systematic review and meta-analysis confirmed that CD3+ and CD8+ TILs have a favorable and prognostic role in HNSCC clinical outcomes and found that FoxP3+ TILs also correlate with improved OS22.
By comparing the peripheral blood mononuclear cells (PBMC) of HNSCC patients and healthy controls, one study showed that there was no quantitative difference in the proportion of T cell subsets, but patient T cells had a qualitative difference from controls23. When these PBMC T cells were stimulated by peptide pools of viral antigens, T cells from the HNSCC patients produced a significantly weaker IFN-γ recall response compared to the controls23, suggesting a systemic immunosuppressive condition in HNSCC patients. While peritumoral CD8+ TILs may indicate an adaptive immune response, studies have shown that dysfunctional TILs are commonly present, which exhibit decreased cytokine production and proliferation ability and lack of cytotoxic functions24,25. Exhausted and dysfunctional TILs in HNSCC cases have been characterized by the upregulation of several checkpoint markers, such as programmed cell death 1 (PD-1), lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin mucin protein-3 (TIM-3), cytotoxic T lymphocyte–associated protein 4 (CTLA-4), and 2B426–29 (Figure 1). The cause and mechanisms by which the T cells become exhausted and dysfunctional in HNSCCs are discussed in detail below.
PD-1 is a co-inhibitory receptor expressed on activated CD4+ or CD8+ T cells, and PD-1 has two ligands, PD ligand 1 (PD-L1) and 2 (PD-L2)30,31. PD-1 is also considered a marker for exhausted T cells and is often upregulated in CD4+ and CD8+ TILs in various types of cancers30,31. CD8+ TILs of human HNSCCs were found to express increased levels of PD-1 on their surface32, and 66% to 79% of SCC patients had PD-L1-positive tumor cells33–36. In addition, we previously showed that CD8+ TILs in a mouse SCC model highly expressed PD-1 and LAG-337. Engaging PD-1 by binding to PD-L1 inhibits the functions of T cells or promotes their apoptosis30,31. Studies have shown that increased PD-L1 expression on tumor cells significantly decreased the level of CD8+ T cells at the regional level in HNSCC TME through apoptotic mechanisms38. In addition, PD-L1 expression was detected in exosomes isolated from plasma samples of HNSCC patients, and circulating PD-L1high exosomes associated with disease progression in HNSCCs39. Of note, HNSCCs may also induce CD8+ T cell apoptosis by producing Fas-L+ microvesicles detected in serum samples of OSCC patients40. Altogether, these mechanisms may provide an explanation for a systemic immunosuppressive condition in HNSCC patients.
Another mechanism that causes the dysfunction of CD8+ T cells in HNSCCs is the elevated levels of both circulating and tumor-infiltrating T regulatory (Treg) and Th17 cells41,42. Furthermore, Th17 cell proliferation is likely caused by the increased levels of IL-23 and IL-6 released by HNSCC cancer cells42. Tregs account for less than 5% of a subset of CD4+ T cells in peripheral blood, which are characterized as CD4+CD25+FOXP3+. Tregs regulate excessive immune reactivity toward other immune cells to prevent autoimmunity; however, this function also limits the immune system to target neoplastic cells43,44. Moreover, peripheral Tregs can be recruited into the TME, where TGF-β causes them to differentiate and become more immunosuppressive45 (Figure 1). Elevated level of TGF-β has been found during the latter phase of HNSCC progression, which seems to disrupt the ratio of Th17 vs. Tregs46. This disruption induced tumor-promoting Treg differentiation, and increased anti-inflammatory cytokine IL-10 production in HNSCCs47,48. On the other hand, a high level of FoxP3+ Treg infiltration in HNSCCs was associated with longer RFS and OS22,49. This is probably because the presence of a high level of FoxP3+ Treg in tumors indicates an on-going robust anti-tumor immune response, which contributes to inhibition of tumor growth.
In addition to dysfunctional T cells, decreased expression of major histocompatibility complexes (MHC), called HLA in humans, may also help the tumor cells evade adaptive immunity. MHC class I is essential for presenting peptides to CD8+ T cells and for CD8+ cytotoxic T lymphocytes (CTL) to recognize tumor cells expressing tumor-associated or tumor-specific antigens50. About 50% of HNSCC tumors might evade immune surveillance through the downregulation of MHC class I molecules38. While 12.7% of HNSCC cases showed no detectable expression of MHC class I, others had reduced MHC class I expression38. However, the mechanisms of MHC class I reduction in HNSCCs remain less well-understood. It has been found that during the early stages of HNSCC tumor development, the interaction of CD8+ T cells and MHC class I in primary HNSCCs led to either tumor rejection or immune escape13,51. Decreased expression of MHC class I would allow tumor cells to escape the detection by CD8+ T cells and avoid the activation of CD8+ CTL-mediated anti-tumor immune responses52.
Myeloid Cells
Tumor-associated macrophages (TAMs) are common in HNSCCs, and are classified into two subpopulations: M1, which mediates pro-inflammatory and anti-tumor responses, and M2, which is immunosuppressive and has pro-tumor properties53 (Figure 1). M1 produces pro-inflammatory cytokines such as IL-12, IL-23, and IFN-γ, and has been shown to have anti-tumor immune properties16,54,55. In contrast, M2 exhibits immunosuppressive characteristics by not only producing IL-10 and TGF-β, shown to be suppressive cytokines, but also inhibits M1’s anti-tumor cytotoxic effects56,57. A higher level of TAMs in the TME has been shown to correlate with lymph node metastasis and advanced stage of HNSCCs53,58–61. A recent study has shown that HNSCC TME largely encompasses M2 TAMs and exhibits an increased level of TGF-β62. A likely mechanism that TAMs contribute to inflammation and tumorigenesis is by producing reactive oxygen species (ROS), reactive nitrogen species (RNS), and prostaglandins (PGs). Furthermore, TAMs have been found to produce high levels of inflammatory cytokines (e.g., TNF-α and IL-1β) and macrophage migration inhibitory factor (MIF)54,63–65. MIFs are ligands of chemokine receptor CXCR2, and have been shown to promote migration of SCCs, cell-matrix adhesion54,66, as well as neutrophil recruitment to HNSCCs67. The recruited neutrophils can release hepatocyte growth factor (HGF), which in turn increases invasiveness of the tumor cells by feedback mechanisms of the HGF pathway68 that regulates cell growth, morphogenesis and motility.
The effects of TAM reduction were investigated using mouse SCC models69. Macrophages were depleted by treating recipient mice bearing K15.KrasG12D/Smad4−/− SCC tumors with clodronate, and it was observed that TAM reduction resulted in reduced SCC tumor volume69. Tumors with clodronate treatment increased SCC apoptosis but did not attenuate SCC proliferation and metastasis69. Given the immunosuppressive properties of these myeloid cells in the TME, quantifying TAMs may thus be useful for prognostic stratification in HNSCCs. Furthermore, strategies for targeting TAMs (through inhibition or reprogramming) in combination with checkpoint inhibitors in ongoing clinical trials have shown promising results in melanoma, lung cancer, colon cancer, and breast cancer59,63,70–72. While some studies have suggested that increased density of TAMs are correlated with poor clinicopathologic markers in HNSCCs, strategies for targeting TAMs in HNSCC patients have yet to be explored.
MDSC
Previous studies suggest that myeloid derived suppressor cells (MDSC) may play a pivotal role in predicting tumor response to various tumor immunotherapies14,65,73–76. MDSCs circulate in peripheral blood, draining lymphoid tissue, as well as in HNSCC tumor tissue77–79. Many studies have demonstrated that MDSCs downregulate immune responses during infection, inflammation and tumor development14,67,79–85. MDSCs are CD11b+ cells that are phenotypically subdivided into two groups, polymorphonuclear MDSC (PMN-MDSC) and monocytic MDSC (M-MDSC)86–88. PMN-MDSCs are similar to immature polymorphonuclear cells and phenotypically express Ly6Clo and Ly6G+. M-MDSCs are immature mononuclear cells characterized by cell surface markers, Ly6Chi and Ly6G−, and can differentiate into TAMs as a result of signal transducer and activator of transcription 3 (STAT3) downregulation64,89–91. One study on murine lymphoma and colon carcinoma showed that intratumoral MDSCs had significantly lower density of activated STAT3 than peripheral blood or spleen MDSCs90. The downregulation of STAT3 activities was suggested to facilitate higher frequencies of MDSC differentiation into TAMs, but further studies are needed to verify whether lowered STAT3 activation is observed in the MDSCs of HNSCCs.
Studies have found that the frequency of M-MDSC is negatively correlated to the response of chemotherapy in SCCs, and M-MDSCs are highly immunosuppressive via an antigen-nonspecific mechanism59,80,81. While PMN-MDSCs are also immunosuppressive, they have been found to promote T cell tolerance in an antigen-specific manner80. It has been shown that peripheral MDSCs from lymphoid organs suppress antigen-specific CD8+ T cells, while TME MDSCs can suppress both antigen-specific and antigen-non-specific T cell function80,82,92. Peripheral MDSCs can suppress antigen-specific immune responses of T cells, which requires close cell-to-cell contact, via the production of nitric oxide (NO) and ROS80. In addition, peripheral MDSCs can reduce T cell proliferation by depleting essential amino acid levels, such as L-arginine and tryptophan, attributed to the activity of Arginase-1 and indole amine 2,3 dioxygenase (IDO), respectively79,80,83,91. However, TME MDSCs in HNSCC patients not only exhibit more potent antigen-specific immunosuppressive properties through NO production and Arg1 activities, but also suppress antigen-specific and -non-specific T cell response via inhibiting CD3/CD28 expression on T cells92,93. Another way for MDSCs to suppress cytotoxic CD8+ T cell responses is to induce immunosuppressive cytokines (e.g. IL-10 and TGF-β) and Tregs46,47, which downregulate effector T cell proliferation and activation, thereby leading to tumor growth and metastasis.
A recent study has discovered that tumor-associated hypoxia caused an increase of PD-L1 expression on tumor-infiltrating MDSCs94. This upregulation of PD-L1 on MDSCs was due to the transcription factor, hypoxia-inducible factor 1-α (HIF-1α), which resulted in more potent immunosuppressive effects of MDSCs94. Another mechanism that can increase the immunosuppressive activities of MDSCs is the upregulation of CD38 on MDSCs. MDSCs that express a higher level of CD38 evidently have a greater capacity to promote tumor growth and exhibit more potent immunosuppressive effects95. CD38 also promotes the expansion of M-MDSC populations95,96. Furthermore, it has been found that IFN-γ, TNF-α, and other cytokines can induce CD38 expression in MDSCs97,98, suggesting a negative feedback loop that prevents anti-tumor immunity. Studies have detected a delay in tumor progression in murine models of myeloma and hematological tumors after administering anti-CD38 mAb that could target CD38+ MDSCs97,99–101. Thus, we suggest that targeted therapies that selectively inhibit certain subtypes and aspects of MDSCs may provide a functional remedy to combat HNSCCs with a high density of MDSCs.
Current and future immunotherapies in HNSCC
When HNSCC is diagnosed at an early stage, it is typically treated with surgery or radiotherapy; however, treatment for HNSCC can be quite morbid due to significant functional impairments and aesthetic deformities to the patients. HNSCCs are genetically heterogeneous that can hinder the classification and development of specific targeted therapies102. Current treatment strategies for HNSCCs are not adequate and may lead to resistance. Thus, to rectify this issue, new treatment strategies and specific molecular or cellular markers need to be developed and identified to improve and better predict treatment outcome and survival of HNSCC patients.
The breakthrough findings that blocking certain immune checkpoints can help rescue T cell responses have prompted investigators to develop treatments to restore anti-tumor immune responses30,31,103. In recent years, immune checkpoint blockade therapies in HNSCCs, such as pembrolizumab, nivolumab, and durvalumab, that target PD-1/PD-L1, have been shown to have limited advantageous safety profile and response rate104–107. Although anti-PD-1/PD-L1 has been approved by FDA for treating HNSCCs, the overall response rate remains low104–107. The mechanisms underlying the unresponsiveness to anti-PD-1/anti-PD-L1 remains poorly understood and the markers to predict responses are not well-characterized. There are many ongoing HNSCC clinical trials evaluating the various indications of anti-PD-1/anti-PD-L1 alone or in combination with radiation, targeted therapy and chemotherapy3,105,106,108, but their curative effect and safety profile need further experimental investigation and verification.
A likely key factor that can predict responsiveness to PD-1 blockade in HNSCCs is the production of IFN-γ. In a 2017 study, it was identified that IFN-γ signature score can predict clinical response to PD-1 blockade in HNSCCs109. Furthermore, it was shown that the T cell-inflamed gene expression profiles, which contain IFN-γ-responsive genes, also predicted clinical responses to PD-1 blockade109. However, these features associated with T cell-inflamed phenotypes were necessary, but not always sufficient, for clinical benefit, because one category of non-responders to PD-1 blockade clearly exhibited a high level of IFN-γ signature score and T cell inflammatory gene expression, yet, these patients had no clinical benefit109. Hence, we need to better understand the resistance mechanisms in tumors that exhibit evidence of T cell-inflamed phenotypes yet still lack clinical response. In this regard, activated T cells and NK cells in the TME can produce IFN-γ, which can directly upregulate PD-L1 and PD-L2 on tumor cells or other cells in the TME to induce a feedback inhibition loop by activating PD-1 on TILs (Figure 1). Therefore, blocking PD-1 or PD-L1 can disrupt this feedback inhibition loop and control tumor growth. However, IFN-γ production may also trigger inhibitory feedback loops that involve additional pathways other than PD-1/PD-L1, for instance, other immunosuppressive molecules such as LAG-3 and IDO1 could be overexpressed in an IFN-γ rich TME.
HNSCCs in heavy smokers often harbor extensive DNA damage, and respond poorly to conventional therapies110. To solve the challenge and enhance the sensitivity of HNSCCs to immune checkpoint inhibitors, new therapeutic strategies are needed such as developing vaccine-based immunotherapy for HNSCCs using neoantigens. Neoantigens are newly formed antigens by somatic mutations in cancers; thus, they are absent in the normal host tissues111–114. Neoantigens hold great promise to induce cancer-specific immune responses that can potentially eradicate cancers, while sparing normal tissues, thereby minimizing side-effects. Recent advances in next-generation sequencing (NGS) and epitope prediction algorithms have made the identification of tumor-specific neoantigens feasible111,115. By combining genomic, bioinformatics, and immunological approaches, neoantigens were identified in both mouse models and human patient samples111,116–123. Preclinical studies of mouse models have indicated the potential benefits of tumor-specific neoantigens in immunotherapy116,121,122. Results of the therapeutic use of neoantigens in human cancers are also encouraging124–127. It would be of great interest to test whether HNSCCs harbor neoantigens and whether neoantigen-based immunotherapy can be effective in HNSCCs.
Another novel advance in adoptive immunotherapy is the administration of genetically modified antigen-specific T cells that target antigens expressed on the surface of tumor cells. In recent years, synthetic chimeric antigen receptor (CAR)-T cell therapy has encouraging therapeutic potential in HNSCCs and hematological cancers128–130. A previous study identified nine overexpressed genes on the surface of HNSCCs as potential targets for CAR-T cell therapy128, but only a few targeted antigens have shown favorable results. Pre-clinical and clinical data on HER2-specific, CD70-specific, and T4-immunotherapy (T1E28ζ/4αβ) CAR-T cell therapy on HNSCCs demonstrate potent anti-tumor activity, but the risk of on-target off-tumor toxicity remains a challenge to overcome128,129,131. T4+ CAR T-cells are retrovirally transduced to co-express (a) T1E28ζ, a CAR coupling ErbB ligand derived from EGF and TGFα to a fused CD28/CD3ζ endodomain; and (b) 4αβ, a chimeric cytokine receptor containing the IL-4Rα ectodomain coupled to the IL-2Rβ endodomain131.
In addition to T cell-specific immunotherapy, recent studies have seen the potential importance of targeting MDSC populations in cancers. Studies have determined the immunosuppressive properties of tumor infiltrating MDSCs, and by inhibiting them directly or targeting CD38+ MDSCs by administering anti-CD38 mAb have emerged as potential immunotherapies for MDSC-high cancers in mouse models of myeloma or other hematological cancers99,101,132. Furthermore, other immunotherapies are being studied that can potentially extend survival and suppress tumor growth. For example, recent investigations on targeting vascular endothelial growth factor, HIF-1α and nuclear factor-κB as therapeutic targets in the TME have been promising92,94,133. However, all these immunotherapies face multiple factors that can be challenging, such as the need to identify tumor-associated antigens that are specifically overexpressed on the tumor cells and not in normal tissues.
Acknowledgments
This work was supported by University of Colorado School of Medicine and Cancer Center startup funds and a THI pilot grant to J.H.W., NIH R01-DE027329 and R01-DE028420 to J.H.W., and a fund from American Cancer Society (ACS IRG #16-184-56) to Z.C. X.G.W. was supported by an AAI Careers in Immunology Fellowship. R.A.W. is supported by a NIH F31 fellowship (F31DE027854). S.M.Y.C. is supported by a NIH T32 fellowship (T32 AI007405).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nature Reviews Clinical Oncology. 2014;11(8):460–472. [DOI] [PubMed] [Google Scholar]
- 3.Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16(11):669–683. [DOI] [PubMed] [Google Scholar]
- 4.Liang C, Marsit CJ, McClean MD, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72(19):5004–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Xia RH, Ye DX, Li J. Human Papillomavirus 16 Infection and TP53 Mutation: Two Distinct Pathogeneses for Oropharyngeal Squamous Cell Carcinoma in an Eastern Chinese Population. PLoS One. 2016;11(10):e0164491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canning M, Guo G, Yu M, et al. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front Cell Dev Biol. 2019;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing Y, Zhang J, Lin H, et al. Relation between the level of lymph node metastasis and survival in locally advanced head and neck squamous cell carcinoma. Cancer. 2016;122(4):534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck. 2006;28(5):462–470. [DOI] [PubMed] [Google Scholar]
- 9.Park YJ, Song B, Kim YS, et al. Tumor microenvironmental conversion of natural killer cells into myeloid-derived suppressor cells. Cancer Res. 2013;73(18):5669–5681. [DOI] [PubMed] [Google Scholar]
- 10.Curry JM, Sprandio J, Cognetti D, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41(2):217–234. [DOI] [PubMed] [Google Scholar]
- 11.Meissner MRT, Kunkel M, Gooding W, Whiteside TL, Ferrone S, Seliger B. Defects in the Human Leukocyte Antigen Class I Antigen Processing Machinery in Head and Neck Squamous Cell Carcinoma Association with Clinical Outcome. Clin Cancer Res. 2005;11(7):2552–2560. [DOI] [PubMed] [Google Scholar]
- 12.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saloura V, Fatima A, Zewde M, et al. Characterization of the T-Cell Receptor Repertoire and Immune Microenvironment in Patients with Locoregionally Advanced Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2017;23(16):4897–4907. [DOI] [PubMed] [Google Scholar]
- 14.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron. 2013;6(2):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katou F, Ohtani H, Watanabe Y, Nakayama T, Yoshie O, Hashimoto K. Differing phenotypes between intraepithelial and stromal lymphocytes in early-stage tongue cancer. Cancer Res. 2007;67(23):11195–11201. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016;2016:6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nirmal AJ, Regan T, Shih BB, Hume DA, Sims AH, Freeman TC. Immune Cell Gene Signatures for Profiling the Microenvironment of Solid Tumors. Cancer Immunol Res. 2018;6(11):1388–1400. [DOI] [PubMed] [Google Scholar]
- 18.Peltanova B, Raudenska M, Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol Cancer. 2019;18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanker A, Thounaojam MC, Mishra MK, Dikov MM. Innate-Adaptive Immune Crosstalk 2016. J Immunol Res. 2017;2017:3503207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47(12):1148–1153. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen N, Bellile E, Thomas D, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(7):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6(11):e1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upreti D, Zhang ML, Bykova E, Kung SK, Pathak KA. Change in CD3zeta-chain expression is an independent predictor of disease status in head and neck cancer patients. Int J Cancer. 2016;139(1):122–129. [DOI] [PubMed] [Google Scholar]
- 24.Li H, van der Leun AM, Yofe I, et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell. 2019;176(4):775–789 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 targets LAG-3 and 4–1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med. 2017;214(2):381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Mao L, Liu JF, et al. Blockade of TIGIT/CD155 Signaling Reverses T-cell Exhaustion and Enhances Antitumor Capability in Head and Neck Squamous Cell Carcinoma. Cancer Immunol Res. 2019;7(10):1700–1713. [DOI] [PubMed] [Google Scholar]
- 27.Jie HB, Gildener-Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. British Journal of Cancer. 2013;109(10):2629–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. Journal of Clinical Oncology. 2015;33(29):3293–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perri F, Ionna F, Longo F, et al. Immune Response Against Head and Neck Cancer: Biological Mechanisms and Implication on Therapy. Transl Oncol. 2019;13(2):262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8(328):328rv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider S, Kadletz L, Wiebringhaus R, et al. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis – impact on clinical outcome. Histopathology. 2018;73(4):573–584. [DOI] [PubMed] [Google Scholar]
- 33.Zheng A, Li F, Chen F, et al. PD‑L1 promotes head and neck squamous cell carcinoma cell growth through mTOR signaling. Oncology reports. 2019;41(5):2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strome SE DH, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–6505. [PubMed] [Google Scholar]
- 35.Hsieh C-C, Hsu H-S, Li AF-Y, Chen Y-J. Clinical relevance of PD-L1 and PD-L2 overexpression in patients with esophageal squamous cell carcinoma. Journal of Thoracic Disease. 2018;10(7):4433–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattox AK, Lee J, Westra WH, et al. PD-1 Expression in Head and Neck Squamous Cell Carcinomas Derives Primarily from Functionally Anergic CD4(+) TILs in the Presence of PD-L1(+) TAMs. Cancer research. 2017;77(22):6365–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra Ameet K. K T, Wang Xiaoguang, Driver Emily, Chen Zhangguo W X-J, Wang Jing H.. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of cd8 T cells co-expressing pd-1 and lag-3 inhibitory receptors. Oncotarget. 2016;7(49): 81341–81356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo SH, Keam B, Ock CY, et al. Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci Rep. 2019;9(1):7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin Cancer Res. 2018;24(4):896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11(3):1010–1020. [PubMed] [Google Scholar]
- 41.Liu Z, McMichael EL, Shayan G, et al. Novel Effector Phenotype of Tim-3(+) Regulatory T Cells Leads to Enhanced Suppressive Function in Head and Neck Cancer Patients. Clin Cancer Res. 2018;24(18):4529–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br J Cancer. 2010;103(8):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boucek J, Mrkvan T, Chovanec M, et al. Regulatory T cells and their prognostic value for patients with squamous cell carcinoma of the head and neck. J Cell Mol Med. 2010;14(1–2):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Echarti A, Hecht M, Buttner-Herold M, et al. CD8+ and Regulatory T cells Differentiate Tumor Immune Phenotypes and Predict Survival in Locally Advanced Head and Neck Cancer. Cancers (Basel). 2019;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jie HB, Schuler PJ, Lee SC, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75(11):2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maggioni D, Pignataro L, Garavello W. T-helper and T-regulatory cells modulation in head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(7):e1325066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu L, Yang F, Zhang F, et al. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018;9(9):905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Medeiros Marcell Costa B R, Liu Min, Anovazzi Giovana, Nisha J, Junior DSaCR. HNSCC subverts PBMCs to secrete soluble products that promote tumor cell proliferation. Oncotarget. 2017;8(37):60860–60874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seminerio I, Descamps G, Dupont S, et al. Infiltration of FoxP3+ Regulatory T Cells is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers (Basel). 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maleki S, Schlecht NF, Keller C, et al. Lymphocytic host response to oral squamous cell carcinoma: an adaptive T-cell response at the tumor interface. Head Neck Pathol. 2011;5(2):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grandis JR FD, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck clinical and immunogenetic consequences. Clin Cancer Res. 2000;6(7):2794–2802. [PubMed] [Google Scholar]
- 52.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosn EE, Cassado AA, Govoni GR, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107(6):2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dumitru CA, Gholaman H, Trellakis S, et al. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Cancer. 2011;129(4):859–869. [DOI] [PubMed] [Google Scholar]
- 55.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125(9):3365–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front Immunol. 2017;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jayaraman P, Parikh F, Newton JM, et al. TGF-beta1 programmed myeloid-derived suppressor cells (MDSC) acquire immune-stimulating and tumor killing activity capable of rejecting established tumors in combination with radiotherapy. Oncoimmunology. 2018;7(10):e1490853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao L, Zhang W, Zhong WQ, et al. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol Rep. 2018;40(5):2558–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar AT, Knops A, Swendseid B, et al. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front Oncol. 2019;9:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roszer T Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa NL, Valadares MC, Souza PP, et al. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013;49(3):216–223. [DOI] [PubMed] [Google Scholar]
- 63.Cassetta L, Kitamura T. Targeting Tumor-Associated Macrophages as a Potential Strategy to Enhance the Response to Immune Checkpoint Inhibitors. Front Cell Dev Biol. 2018;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schouppe E, Mommer C, Movahedi K, et al. Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events. Eur J Immunol. 2013;43(11):2930–2942. [DOI] [PubMed] [Google Scholar]
- 66.Steele CW, Karim SA, Leach JDG, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016;29(6):832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothenberger NJ, Stabile LP. Hepatocyte Growth Factor/c-Met Signaling in Head and Neck Cancer and Implications for Treatment. Cancers (Basel). 2017;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu FL, Nolan K, Strait AA, et al. Macrophages Promote Growth of Squamous Cancer Independent of T cells. J Dent Res. 2019;98(8):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8(7):1596004–1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber R, Fleming V, Hu X, et al. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front Immunol. 2018;9:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fleming V, Hu X, Weber R, et al. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol. 2018;9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He W, Liang P, Guo G, et al. Re-polarizing Myeloid-derived Suppressor Cells (MDSCs) with Cationic Polymers for Cancer Immunotherapy. Sci Rep. 2016;6:24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun L, Clavijo PE, Robbins Y, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gubin MM, Esaulova E, Ward JP, et al. High-Dimensional Analysis Delineates Myeloid and Lymphoid Compartment Remodeling during Successful Immune-Checkpoint Cancer Therapy. Cell. 2018;175(4):1014–1030 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sumi S, Umemura N, Takayama E, et al. Metastasized murine oral squamous cell carcinoma cells induce intratumoral polymorphonuclear myeloid derived suppressor cells. Oncol Rep. 2017;37(5):2897–2904. [DOI] [PubMed] [Google Scholar]
- 79.Younis RH, Han KL, Webb TJ. Human Head and Neck Squamous Cell Carcinoma-Associated Semaphorin 4D Induces Expansion of Myeloid-Derived Suppressor Cells. J Immunol. 2016;196(3):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37(3):208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noman MZ, Janji B, Hu S, et al. Tumor-Promoting Effects of Myeloid-Derived Suppressor Cells Are Potentiated by Hypoxia-Induced Expression of miR-210. Cancer Res. 2015;75(18):3771–3787. [DOI] [PubMed] [Google Scholar]
- 83.Ohl K, Tenbrock K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front Immunol. 2018;9:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22(4):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 2019;40(1):4–7. [DOI] [PubMed] [Google Scholar]
- 86.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hochst B, Mikulec J, Baccega T, et al. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS One. 2015;10(3):e0119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–983. [DOI] [PubMed] [Google Scholar]
- 89.Bu LL, Yu GT, Wu L, et al. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J Dent Res. 2017;96(9):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar V, Cheng P, Condamine T, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44(2):303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su YL, Banerjee S, White SV, Kortylewski M. STAT3 in Tumor-Associated Myeloid Cells: Multitasking to Disrupt Immunity. Int J Mol Sci. 2018;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corzo CA, Condamine T, Lu L, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol. 2011;90(1):31–36. [DOI] [PubMed] [Google Scholar]
- 94.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karakasheva TA, Waldron TJ, Eruslanov E, et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015;75(19):4074–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karakasheva TA, Dominguez GA, Hashimoto A, et al. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight. 2018;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen L, Diao L, Yang Y, et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018;8(9):1156–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blacher Eran L A, Baruch Bar Ben, Green Keith D., Garneau-Tsodikova Sylvie, Fridman Micha, Stein Reuven. Targeting CD38 in the tumor microenvironment; a novel approach to treat glioma. Cancer Cell & Microenvironment. 2015. [Google Scholar]
- 99.van de Donk NW JM, Mutis T, Lammerts van Bueren JJ, Ahmadi T, Sasser AK, Lokhorst HM, Parren PW. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levy A, Blacher E, Vaknine H, Lund FE, Stein R, Mayo L. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro Oncol. 2012;14(8):1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F. CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma. Front Immunol. 2018;9:2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin Daniel A MC, Molinolo Alfredo A., Vitale-Cross Lynn, Wang Zhiyong Z M, Delic Naomi C., Samuels Yardena, Lyons J. Guy, Gutkind JS. The head and neck cancer cell oncogenome A platform for the development of precision molecular therapies. Oncotarget. 2014;5:8906–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forster MD, Devlin MJ. Immune Checkpoint Inhibition in Head and Neck Cancer. Front Oncol. 2018;8:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saleh K, Eid R, Haddad FG, Khalife-Saleh N, Kourie HR. New developments in the management of head and neck cancer - impact of pembrolizumab. Ther Clin Risk Manag. 2018;14:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. [DOI] [PubMed] [Google Scholar]
- 106.Alsahafi E, Begg K, Amelio I, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10(8):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferris RL, Blumenschein G Jr., Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moskovitz JM, Moy J, Seiwert TY, Ferris RL. Immunotherapy for Head and Neck Squamous Cell Carcinoma: A Review of Current and Emerging Therapeutic Options. Oncologist. 2017;22(6):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. The Journal of clinical investigation. 2015;125(9):3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nature reviews Cancer. 2014;14(2):135–146. [DOI] [PubMed] [Google Scholar]
- 113.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nature reviews Cancer. 2005;5(8):615–625. [DOI] [PubMed] [Google Scholar]
- 114.Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. The EMBO journal. 2013;32(2):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68(3):889–892. [DOI] [PubMed] [Google Scholar]
- 116.Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nathanson T, Ahuja A, Rubinsteyn A, et al. Somatic Mutations and Neoepitope Homology in Melanomas Treated with CTLA-4 Blockade. Cancer immunology research. 2017;5(1):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johanns TM, Ward JP, Miller CA, et al. Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer immunology research. 2016;4(12):1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johanns TM, Ward J, Wilson C, et al. 143 Identification of Neoantigen-specific CD8+ T Cells in Two Murine Orthotopic Glioblastoma Models Using Cancer Immunogenomics. Neurosurgery. 2016;63 Suppl 1:158. [Google Scholar]
- 120.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. [DOI] [PubMed] [Google Scholar]
- 123.Linnemann C, van Buuren MM, Bies L, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nature medicine. 2015;21(1):81–85. [DOI] [PubMed] [Google Scholar]
- 124.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. The New England journal of medicine. 2016;375(23):2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park YP, Jin L, Bennett KB, et al. CD70 as a target for chimeric antigen receptor T cells in head and neck squamous cell carcinoma. Oral Oncol. 2018;78:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Townsend MH, Shrestha G, Robison RA, O’Neill KL. The expansion of targetable biomarkers for CAR T cell therapy. Journal of Experimental & Clinical Cancer Research. 2018;37(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, & Ganguly N CAR T cell therapy: A new era for cancer treatment (Review). Oncology Reports,. 2019;42:2183–2195. [DOI] [PubMed] [Google Scholar]
- 131.Papa S, Adami A, Metoudi M, et al. A phase I trial of T4 CAR T-cell immunotherapy in head and neck squamous cancer (HNSCC). Journal of Clinical Oncology. 2018;36(15_suppl):3046–3046. [Google Scholar]
- 132.van de Donk N, Usmani SZ. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front Immunol. 2018;9:2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16(7):447–462. [DOI] [PubMed] [Google Scholar]


