Abstract
Background & Aims:
Glypican 3 (GPC3) is an oncofetal antigen involved in Wnt-dependent cell proliferation that is highly expressed in hepatocellular carcinoma (HCC). We investigated whether the functions of chimeric antigen receptors (CARs) that target GPC3 are affected by their antibody-binding properties.
Methods:
We collected peripheral blood mononuclear cells from healthy donors and patients with HCC and used them to create CAR T cells, based on the hYP7 and HN3 antibodies, which have high affinities for the C-lobe and N-lobe of GPC3, respectively. NSG mice were given intraperitoneal injections of luciferase-expressing (Luc) Hep3B or HepG2 cells and after xenograft tumors formed, mice were given injections of saline or untransduced T cells (controls), or CAR (HN3) T cells or CAR (hYP7) T cells. In other NSG mice, HepG2-Luc or Hep3B-Luc cells were injected into liver, and after orthotopic tumors formed, mice were given 1 injection of CAR (hYP7) T cells or CD19 CAR T cells (control). We developed droplet digital PCR and genome sequencing methods to analyze persistent CAR T cells in mice.
Results:
Injections of CAR (hYP7) T cells eliminated tumors in 66% of mice by week 3, whereas CAR (HN3) T cells did not reduce tumor burden. Mice given CAR (hYP7) T cells remained tumor free after re-challenge with additional Hep3B cells. The CAR T cells induced perforin- and granzyme-mediated apoptosis and reduced levels of active β-catenin in HCC cells. Mice injected with CAR (hYP7) T cells had persistent expansion of T cells and subsets of polyfunctional CAR T cells via antigen-induced selection. These T cells were observed in the tumor microenvironment and spleen for up to 7 weeks after CAR T cell administration. Integration sites in pre-infusion CAR (HN3) and CAR (hYP7) T cells were randomly distributed, whereas integration into NUPL1 was detected in 3.9% of CAR (hYP7) T cells 5 weeks after injection into tumor-bearing mice and 18.1% of CAR (hYP7) T cells at week 7. There was no common site of integration in CAR (HN3) or CD19 CAR T cells from tumor-bearing mice.
Conclusions:
In mice with xenograft or orthoptic liver tumors, CAR (hYP7) T cells eliminate GPC3-positive HCC cells, possibly by inducing perforin- and granzyme-mediated apoptosis or reducing Wnt signaling in tumor cells. GPC3-targeted CAR T cells might be developed for treatment of patients with HCC.
Keywords: hepatic, immunotherapy, tumor-specific T cells, lymphocyte
Graphical Abstract
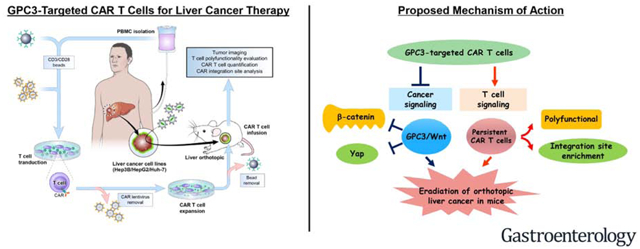
Lay Summary:
We engineered immune cells to recognize and kill liver cancer cells and cause regression of liver tumors in mice. This approach might be useful for treating patients with liver cancer.
Introduction
Robust efficacy of chimeric antigen receptor (CAR) T cells targeting CD19 in B cell malignancies has led to the approval of two CD19 CAR T cell products by the Food and Drug Administration (FDA)1, 2. However, the translation of CAR T cells for solid tumors remains an unmet challenge. Glypican 3 (GPC3) is a glycosylphosphatidylinositol-anchored cell surface protein consisting of a core protein and two heparan sulfate (HS) chains3. GPC3 is an oncofetal protein expressed in over 70% of hepatocellular carcinoma (HCC)4, 5 and other solid tumors including hepatoblastoma and lung squamous cell carcinoma6, 7. Its expression is not detected in nonmalignant adult tissues including normal liver6, 8. Mechanistically, GPC3 can promote tumor growth by modulating the Wnt/Frizzled signaling complex on HCC cells9–11. Various antibody-based therapies including immunotoxins12, 13, antibody-drug conjugates (ADCs)14, CAR T cells or bispecific antibodies targeting GPC315–21 have been developed. Recent evidence indicated that CAR T cells targeting GPC3 could inhibit the growth of HCC cells15, 16, 18. Preliminary results showed a modest response with GPC3-targeted CAR T cells in a Phase I trial of 13 patients in China22. To improve its anti-tumor activity, their research group is modifying the CAR construct via co-expressing a soluble PD-1 protein21.
Considerable progress has been made in improving the signaling domains of CARs. However, another challenge in developing effective CAR T cell therapy is the functional optimization of antibody domains. We hypothesize that the function of CARs is affected by their epitope specificity, affinity, and functional activity. In the present study, we used the antibodies HN323 and humanized YP7 (hYP7)24, 25 with comparable high affinity for the N-lobe and C-lobe of GPC3, respectively, to engineer CAR T cells and evaluated their antitumor properties. We developed the methods to analyze tissue persistence and genomic integration sites of the CAR T cells.
Material and Methods
Generation of GPC3-targeted CAR T cells
The antigen recognition region from the HN3, hYP7, and the anti-CD19 antibody FMC63 was subcloned into the 2nd generation (2G) CAR construct, which contains expressing cassettes encoding the CD8α hinge and transmembrane region, a 4–1BB costimulatory domain, the intracellular CD3ζ, the self-cleaving T2A sequence, and the truncated human epidermal growth factor receptor (hEGFRt) for cell tracking and ablation. The hEGFRt lacks the domains essential for ligand binding and tyrosine kinase activity, but it retains the binding epitope of anti-EGFR monoclonal antibody cetuximab26. A 3rd generation (3G) CAR (hYP7) construct containing both 4–1BB and CD28 costimulatory domains was also made. The CAR T cells were produced as described previously27.
Animal studies
5-week-old female NOD/SCID/IL-2Rgcnull (NSG) mice (NCI Frederick) were housed and treated under the protocol (LMB-059) approved by the Institutional Animal Care and Use Committee at the NIH. For the peritoneal Hep3B model, 3 million luciferase-expressing Hep3B (Hep3B-Luc) cells were intraperitoneally (i.p.) injected into mice. Mice with established tumors were then randomly allocated into six groups and i.p. infused once with varying conditions as follows: (a) saline only (PBS); (b) 5 million un-transduced T cells (Mock); (c) 5 million CAR (HN3) T cells; (d) 5 million CAR (hYP7) T cells; (e) 10 million CAR (hYP7) T cells; (f) 20 million CAR (hYP7) T cells. For the peritoneal HepG2 model, 2 million HepG2-Luc cells were i.p. injected into male mice. Mice with established tumors were randomly allocated into two groups and i.p. infused once with mock T cells or CAR (hYP7) T cells. For the orthotopic HepG2 or Hep3B model, mice were inoculated with 0.5 million HepG2-Luc or Hep3B-Luc tumor cells in the liver. After 2 or 3 weeks of tumor establishment, mice were intravenously (i.v.) infused once with CAR (hYP7) T cells. The CD19 CAR T cells were used as control. For the Hep3B re-challenge model, 4 weeks after CAR (hYP7) T cell administration, mice were re-challenged with 0.5 million Hep3B-Luc tumor cells (i.p.) and followed for two weeks. Tumors were measured by total bioluminescent flux using a Xenogen IVIS Lumina (PerkinElmer).
Droplet digital PCR (ddPCR)
Genomic DNA from cells was isolated using the FlexiGene DNA kit (QIAGEN). ddPCR experiments were performed on a QX200 ddPCR system (Bio-Rad) according to the manufacturer’s instructions. CAR vector specific primers and probe were multiplexed with either a human (myocardin-like protein 2, MKL2) or mouse (Transferrin Receptor, Tfrc) reference gene assay. The primers and probes sequences were listed in Supplementary Methods.
Integration site analysis
CAR lentivector integration site analysis was performed using linker-mediated PCR adapted from a procedure described previously for measuring viral infection in HIV patients28. Briefly, sample DNA is randomly sheared, end-repaired, and ligated to a linker. The integration site is amplified with one primer specific to the lentivector LTR and another primer specific to the linker. The amplified product is subjected to high-throughput Illumina Sequencing. Integration sites in the sample are identified and quantified for further analysis. The primer sequences designed for the present study are listed in Supplementary Methods. The raw data for all CAR integration sites identified in the present study are provided as Supplementary Data Sheets 1–3.
Results
CAR (hYP7) T cells are more potent than CAR (HN3) T cells
To evaluate the effect of GPC3 epitopes in CAR T cell killing, we compared the HN323 and hYP724, 25 antibodies that recognizes the N-lobe and C-lobe of GPC3, respectively (Figure 1A). The HN313 and YP7 antibodies are highly tumor specific for their binding on tumor cells and tissues (Supplementary Figure 1 to 4). To produce CAR T cells for testing in HCC cell and animal models (Figure 1B), the antigen recognition region of the HN3 or the hYP7 antibody was cloned into lentiviral vectors encoding either the 2G CAR with 4–1BB costimulatory domain or the 3G CAR with 4–1BB and CD28 costimulatory domains (Figure 1C). The expression of CARs in transduced T cells was detected through recombinant GPC3 protein staining and cell surface hEGFRt expression (Supplementary Figure 5). In view of potential off-target toxicity induced by the 3G CAR (hYP7) T cells (Supplementary Figure 6A), the 2G CAR construct was used for the rest of our study unless otherwise noted.
Figure 1.
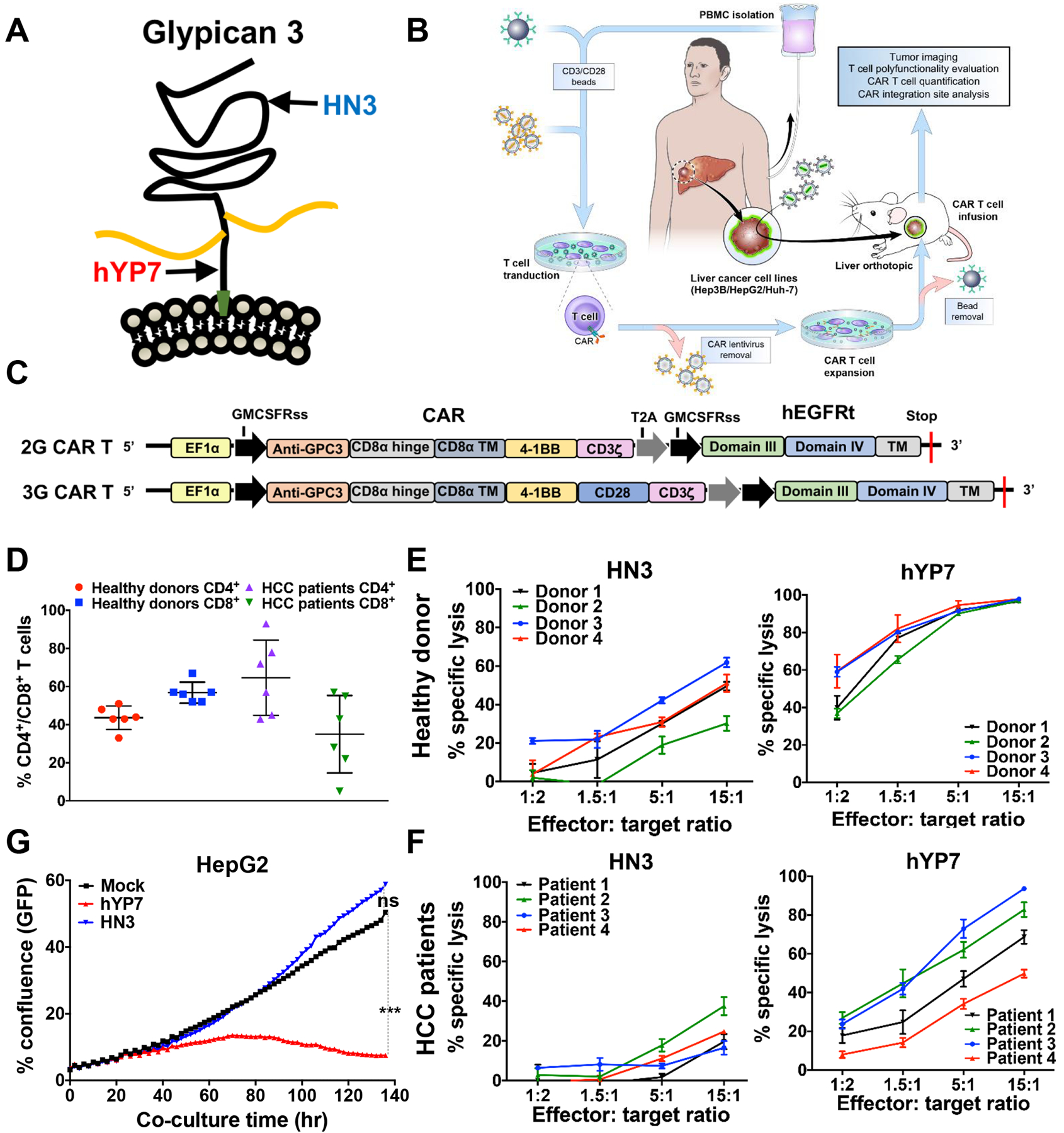
GPC3-targeted CAR T cells kill GPC3-positive HCC cells in vitro. (A) The binding of two antibodies to GPC3. HN3 binds to the N-lobe of GPC3 around residue 41 (phenylalanine). YP7 binds to the C-lobe of GPC3 (residue 521–530). (B) Schematic of CAR T cell production and evaluation in mouse models. (C) Schematic of the 2nd generation (2G) and 3rd generation (3G) CAR constructs. (D) CD4+ and CD8+ T cell analysis of CAR (hYP7) T cells from 6 healthy donors and 6 HCC patients. (E-F) Cytolytic activity of CAR (HN3) T cells and CAR (hYP7) T cells from healthy donors (E) and HCC patients (F) after 24 hours of co-culture with Hep3B cells. (G) GPC3-targeted CAR T cells-mediated killing of HepG2 cells as determined by using IncuCyte zoom. HepG2 cells were incubated with CAR T cells at the E:T ratio of 2:1 up to 140 hours. GFP, green fluorescent protein. Values represent mean ± SEM. ***P < .001, ns, not significant.
Next, we evaluated the expansion of GPC3-targeted CAR T cells from healthy donors and HCC patients (Supplementary Figure 6B). After expansion, CAR (hYP7) T cells derived from healthy donors were comprised of similar ratios of CD4+ and CD8+ T cell subsets (Figure 1D). Interestingly, we observed substantial variations in the frequency of CD4+ (43–93%) and CD8+ T cells (7–57%) in HCC patients. We then compared the cytolytic capability of CAR (HN3) and CAR (hYP7) T cells in HCC cells. As shown in Figure 1E and F, CAR (hYP7) T cells displayed higher lytic activity than CAR (HN3) T cells in Hep3B cells. At the E:T ratio of 5, lytic activity of HCC patient-derived CAR (hYP7) T cells ranged from 34% to 73%, with an average of 54%, which was lower than the average activity (92%) of healthy donor-derived CAR (hYP7) T cells, possibly due to low CD8+ T cell number in HCC patients. Minimal cell lysis was observed in Hep3B cells treated with mock T cells (Supplementary Figure 6C) or the GPC3 knockout Hep3B cells treated with CAR (hYP7) T cells (Supplementary Figure 6D), demonstrating target-dependent specificity. Furthermore, additional liver cancer cell lines including HepG2 and Huh-7 were also lysed by CAR (HN3) and CAR (hYP7) T cells (Supplementary Figure 6E and F). CAR (hYP7) T cells were significantly more potent in eliminating HepG2 cells compared with CAR (HN3) T cells during the period of 140 hours (Figure 1G). In addition, CAR (hYP7) T cells produced more cytokines and chemokines than CAR (HN3) T cells in the presence of Hep3B or HepG2 cells (Supplementary Figure 7). Collectively, CAR (hYP7) T cells exhibit better cytolytic ability than CAR (HN3) T cells. CAR T cells derived from HCC patients are able to kill GPC3-positive HCC cells.
CAR (hYP7) T cells are highly polyfunctional
Polyfunctional T cells, a subset of T cells capable of co-producing two or more cytokines/chemokines at the single-cell level, are recently reported for their association with long-term immune responses in the clinical settings29–31. Here, we used the 32-plex panel that included the key immune elements of T cells (Figure 2A). The polyfunctional strength index (PSI) values are defined by cytokine function to highlight the contribution of each group to the overall polyfunctionality of the sample29. As shown in Figure 2B and C, CAR (hYP7) T cells from a healthy donor showed remarkably higher PSI than CAR (HN3) T cells when stimulated with Hep3B (11-fold) or G1 (77-fold) cells, whereas no increase of PSI was shown in CAR (hYP7) T cells stimulated by antigen-negative A431 (Figure 2C). Moreover, CD8+ CAR (hYP7) T PSI is 8 and 11 times higher than CD4+ CAR (hYP7) T PSI when co-cultured with Hep3B and G1 cells, respectively, indicating CD8+ CAR T cells were more polyfunctional than CD4+ CAR T cells. To distinguish the polyfunctional subsets within each sample, we used a polyfunctional heat map visualization to display major secreted functional subsets. As shown in Figure 2D, a 4-plex group containing granzyme B, INF-γ, perforin and s4–1BB was only expressed by a small subset of CD8+ CAR (hYP7) T cells upon Hep3B stimulation. The G1-stimulated CD8+ CAR (hYP7) T cells were more polyfunctional with a small subset secreting a 7-plex group containing granzyme B, INF-γ, CCL-3, CCL-4, perforin, TNF-α and s4–1BB (Figure 2E).
Figure 2.
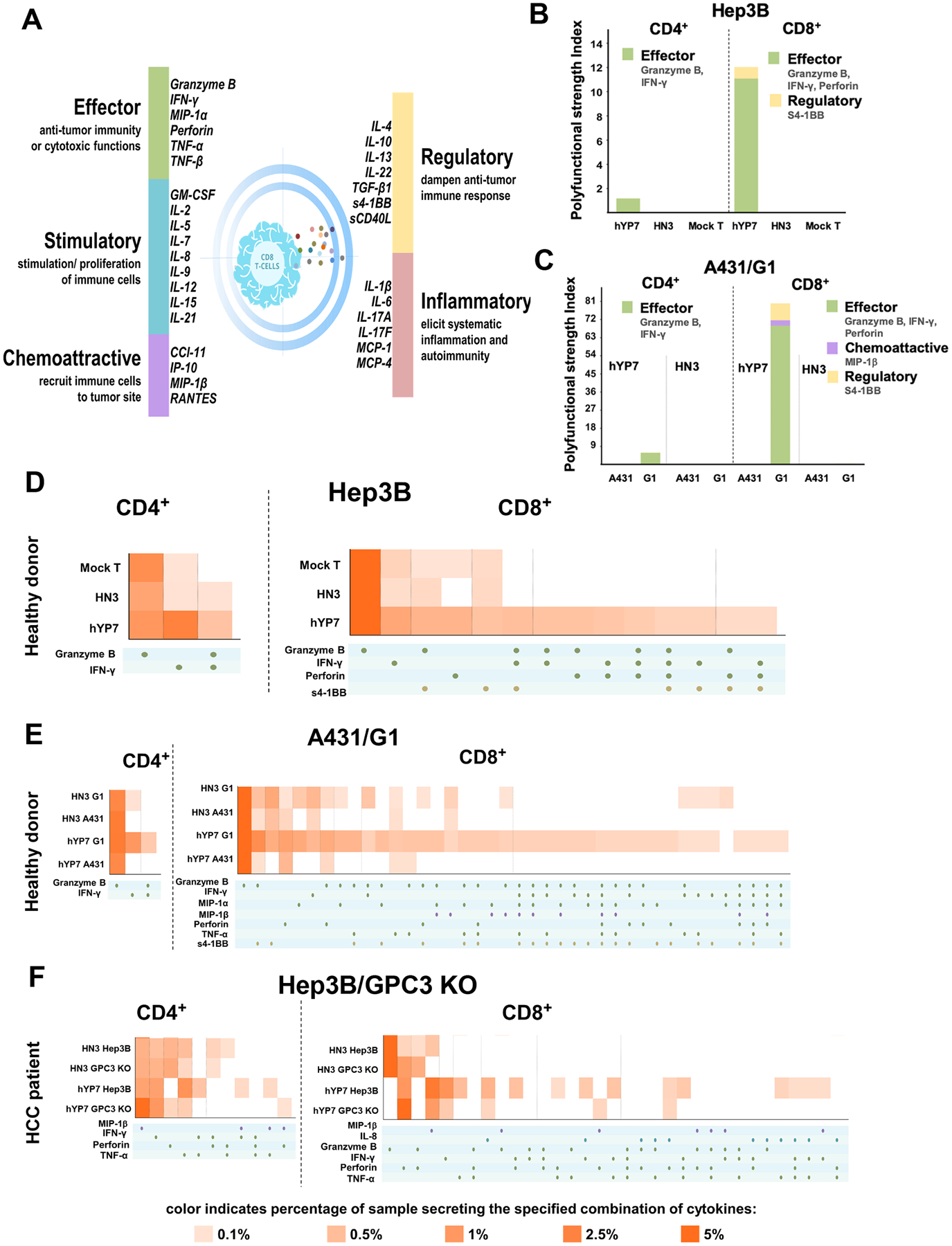
Cytokine/chemokine profiles of polyfunctionality of T cells redirected with GPC3. (A) The validated 32-plex panel including five groups of cytokines: effector, stimulatory, chemoattractive, regulator and inflammatory. (B-C) PSI computed for healthy donor-derived GPC3-targeted CAR T cells co-cultured with Hep3B (B) and G1/A431 (C) cells for 20 hours at the single-cell level. (D-E) Polyfunctional heat map displaying major functional cytokines/chemokines secreted across GPC3-specific CAR T cells from healthy donor upon Hep3B (D) and G1/A431 (E) cell stimulation. (F) Polyfunctional heat map of HCC patient-derived GPC3-specific CAR T cells upon Hep3B and GPC3 knockout Hep3B cell stimulation for 20 hours.
We also examined the polyfunctionality of CAR T cells from a HCC patient (#3, Supplementary Table 1) after stimulated with Hep3B cells. Consistent with polyfunctional profiles of healthy donor-derived CAR T cells, the pronounced upregulation of polyfunctional groups was only found in CAR (hYP7) product, predominantly in CD8+ T cells, but not in CAR (HN3) product from the HCC patient (Figure 2F). Interestingly, the HCC patient-derived polyfunctional CD8+ CAR (hYP7) T cells were not only composed of effector cytokines (granzyme B, INF-γ, perforin), but also additional cytokines/chemokines (TNF-α, MIP-1β and IL-8) that were not secreted by CD8+ CAR (hYP7) T cells from the healthy donor (Figure 2D). Taken together, CAR (hYP7) derived from both the healthy donor and the HCC patient stimulated robust activation and expansion of polyfunctional T cells, particularly through small subsets of CD8+ cytotoxic T cells which lyse tumor cells by inducing perforin/granzyme apoptosis pathway.
CAR T cells targeting GPC3 inhibit Wnt signaling in HCC
Previous studies have shown that GPC3 interacts with Wnt ligands and promotes HCC cell proliferation by facilitating Wnt/Frizzled binding9–12. To determine if GPC3-targeted CAR T cells affected Wnt signaling in HCC cells, we measured active- and total β-catenin levels. CAR (hYP7) T cells from a healthy donor significantly reduced the expression of active-β-catenin and total β-catenin after co-cultured with Hep3B cells, while CAR (HN3) T cells and CD19 CAR T cells failed to inhibit β-catenin expression (Figure 3A). Interestingly, we found that CAR (hYP7) T cells from the #3 HCC patient dramatically suppressed the expression of active-β-catenin to a greater extent than the healthy donor-derived CAR (hYP7) T cells (Figure 3B). In addition, CAR (hYP7) T cells were able to inhibit β-catenin expression in HepG2 cells (Figure 3C), but not GPC3-negative A431 cells (Figure 3D). Furthermore, previous reports have shown that GPC3 regulates the expression of Yes-associated protein (YAP), a key effector molecule in the Hippo pathway in HCC23, 32. Here, we observed the increased phosphorylated YAP (p-YAP) and reduced total YAP (T-YAP) expression in Hep3B cells treated with CAR (hYP7) T cells from the HCC patient (Figure 3E). Together, our results indicate that targeting GPC3 by CAR (hYP7) T cells can suppress the Wnt/β-catenin and Yap signaling in HCC cells.
Figure 3.
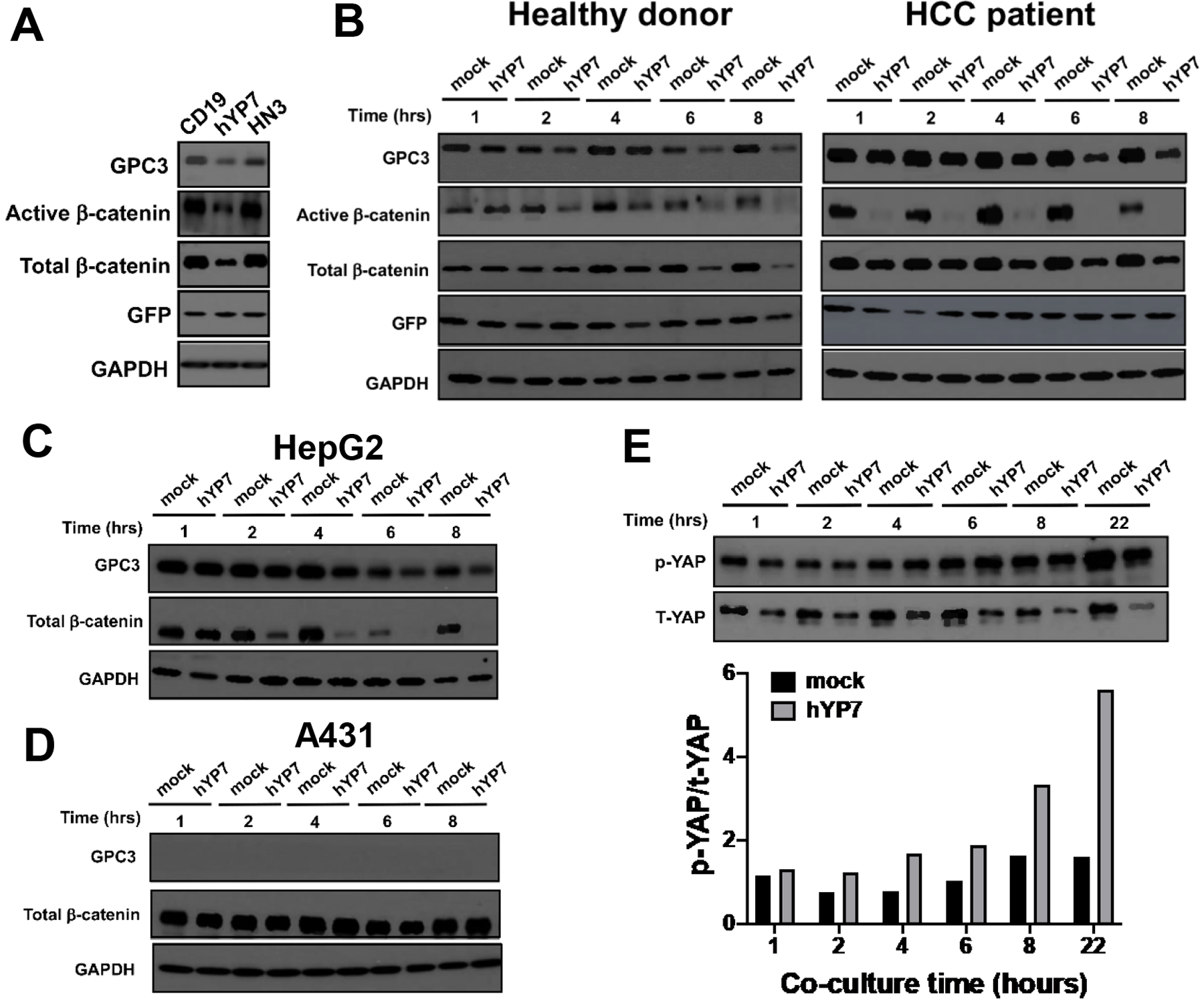
Inhibition of Wnt/β-catenin and YAP signaling by CAR (hYP7) T cell administration. (A) CAR (hYP7) T cells from healthy donor suppressed the expression of β-catenin in Hep3B cells after 6 hours of administration. (B) CAR (hYP7) T cells from healthy donor and HCC patient inhibited the expression of β-catenin in Hep3B cells in a time-dependent manner. (C-D) CAR (hYP7) T cells from healthy donor suppressed the expression of β-catenin in HepG2 cells (C), but not in A431 cells (D). (E) CAR (hYP7) T cells from HCC patient inhibited YAP signaling as evidenced by the increase of phospho-YAP expression and decrease of total-YAP expression after co-culture with Hep3B cells.
CAR (hYP7) T cells suppress the growth of HCC xenografts in mice
To evaluate the antitumor activities of GPC3-specific CAR T cells in vivo, NSG female mice were intraperitoneally (i.p.) injected with Hep3B-Luc cells (Figure 4A). Various doses of CAR (hYP7) T cells all showed reduced tumor burden compared with the mock group (Figure 4B and C). No significant tumor growth inhibition was seen in the mice treated with CAR (HN3) T cells. Mice receiving 20 million CAR (hYP7) T cells were all alive without recurrence by day 70, compared with 50% survival in the 5 million CAR (hYP7) T cell group (Figure 4D). Although GPC3-targeted CAR T cells initially caused a loss in body weight, mice gradually regained weight (Supplementary Figure 8). Moreover, the serum alpha-fetoprotein (AFP) levels in mice treated with 5 million (mean: 429 ng/mL) or 10 million (mean: 296 ng/mL) CAR (hYP7) T cells were significantly lower than the levels in mock T cell-treated mice (mean: 21,467 ng/mL) after 2 weeks of administration (Figure 4E). Notably, the AFP levels in mice treated with 20 million CAR (hYP7) T cells were in the range of 25–78 ng/mL, which is close to the cut-off value (20 ng/mL) in human adults33. Robust in vivo expansion and survival of genetically modified T cells are considered critical predictors of durable clinical remissions in cancer patients. We assessed the percentage of CAR T cells using ddPCR, which allows measurement of absolute gene copy number to determine CAR vector-positive cells. As shown in Figure 4F, 10.1% of cells were CAR vector-positive in the 5 million CAR (hYP7) group, whereas only 1.3% of cells were CAR vector-positive in the 5 million CAR (HN3) group after 3 weeks of administration. In the 10 million CAR (hYP7) group, 26.5% of CAR vector-positive cells were detected, demonstrating an inverse correlation between tumor burden and T cell persistence over time.
Figure 4.
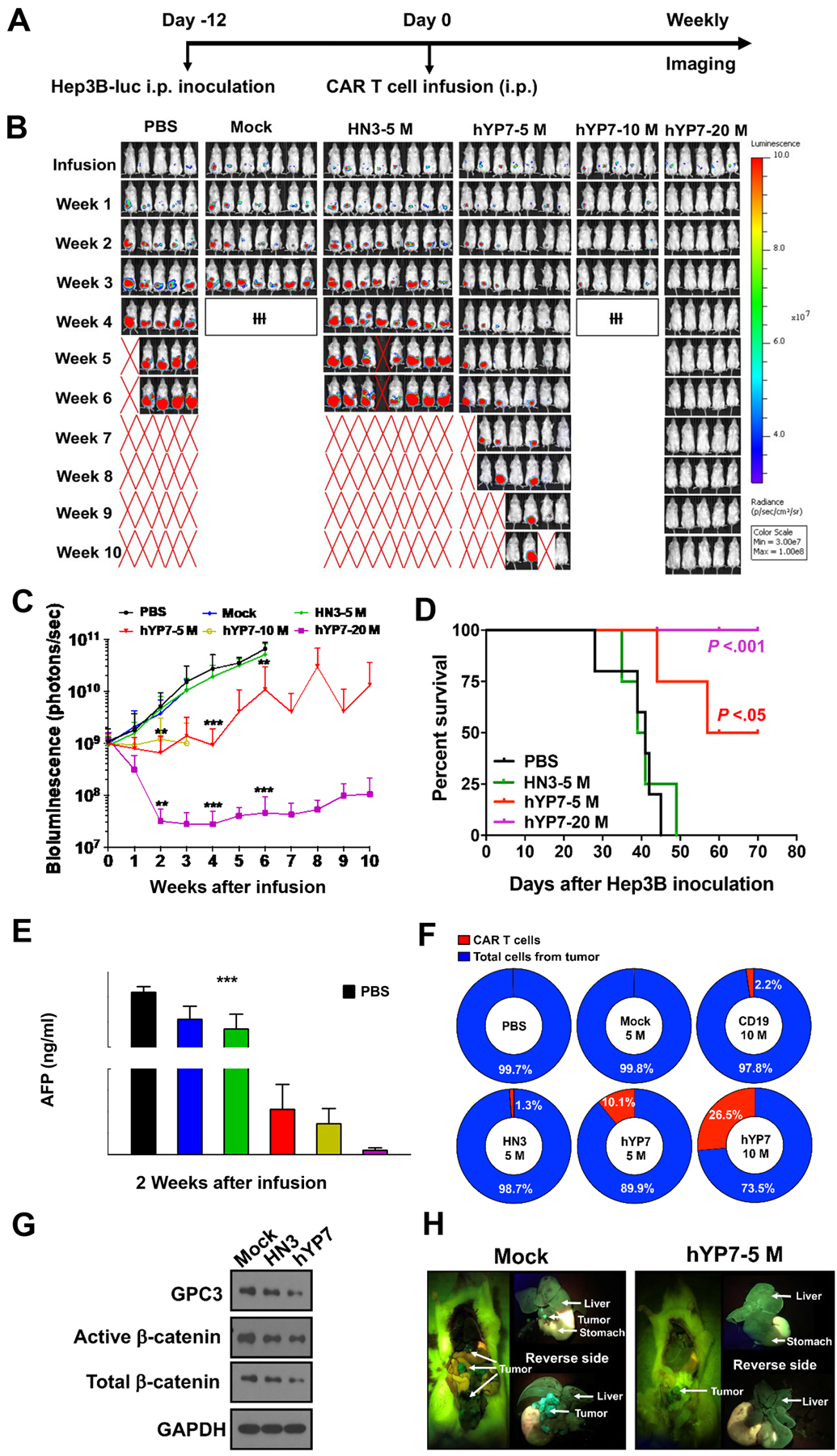
CAR (hYP7) T cells eradicate tumors in the peritoneal Hep3B xenograft mouse model. (A) Experimental schematic. Hep3B tumor-bearing NSG mice were i.p. injected with mock T cells (mock), 5 million CAR (HN3) T cells (HN3–5M), and 5, 10 and 20 million CAR (hYP7) T cells (hYP7–5M, hYP7–10M, hYP7–20M). (B) CAR (hYP7) T cells regressed established Hep3B xenografts at high dose (20M) and inhibited tumor growth at low doses (5M and 10M), whereas CAR (HN3) T cells did not inhibit tumor growth. Symbol ‘ƗƗƗ’ indicated that mice were euthanized in advance for analysis. (C) Tumor bioluminescence in mice treated in Figure 4B. (D) Kaplan–Meier survival curve of mice after infusion. (E) AFP levels in serum collected from groups shown in Figure 4B after 2 weeks of injection. (F) Detection of CAR vector-positive cells in xenograft tumor tissues after 3 weeks of injection. (G) CAR (hYP7) T cells caused the reduction of active- and total-β-catenin levels as compared with mock T cell-treated mice. (H) Representative pictures of mouse from mock and hYP7–10M group. Values represent mean ± SEM. *P < .05, **P < .01, ***P < .001, ns, not significant.
Consistent with the inhibitory effect of silencing GPC3 on Wnt signaling in vitro, CAR (hYP7) T cell administration downregulated active- and total-β-catenin levels compared with the mock group in vivo (Figure 4G). From the peritoneal Hep3B xenograft mouse model, we found that mice developed tumor lesions on the liver and other organs within the abdominal cavity (Figure 4H). Interestingly, Hep3B tumors in the mice treated with 5 million CAR (hYP7) T cells grew locally and were restricted to the adipose tissue away from the mouse liver, suggesting that a low dose of CAR (hYP7) T cells can prevent tumors from seeding and growing in the liver as well as spreading to other organs.
A previous report showed that HCC tumors grew faster and had a higher volume in male than female NSG mice34, which underlines the need for using male mice in liver cancer studies. To evaluate the efficacy of CAR (hYP7) T cells in another liver cancer xenograft mouse model, we i.p. inoculated HepG2 cells into male NSG mice (Figure 5A). Encouragingly, CAR (hYP7) T cells were able to regress tumor growth (Figure 5B and C), indicating that efficacy of GPC3-targeted CAR T cells is gender independent. After 5 weeks of CAR (hYP7) T cell administration, ddPCR detected 35.6% and 19.5% of CAR vector-positive cells from tumor and mouse spleen, respectively (Figure 5D). By contrast, we did not detect CAR vector-positive cells in either tissue from CD19 CAR T cell-treated mouse. Furthermore, we found that human HepG2 cells migrated to the mouse liver and CAR (hYP7) T cells restricted the liver metastasis of tumor cells (Figure 5E), which was similar to our observation in the peritoneal Hep3B xenograft mouse model.
Figure 5.
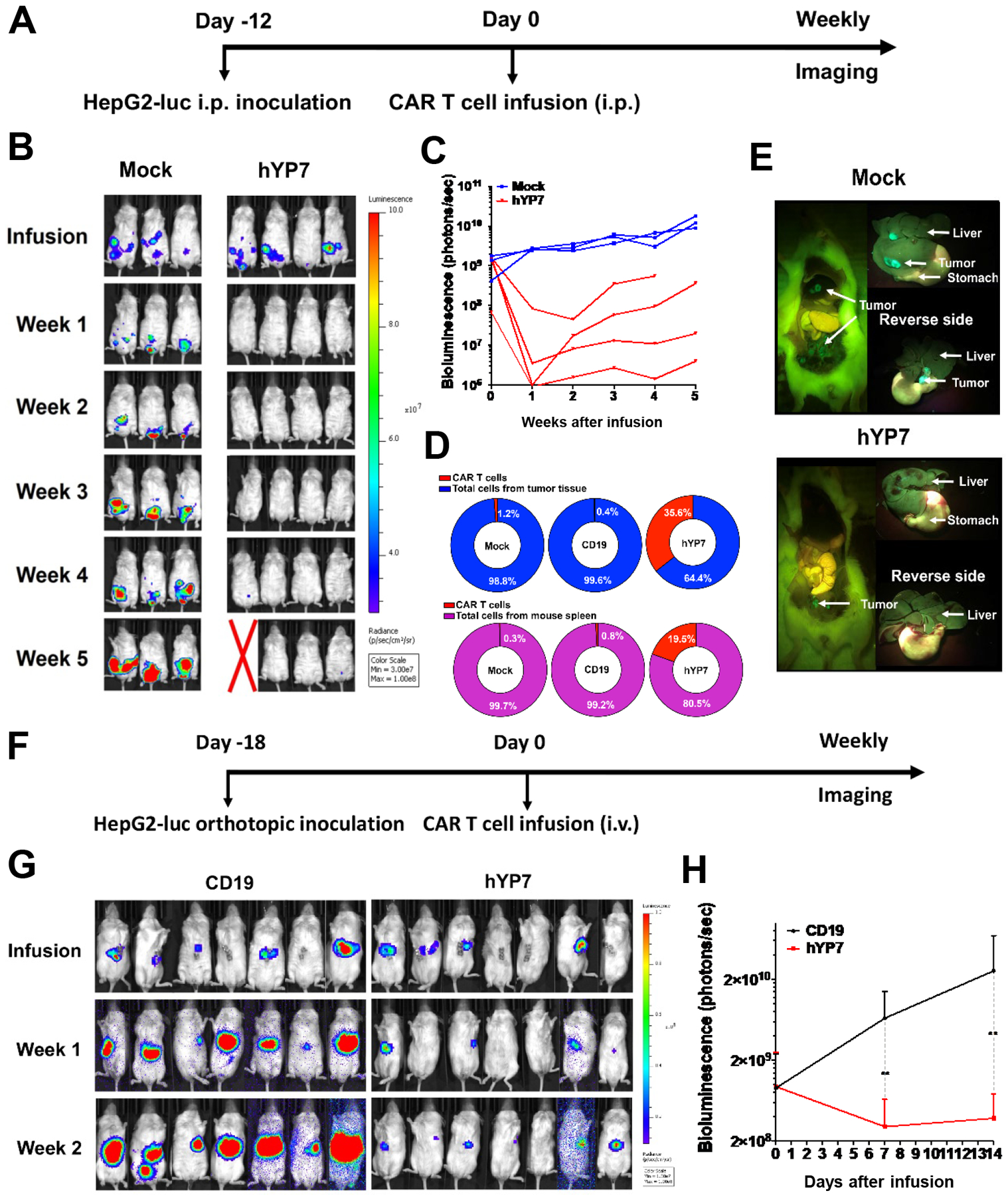
CAR (hYP7) T cells eliminate tumor cells in the HepG2 xenograft mouse models. (A) Experimental schematic of the peritoneal HepG2 xenograft mouse model. HepG2 tumor-bearing male NSG mice were i.p. injected with 20 million mock T cells or CAR (hYP7) T cells. (B) CAR (hYP7) T cells demonstrated potent antitumor activity and mediated eradication of HepG2 xenograft tumors. (C) Tumor bioluminescence in mice treated in Figure 5B. (D) Detection of CAR vector-positive cells in tumor and spleen from mice after 5 weeks of injection. (E) Representative pictures of mouse from mock and hYP7 groups. (F) Experimental schematic of the orthotopic HepG2 xenograft model. HepG2 tumor-bearing female NSG mice were infused with 10 million CD19 CAR T cells and CAR (hYP7) T cells. (G) CAR (hYP7) T cells regressed growth of orthotopic HepG2 tumors. (H) Tumor bioluminescence in mice treated in Figure 5G. Values represent mean ± SEM. **P < .01.
CAR (hYP7) T cells cause tumor regression in orthotopic xenograft models
We further examined antitumor activity of CAR (hYP7) T cells in an orthotopic HCC mouse model. Hep3B-Luc cells were injected into the liver of NSG mice, healthy donor derived-CAR (hYP7) T cells were i.p. or i.v. infused 21 days after tumor inoculation (Figure 6A). Although both routes of administration of CAR (hYP7) T cells led to a reduction in tumor size and suppressed tumor growth compared to the control group, i.v. injection of CAR T cells (hYP7 IV) resulted in greater tumor regression than i.p. injection of CAR T cells (hYP7 IP) (Figure 6B), possibly because of preferred localization of CAR T cells to the liver via blood circulation35. At the end of this study, 75% of mice in the hYP7 IV group were tumor free, whereas all mice in the mock group carried large tumors. Consistently, higher percentage of CAR vector-positive cells were detected in spleen and tumor microenvironment from the hYP7 IV group than tissues from the hYP7 IP group (Supplementary Figure 9). We also conducted toxicology analysis of CAR (hYP7) T cells by examining blood counts, serum chemistry and organ weights in mice (Supplementary Table 2).
Figure 6.
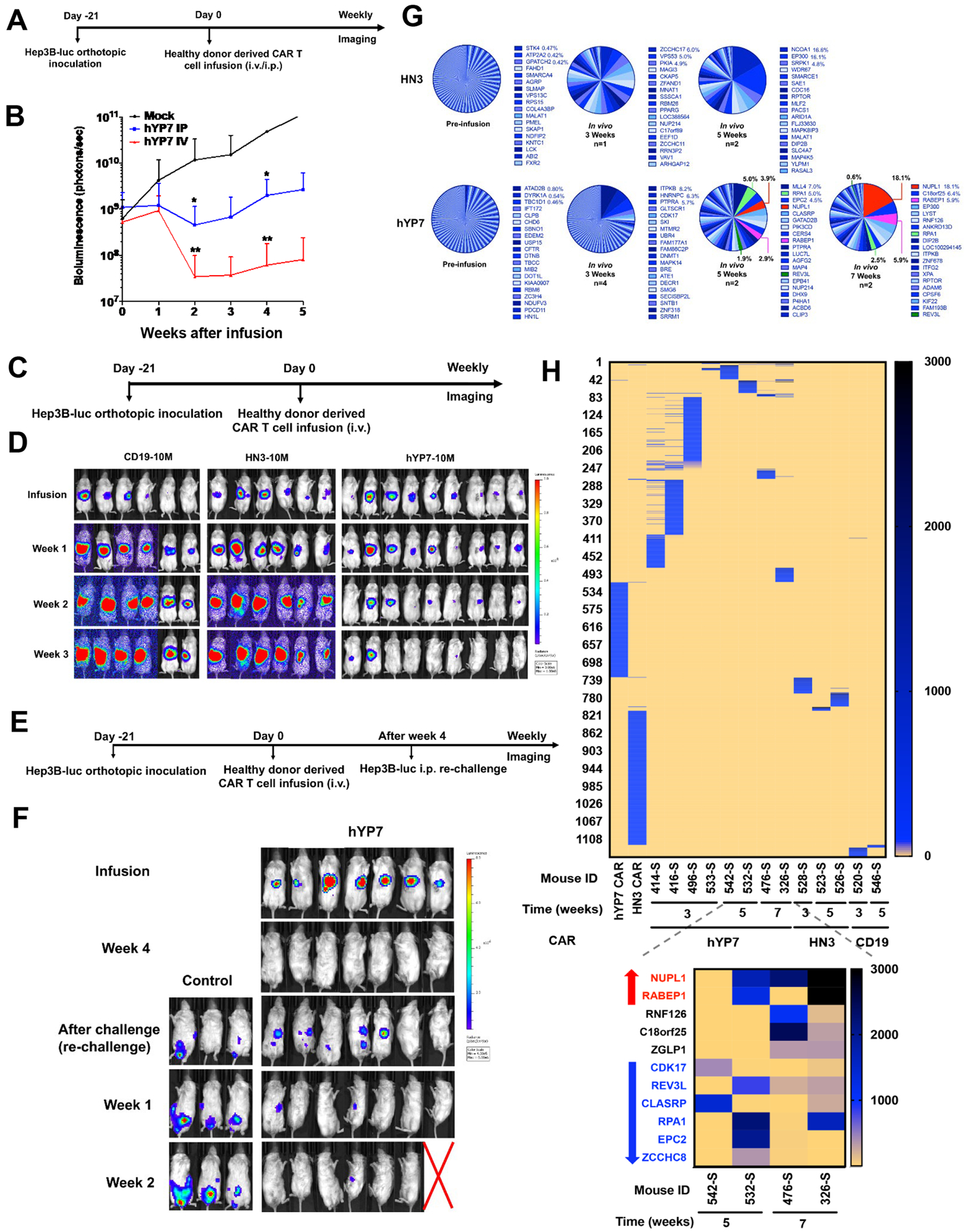
Tumor eradication in the orthotopic Hep3B xenograft mouse model by CAR (hYP7) T cells from a healthy donor. (A) Experimental schematic. The orthotopic Hep3B tumor-bearing NSG mice were i.p. or i.v. injected with 20 million CAR (hYP7) T cells. (B) Mice treated with CAR (hYP7) T cells via tail vein (hYP7 IV) demonstrated tumor eradication, while intraperitoneal infusion (hYP7 IP) resulted in tumor growth inhibition. (C) Experimental schematic. The orthotopic Hep3B tumor-bearing NSG mice were i.v. injected with 10 million CD19 CAR, CAR (HN3) and CAR (hYP7) T cells. (D) CAR (hYP7) T cell administration regressed Hep3B tumor growth in mice, while CAR (HN3) T cells failed to inhibit tumor growth. (E) Experimental schematic of Hep3B tumor re-challenge. CAR (hYP7)-treated mice that showed no detectable tumor were i.p. implanted with 0.5 million Hep3B cells. As control, naïve mice were implanted with Hep3B cells following an injection of mock T cells. (F) CAR (hYP7)-treated mice resisted Hep3B tumor re-challenge. (G) Frequency of integrated genes in spleen (S) of mice treated with CAR (HN3) and CAR (hYP7) T cells at 3 weeks, 5 weeks and 7 weeks post-infusion. The top twenty genes were listed. (H) Distribution of integration sites in spleen of mice treated with CD19 CAR, CAR (HN3) and CAR (hYP7) T cells over 3–7 weeks. The shared integrated genes in mice after 5 to 7 weeks of CAR (hYP7) T cell injection was highlighted in a separate heatmap. Values represent mean ± SEM. *P < .05, **P < .01.
Next, we compared the efficacy of healthy donor-derived CAR (HN3) and CAR (hYP7) T cells in the same orthotopic Hep3B xenograft mouse model (Figure 6C). As shown in Figure 6D, CAR (hYP7) T cells suppressed tumor growth and eliminated the tumor in 66% of mice by week 3, whereas CAR (HN3) T cells failed to reduce tumor burden. To determine the ability of persistent CAR (hYP7) T cells to prevent tumor relapse, mice that cleared initial tumor burden were re-challenged with Hep3B tumor cells (Figure 6E). While tumors grew rapidly in control mice, mice previously treated with CAR (hYP7) T cells remained tumor free after Hep3B tumor re-challenge (Figure 6F). Moreover, efficacy of CAR (hYP7) T cells was also validated in another orthotopic (HepG2) xenograft mouse model (Figure 5F–H).
To analyze the molecular determinants of CAR (hYP7) T cell efficacy and persistence, we analyzed the integration sites from the spleens of mice (showed in Figure 6D) treated with CAR (hYP7) T cells (n=8), CAR (HN3) (n=3) and CD19 CAR (n=2) from 3 to 7 weeks. As we expected, the integration sites from pre-infusion CAR (HN3) and CAR (hYP7) T cells were randomly distributed (Figure 6G). The integration into NUPL1 was detected at a frequency of 3.9% 5 weeks after injection and increased to 18.1% at week 7, whereas no integration site was shared and enriched in mice treated with CAR (HN3) T cells. Analysis of all the integration sites from each individual mouse identified 11 integration sites shared among different mice from 5 to 7 weeks (Figure 6H, Supplementary Table 3 and Supplementary Data Sheet 1). Some of these integration sites (e.g. NUPL1 and RABEP1) were enriched from 5 to 7 weeks, while some (e.g. REV3L and RPA1) became gradually diminished. We also found largely different integration sites between a complete responder and a partial responder after 7 weeks of CAR (hYP7) T cell injection (Supplementary Figure 10 and Supplementary Data Sheet 2). Interestingly, the two shared integration sites (NUPL1 and RABEP1) decreased in the partial responder at week 7.
Finally, to evaluate the efficacy of CAR (hYP7) T cells derived from HCC patients, we again chose to use primary T cells isolated from the #3 HCC patient, who was a 64-year-old male that had been treated with sorafenib for 6 months until his disease progressed. We produced CAR (hYP7) and CD19 CAR T cells from this HCC patient and used these cell products to treat mice engrafted orthotopically with Hep3B-Luc cells (Figure 7A). The patient-derived CAR (hYP7) T cells effectively eradicated Hep3B tumors in 77% of mice, whereas mice treated with CD19 CAR T cells succumbed to tumors 3 weeks post T cell transfusion (Figure 7B). The same HCC patient-derived CAR (hYP7) T cells were able to eliminate Hep3B tumors in 75% of mice from a repeated experiment (Supplementary Figure 11). Furthermore, we characterized the function of persistent CAR (hYP7) T cells recovered from the spleen of treated mouse named as mCAR (hYP7) T cells. As shown in Figure 7C, CD8+ mCAR (hYP7) T cells exhibited significantly higher lytic activity than CD4+ mCAR (hYP7) T cells against Hep3B cells. Furthermore, we found a profound increase of polyfunctionality in CD8+ mCAR (hYP7) T cells (Figure 7D). The polyfunctional CAR T cell subsets with combination of 3 or more cytokine secretions (MIP-1β, IL-8, granzyme B, INF-γ, perforin, TNF-α) in both CD4+ and CD8+ T cells are largely increased only when co-cultured with Hep3B cells and not with GPC3 knockout Hep3B cells, further demonstrating the specificity of CAR (hYP7) after in vivo passage.
Figure 7.
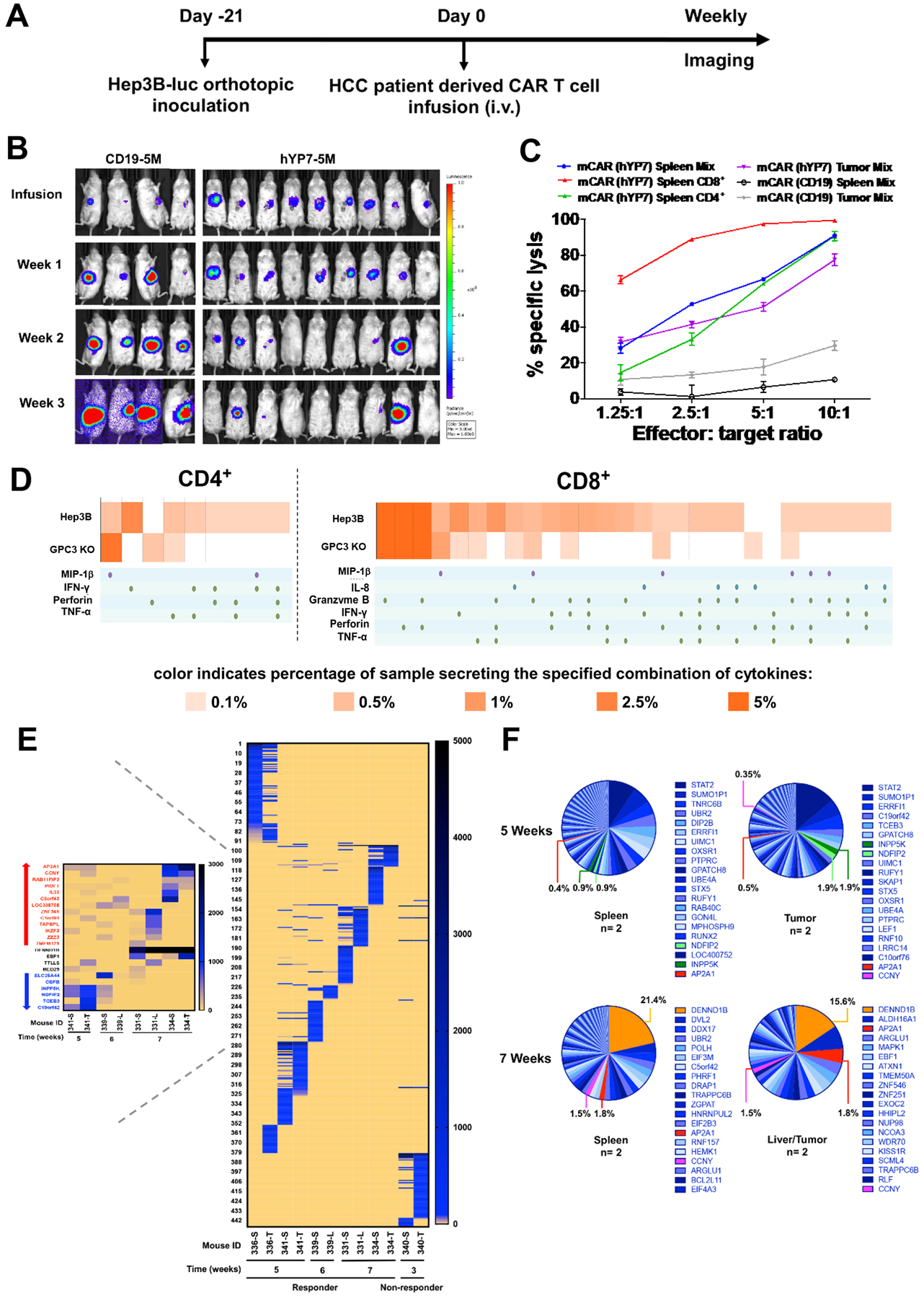
Persistent polyfunctional CAR (hYP7) T cells from a HCC patient eradicate orthotopic Hep3B xenograft tumors. (A) Experimental schematic. The orthotopic Hep3B tumor-bearing NSG mice were i.v. injected with 5 million CD19 CAR and CAR (hYP7) T cells. (B) CAR (hYP7) T cells regressed Hep3B tumor growth in mice. (C) CAR (hYP7) T cells recovered from mouse spleen after 4 weeks of injection named as mCAR (hYP7) T cells were co-cultured with Hep3B cells, and cytolytic activity was analyzed 24 hours after co-culture. (D) mCAR (hYP7) T cells showed profound increase of polyfunctionality by the stimulation of Hep3B cells, but not the GPC3 knockout Hep3B cells. (E) Distribution of integration sites in individual mouse treated with CAR (hYP7) T cells for 3–7 weeks. The shared integrated genes in mice after 5 to 7 weeks of CAR (hYP7) T cell injection was highlighted in a separate heatmap. S, T and L represent spleen, tumor and liver, respectively. (F) Frequency of integrated genes in spleen and liver/tumor of mice treated with CAR (hYP7) T cells from 5 weeks to 7 weeks post-infusion. The top twenty genes were listed. Values represent mean ± SEM.
We also analyzed the integration sites in mice treated with this HCC patient-derived CAR (hYP7) T cells from 3 to 7 weeks. As shown in Figure 7E, CAR (hYP7) showed a strong integration preference into distinct genes between responders and non-responder. Notably, the integrated sites were largely shared between different tissues (e.g. spleen, liver) of the same mouse, indicating clonal expansion of CAR (hYP7) T cells in mice. Comparing with low abundancy at 5 weeks post infusion, CAR (hYP7) integration into particular genes (e.g. AP2A1, CCNY) were enriched at 7 weeks while some integration events (e.g. INPP5K, NDFIP2) became diminished at 7 weeks. The details of all 23 shared integrated genes can be found in Supplementary Table 4 and Supplementary Data Sheet 3. Moreover, the CAR (hYP7) integration into DENND1B became the most dominant event at 7 weeks (spleen 21.4% and liver 15.6%) (Figure 7F). Taken together, we found that persistent CAR (hYP7) T cells had integration sites related to specific genes shared in different mice at different time points, indicating a potential selection pressure for integration sites in the genome for CAR T cell activation, survival and clonal expansion in vivo.
Discussion
In the present study, we used the antibodies hYP7 and HN3 specifically for a membrane-proximal C-lobe epitope and a membrane-distal N-lobe epitope of GPC3 to make CAR T cells and analyzed their antitumor activities. The CAR (hYP7) T cells targeting the C-lobe of GPC3 close to the cell membrane showed superior antitumor activity by producing CAR T cells that not only induce perforin/granzyme-mediated apoptosis, but also inhibit Wnt/β-catenin signaling in tumor cells. We also used ddPCR and genomic sequencing methods to analyze the persistence of CAR T cells in mice and demonstrated a subset of the CAR (hYP7) T cell clones that were enriched and expanded over 7 weeks after injection.
Various antibody-based therapeutic strategies including immunotoxins and ADCs targeting GPC3 have been developed for treating HCC12–14. The immunogenicity of immunotoxins and the likelihood of multidrug resistance to ADC treatment are potential limitations for them to be used as single agents for reaching long-term tumor remission. An optimized GPC3-targeted CAR T cell therapy for treating liver cancer may hold greater potential for achieving sustained tumor remission despite the current challenge for treating solid tumors in general. The GPC3-targeted CAR T cells based on the GC33 antibody36 have been made and are currently being tested for the treatment of HCC15, 18, 22. Although GC33 and YP7 are known to bind the C-lobe of GPC3, they recognize two distinct epitopes (Supplementary Figure 12A). Specifically, the GPC3 fragment (amino acid 521–530) is crucial for the binding ability of YP7, while GC33 does not bind to the same fragment. The CAR (hYP7) expressed on Jurkat T cells bind to GPC3 with higher affinity than GC33 CAR (Supplementary Figure 12B). We have compared 2G CAR (hYP7) and CAR (HN3) with the reported 3G CAR (GC33)15 using the Huh-7 i.p. dissemination xenograft mouse model. We found that the CAR (hYP7) T cells reduced tumor burden more effectively than CAR (GC33) T cells (Supplementary Figure 13). It would be interesting to compare the efficacy and safety of CAR (hYP7) T cell therapy with CAR (GC33) T cell therapy in future clinical studies.
Using single cell-based functional analysis, we found that the CD8+ T cells showed a greater increase in polyfunctionality than CD4+ T cells in CAR (hYP7) T cells from healthy donor and HCC patient. Moreover, HCC patient-derived CD8+ CAR (hYP7) T cells recovered from mouse spleen showed a greater increase in percentages of polyfunctional T cells than the same patient-derived CD8+ CAR (hYP7) T cells cultured in vitro by Hep3B stimulation (Figure 2F and 7D). Future studies of evaluating HCC patient-derived CAR T cells in the same patient-derived xenograft (PDX) model, as well as a murine HCC model37 by hydrodynamic injection of oncogenes (e.g., MYC) and GPC3, may further validate the efficacy of GPC3-targeted CAR T cells in the tumor microenvironment.
Membrane-proximal epitopes can facilitate efficient T cell synapse formation38, 39, which may explain the superior efficacy of CAR (hYP7) compared with CAR (HN3). In addition to its unique epitope specificity, the YP7 antibody may have the highest binding affinity among all known GPC3 antibodies, including GC33, which may also contribute to the potent efficacy of CAR (hYP7). Furthermore, CAR (hYP7) T cells have significantly higher polyfunctionality than CAR (HN3) T cells. Moreover, a subset of distinct integration sites in the T cell genome are selected and enriched in different mice at different time points after a single injection of CAR (hYP7) T cells, not CAR (HN3) T cells. Taken together, differences in persistence, integration site preferences, and T cell polyfunctionality may contribute to the discrepancy between efficacies of these two CAR constructs. Lastly, it has been shown that GPC3 may function as a co-receptor for Wnt and facilitate the Wnt/Frizzled signaling complex in HCC cells9, 10. Wnt/β-catenin signaling is frequently upregulated in HCC and is implicated in the maintenance of tumor initiating cells, immune escape and resistance37, 40. We previously demonstrated that GPC3 affected Wnt/β-catenin signaling and that GPC3 knockout inhibited HCC tumor growth in cell and mouse models11. In the present study, we found significantly more reduction of GPC3 protein levels induced by the co-culture with GPC3 (hYP7) CAR T cells than GPC3 (HN3) CAR T cells. More reduction of active β-catenin in HCC cells was also observed when co-cultured with GPC3 (hYP7) CAR T cells than the GPC3 (HN3) CAR T cells. Surprisingly, hYP7 showed greater reduction in active β-catenin than HN3 in the CAR format, despite HN3 blocking the Wnt binding site on GPC3 as a naked antibody11, 12. We speculate that the inhibition of Wnt/β-catenin signaling by GPC3-targeted CAR T cells is likely due to the decrease of GPC3 levels in HCC cells via an unknown mechanism rather than the impairment in the direct interaction between GPC3 and Wnt. Interestingly, it has been recently demonstrated that CAR T cells can provoke reversible antigen loss through the trogocytosis mechanism41. The reduction of Wnt signaling in tumor cells may further explain how CAR (hYP7) T cells could eradicate the HCC xenografts in mice.
Recent reports showed CAR T cell associated clonal expansion in a complete remission in patients with leukemia42,43 suggesting it is important to investigate candidate factors driving successful expansion in responders. In the present study, we analyzed the integration sites of CAR (hYP7) from a healthy donor and a HCC patient in mice. Many shared integrated genes were observed in different tissues on the same mouse, likely due to the expansion of selected clones. Several integration events (e.g. NUPL1, DENND1B) have become highly abundant in multiple mice at week 5 and 7 after infusion. Interestingly, some of these integrations were located in the same orientation as the corresponding genes, which could lead to alternative splicing and affect their functions. NUPL1 encodes a nucleoporin in the nuclear pore complex. Interestingly, deletion of NUPL1 was found in some colorectal cancers and knockdown of NUPL1 could promote cell growth44. Therefore, the insertion of CAR (hYP7) into NUPL1 may potentially promote CAR T cell growth. DENND1B plays an important role in regulating TCR internalization in TH2 cells45. The clinical relevance of these integration sites remains unclear. Future studies analyzing persistent CAR T cells in HCC patients will validate shared integration sites (‘hotspots’) in the T cell genome.
In conclusion, we have demonstrated that CAR (hYP7) T cells can induce sustained HCC tumor regression in mice as a GPC3-targeted CAR T cell therapy that might be developed for treatment of patients with HCC.
Supplementary Material
WHAT YOU NEED TO KNOW:
BACKGROUND AND CONTEXT:
Glypican 3 (GPC3) is an oncofetal antigen that promotes Wnt-dependent cell proliferation and is highly expressed in hepatocellular carcinoma (HCC). Chimeric antigen receptors (CARs) that target GPC3 are in development for treatment of HCC.
NEW FINDINGS:
In mice with xenograft or orthoptic liver tumors, GPC3-targeted CAR T cells reduced Wnt signaling in HCC cells and caused tumor regression.
LIMITATIONS:
This study was performed in mice; studies are needed in humans.
IMPACT:
GPC3-targeted CAR T cells might be developed for treatment of patients with HCC.
Acknowledgements:
We thank Dr. Steven Rosenberg (NCI) for valuable advice in the early stage of this study and reading the manuscript. We also thank NIH Fellows Editorial Board, NIH Library Editing Service, Jessica Hong (NCI) and Bryan Fleming (NCI) for editorial assistance. The National Cancer Institute (NCI) holds patent rights to anti-GPC3 antibodies including YP7 and HN3 in many jurisdictions, including the USA (e.g., US Patent 9409994, US Patent 9206257, US Patent 9394364, US Patent 9932406, US Patent Application 62/716169, US Patent Application 62/369861), China, Japan, South Korea, Singapore, and Europe. Claims cover the antibodies themselves as well as conjugates that utilize the antibodies, such as recombinant immunotoxins, antibody-drug conjugates (ADCs), bispecific antibodies, and modified T cell receptors (TCRs)/chimeric antigen receptors (CARs) and vectors expressing these constructs. Anyone interested in licensing these antibodies can contact Mitchell Ho (NCI) for additional information.
Grant Support: This work was supported by the Intramural Research Program of NIH, NCI (Z01 BC010891 and ZIA BC010891) to M. Ho. D.L. was a recipient of a predoctoral fellowship from the China Scholarship Council and supported by the Center for Cancer Research at the NCI and the NIH Graduate Partnerships Program. H.F. was a recipient of a visiting fellowship from the China Scholarship Council. A.K. is a predoctoral fellow supported by the Mayo Clinic (Clinical and Translational Science award UL1 TR000135), the Center for Cancer Research at the NCI and the NIH Graduate Partnership Program. The Leidos Biomedical Research, Inc. was in part supported with federal funds from the NCI, NIH, under Contract No. HHSN261200800001E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
References:
- 1.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filmus J, Shi W, Wong ZM, et al. Identification of a new membrane-bound heparan sulphate proteoglycan. Biochem J 1995;311 (Pt 2):561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res 1997;57:5179–84. [PubMed] [Google Scholar]
- 5.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003;125:89–97. [DOI] [PubMed] [Google Scholar]
- 6.Baumhoer D, Tornillo L, Stadlmann S, et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol 2008;129:899–906. [DOI] [PubMed] [Google Scholar]
- 7.Aviel-Ronen S, Lau SK, Pintilie M, et al. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol 2008;21:817–825. [DOI] [PubMed] [Google Scholar]
- 8.Montalbano M, Georgiadis J, Masterson AL, et al. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma (Review). Oncol Rep 2017;37:1291–1300. [DOI] [PubMed] [Google Scholar]
- 9.Capurro MI, Xiang YY, Lobe C, et al. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005;65:6245–54. [DOI] [PubMed] [Google Scholar]
- 10.Gao W, Kim H, Feng M, et al. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology 2014;60:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Wei L, Liu X, et al. A frizzled-like cysteine rich domain in glypican-3 mediates Wnt binding and regulates hepatocellular carcinoma tumor growth in mice. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Tang Z, Zhang YF, et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun 2015;6:6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming BD, Urban DJ, Hall M, et al. The engineered anti-GPC3 immunotoxin, HN3-ABD-T20, produces regression in mouse liver cancer xenografts via prolonged serum retention. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Urban DJ, Nani RR, et al. Glypican-3-Specific Antibody Drug Conjugates Targeting Hepatocellular Carcinoma. Hepatology 2019;70:563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014;20:6418–28. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Jiang X, Chen S, et al. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front Immunol 2016;7:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiguro T, Sano Y, Komatsu SI, et al. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Guo L, Rathi P, et al. Redirecting T Cells to Glypican-3 with 4–1BB Zeta Chimeric Antigen Receptors Results in Th1 Polarization and Potent Antitumor Activity. Hum Gene Ther 2017;28:437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Li K, Jiang H, et al. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol Immunother 2017;66:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Jiang H, Shi B, et al. Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front Pharmacol 2018;9:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Z, Di S, Shi B, et al. Increased antitumor activities of glypican-3-specific chimeric antigen receptor-modified T cells by coexpression of a soluble PD1-CH3 fusion protein. Cancer Immunol Immunother 2018;67:1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai B, Shi D, Gao H, et al. A phase I study of anti-GPC3 chimeric antigen receptor modified T cells (GPC3 CAR-T) in Chinese patients with refractory or relapsed GPC3+ hepatocellular carcinoma (r/r GPC3+ HCC). Journal of Clinical Oncology 2017;35:3049–3049. [Google Scholar]
- 23.Feng M, Gao W, Wang R, et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A 2013;110:E1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phung Y, Gao W, Man YG, et al. High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening. MAbs 2012;4:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YF, Ho M. Humanization of high-affinity antibodies targeting glypican-3 in hepatocellular carcinoma. Sci Rep 2016;6:33878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Chang WC, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011;118:1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Fu H, Hewitt SM, et al. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc Natl Acad Sci U S A 2017;114:E6623–E6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldarelli F, Wu X, Su L, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014;345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma C, Cheung AF, Chodon T, et al. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov 2013;3:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donia M, Kjeldsen JW, Andersen R, et al. PD-1(+) Polyfunctional T Cells Dominate the Periphery after Tumor-Infiltrating Lymphocyte Therapy for Cancer. Clin Cancer Res 2017;23:5779–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi J, Paczkowski P, Shen YW, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood 2018;132:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao HL, Pan ZJ, Lei CJ, et al. Knockdown of GPC3 inhibits the proliferation of Huh7 hepatocellular carcinoma cells through down-regulation of YAP. J Cell Biochem 2013;114:625–31. [DOI] [PubMed] [Google Scholar]
- 33.Siripongsakun S, Wei SH, Lin S, et al. Evaluation of alpha-fetoprotein in detecting hepatocellular carcinoma recurrence after radiofrequency ablation. J Gastroenterol Hepatol 2014;29:157–64. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Padura I, Agliano A, Marighetti P, et al. Sex-related efficiency in NSG mouse engraftment. Blood 2010;116:2616–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher B, Packard BS, Read EJ, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol 1989;7:250–61. [DOI] [PubMed] [Google Scholar]
- 36.Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res 2008;68:9832–8. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov 2019;9:1124–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Stagg NJ, Johnston J, et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell 2017;31:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013;121:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou Y, Zou Q, Ge R, et al. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res 2012;22:259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019;568:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraietta JA, Nobles CL, Sammons MA, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018;558:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah NN, Qin H, Yates B, et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv 2019;3:2317–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang J, Li Y, Lyon K, et al. Cancer driver-passenger distinction via sporadic human and dog cancer comparison: a proof-of-principle study with colorectal cancer. Oncogene 2014;33:814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CW, Hojer CD, Zhou M, et al. Regulation of T Cell Receptor Signaling by DENND1B in TH2 Cells and Allergic Disease. Cell 2016;164:141–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


