Abstract
Background & Aims:
Visceral hypersensitivity is common in patients with irritable bowel syndrome (IBS). We investigated whether inflammatory molecules, such as histamine and proteases, activate prostaglandin-endoperoxide synthase 2 (PTGS2, also called COX2) to increase the synthesis of prostaglandin E2 (PGE2) by mast cells, which activates the receptor PTGER2 (also called EP2) in the dorsal root ganglia to promote visceral hypersensitivity.
Methods:
We used an ELISA to measure levels of spontaneous release of molecules from mast cells in colonic mucosa from patients with IBS with diarrhea (IBS-D; 18 women and 5 men; ages 28 to 60 y), healthy individuals (controls, n=24), mice, and rats. We measured visceromotor responses to colorectal distension in rodents following intracolonic administration of colon biopsy supernatants, histamine, PGE2, a small interfering RNA against EP2, or an agonist of F2R like trypsin receptor 1 (F2RL1, also called PAR2). We investigated the role of COX2, produced by mast cells, in mediation of visceral hypersensitivity using mice with the Y385F substitution in Ptgs2 (Ptgs2Y385F mice), mast cell-deficient (W/WV) mice, and W/WV mice given injections of mast cells derived from wild-type or Ptgs2Y385F mice.
Results:
Colon biopsies from patients with IBS-D had increased levels of PGE2, based on ELISA, and COX2 mRNA and protein, compared with control biopsies. Immunohistochemistry showed that most of the COX2 was in mast cells. Intracolonic infusions of rats with IBS-D biopsy supernatants generated a 3–4-fold increase in visceromotor responses to colorectal distension; this was associated with significant increases in PGE2, histamine, and tryptase in the colonic mucosa. These increases were prevented by a mast cell stabilizer, COX2 inhibitor, or knockdown of EP2. Intracolonic administration of supernatants from biopsies of patients with IBS-D failed to induce visceral hypersensitivity or increase the level of PGE2 in W/WV and Ptgs2Y385F mice. Reconstitution of mast cells in W/WV mice restored the VH response.
Conclusions:
Abnormal synthesis of PGE2 by colonic mast cells appears to induce visceral hypersensitivity in patients with IBS-D.
Keywords: abdominal pain, inflammation, dorsal root ganglia, tryptase
Introduction
Visceral hypersensitivity (VH) is common among patients with functional bowel disorders. Up to 94% of IBS patients show evidence of VH.1 Rectal balloon inflation at lower volumes produces painful sensations in IBS patients suggestive of visceral afferent dysfunction in IBS.1–3 The mechanisms responsible for VH remain unclear. Both central and peripheral mechanisms may contribute to VH.4
At a local level, mast cells (MC) appear to play an important role in the development of VH in IBS. Specifically located at the host–environment interface, MC are in close proximity to sensory nerves. Electromicroscopic evidence of MC activation is more commonly observed in the colonic mucosa of IBS patients.5,6 Recent studies report that the proximity of activated MC to submucosal nerve fibers correlates with the frequency and severity of abdominal pain in IBS patients.7,8 Other studies show that the colonic mucosa of IBS patients releases increased amounts of MC mediators, including histamine, proteases, and prostaglandin E2 (PGE2).5,6,8,9 These findings, together with the observation that marked excitation of visceral sensory nerves innervating the colon occurs after exposure to IBS mucosal supernatant,10 support a prominent role for MC in the pathogenesis of VH in IBS.
Although both histamine and proteases have been shown to induce VH in experimental animals10–12, we recently demonstrated that administration of EP2 antagonist prevents the development of VH in a rodent IBS model.13 To explain these disparate observations, we hypothesize that inflammatory mediators such as proteases and histamine synergistically activate COX2 to increase the synthesis of PGE2 by MC. PGE2 produced by MC in turn activates sensory neuron EP2 receptors to induce VH.
To test this hypothesis, we investigated the mechanisms by which mediators from MC may sensitize sensory neurons causing VH. We showed that intracolonic administration of IBS-D mucosal supernatants, histamine, and tryptase analogs can induce VH in a manner dependent on synthesis and release of PGE2 from MC. However, these actions fail to cause VH in MC–deficient (W/WV) and COX2 mutant (Ptgs2Y385F) mice. The role of MC–COX2–PGE2 in the mediation of VH was validated by showing that the reconstitution of MC derived from bone marrow of wild-type but not Ptgs2Y385F mice in W/WV mice restored IBS-D supernatant–induced VH.
Materials and Methods
Please refer to the Supplementary Material for comprehensive Materials and Methods.
Patients
IBS-D patients (18 females and 5 males), age 28 to 60 years, were recruited from the University of Michigan, Division of Gastroenterology and Hepatology outpatient and primary care clinics. Twenty-four age-matched healthy subjects served as controls. Consents were obtained from all subjects, and the study was approved by the University of Michigan Human Research Protection Program. The healthy controls (HC) were asymptomatic subjects undergoing colonoscopy for colorectal cancer screening. All subjects had a colonoscopy, and six mucosal biopsies were obtained from the descending colon of each subject. One biopsy was used for immunohistochemistry to exclude microscopic colitis, and five biopsies were used to prepare mucosal supernatants.
Animals
All animal procedures were performed in accordance with National Institutes of Health guidelines and with the approval of the University of Michigan Institutional Animal Care & Use Committee. Wistar male rats (200–250 g) were purchased from Charles River Laboratories. C57B16, MC-deficient WBB6F1/J-Kitw/KitW-V/J (W/WV), and Cox2 mutant B6.129S6(FVB)-Ptgs2tm1.1Fun/J (Ptgs2Y385F) male mice (6 wk old) were obtained from Jackson Laboratory. The Ptgs2Y385F mutant mice have an amino acid substitution that inactivates the cyclooxygenase activity but not the perioxidase activity of the gene product. All animals were housed 3 per cage in a controlled environment (12-h daylight cycle, lights off at 18:00), with free access to food and water.
Collection of mucosal specimens and assays of supernatants
Human mucosal biopsies and experimental animal colon specimens were collected as previously described.14
ELISA assays of PGE2 (#500141, Cayman Chemical, Ann Arbor, MI), histamine (#589651, Cayman Chemical), and tryptase (Pierce Protease Assay Kit, #23263, Thermo Fisher Scientific, Rockford, IL) were performed according to the instructions provided by the manufacturer.
MC reconstitution
Selective reconstitution of MC in MC-deficient W/WV mice was conducted according to the method described by Rijnierse et al15. Bone marrow–derived MC were obtained from wild-type (C57B16) and Ptgs2Y385F mice. Bone marrow was aseptically flushed from femurs and cultured for 4 wk. MC-deficient W/WV mice were injected via the tail vein with 5 × 106 cultured MC and the recipients were studied 4–5 weeks later (see Supplementary Material).
Colorectal distension and electromyography recording
The visceromotor responses (VMR) were recorded by quantifying reflex contractions of the abdominal musculature induced by colorectal distension (CRD). Biopsy supernatants from HC or IBS-D patients (1 mL for rats and 0.15 mL for mice) were administrated intracolonically, proximal to the rectum (75 mm in rats and 30 mm in mice). At set times after the sample administration (0, 1.5, 3, 6, 10, and 24 h; 3, 7, 14, and 21 days), a series of rectal distensions were performed to generate a pressure–response curve. The results of electromyography were digitized, integrated, and analyzed using the Spike2/CED 1401 data-acquisition interface, as described in the Supplementary Material.
Agonists and antagonists
Described in the Supplementary Material.
Isolation of mast cells from rat colon
MC were isolated from rat colon tissue by a sequential combination of washing, enzymatic digestion, and density centrifugation using Percoll, as described in the Supplementary Material.
Mediator release from purified colonic mast cells
Purified colonic MC (2–5 × 104 MC/tube) suspended in 3 mL RPMI 1640 media were stimulated with PAR2 agonist (10 μg/mL) or histamine (10 μM) for 0.5–5 h at 37°C.
Immunohistochemistry
For immunohistochemical staining, colonic biopsy sections were incubated with the following antibodies: rabbit polyclonal anti-COX2 (sc-7951, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA), goat polyclonal anti-MC tryptase (sc-17039, 1:500, Santa Cruz Biotechnology).
Western blot analysis
Western blot against Cox2 (mouse monoclonal #160112, clone CX229, Cayman Chemical) was conducted as described in the Supplementary Material.
Intrathecal ganglionic administration of EP2 receptor siRNAs
Male Wistar rat injections were performed by inserting a 25 gauge 1″ needle connected to a Hamilton syringe into the tissue between the dorsal aspects of L5 and L6. EP2 siRNA (3 μL, sc-45910, Santa Cruz Biotechnology) or scrambled control siRNA (3 μL, sc-37007, Santa Cruz Biotechnology) were mixed with iFect (10 μL, Neuromics, Edina, MN) and injected in a final volume of 10 μL.
Reverse transcriptase–PCR studies
Total RNA extraction. Gene expressions of COX1 and COX2 were measured by RT–PCR. Primer sequences are provided in the Supplementary Material.
Statistical analysis
Differences in quantified data between groups were compared using one-way ANOVA followed by a post hoc Bonferroni test or a Student t test if only two groups were applied. Results are expressed as mean ± SEM, unless otherwise stated. P value < 0.05 was considered statistically significant (see Supplementary Material).
Results
Increased expression of COX2 and PGE2 in colonic mucosa of IBS-D
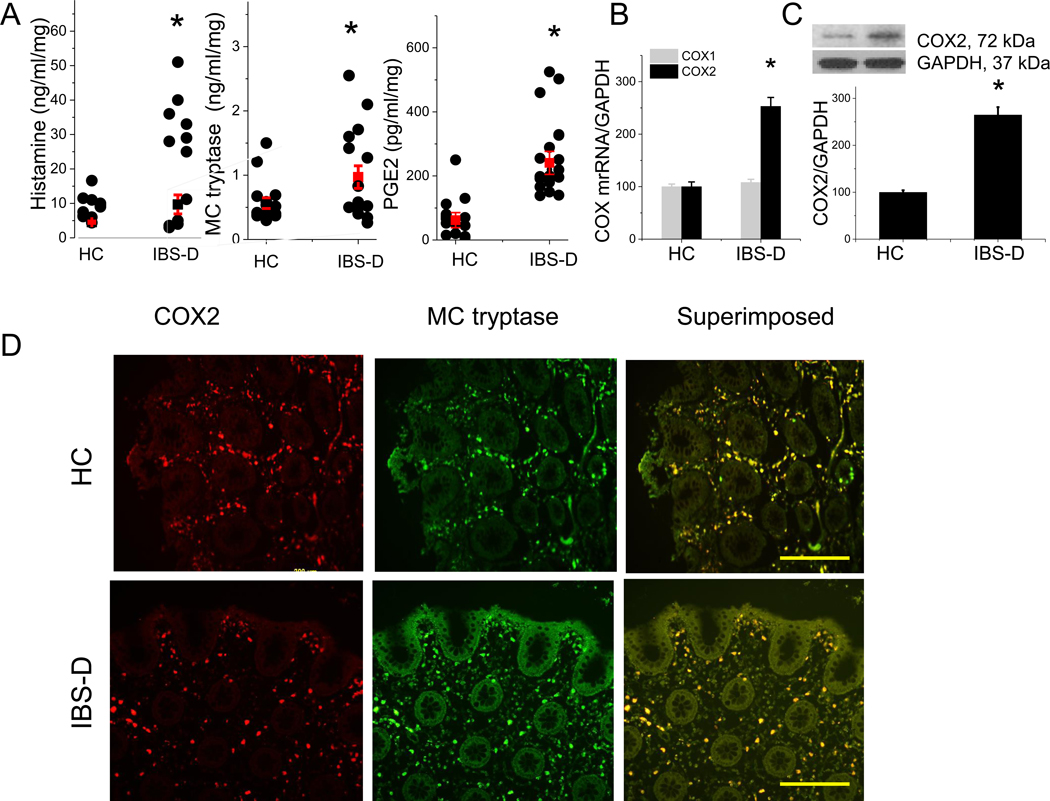
We first examined the spontaneous release of MC mediators, including PGE2, histamine, and tryptase, from the colonic mucosa of IBS-D patients compared to HC. ELISA measurement showed a significant increase in PGE2, histamine, and tryptase release into the supernatant of colonic mucosa of IBS-D patients, compared to the levels observed in HC (Figure 1A). COX1 and COX2 are the rate-limiting enzymes regulating the synthesis of prostanoids, including PGE2.16 Our RT–PCR data showed that the gene expression of COX2 but not COX1 was significantly elevated in the colonic biopsies of IBS-D patients when compared to HC (P < 0.05, n = 9, Figure 1B). This was accompanied by elevated protein expression of COX2 in the colonic mucosa of IBS-D patients (P < 0.05, Figure 1C).
Figure 1. Proinflammatory mediators released from colonic mucosa of IBS-D patients and healthy controls (HC).
(A) Release of histamine, tryptase, and PGE2 from colonic biopsies obtained from IBS-D patients and HC. Results are expressed as mean ± SEM, n = 12–23 in each group. *P < 0.05 from HC, Student t test. (B) RT–PCR of COX1 and COX2 in colonic biopsy samples from IBS-D and HC (n = 9 in each group). Results are expressed as mean ± SEM, *P < 0.05 from HC, Student t test. (C) Western blot of COX2 from IBS-D and HC colonic biopsy samples, quantified by densitometry analysis of the blots. Results are expressed as mean ± SEM, *P < 0.05 from HC, Student t test, n = 5 in each group. (D) Immunofluorescence staining for COX2 (red) and MC tryptase (green) in colonic biopsies from HC and IBS-D patients. Superimposed images demonstrate significant overlap of COX2 and MC tryptase immunoreactivity (yellow). Scale bar, 200 μm.
Immunohistochemical staining of mucosal biopsies obtained from IBS-D patients and HC showed that 87% ± 2.8 (n = 5) and 89% ± 3.1 (n = 5) COX2 immunoreactivity colocalized with MC tryptase in HC and IBS-D patient mucosa, respectively (Figure 1D).
Visceral hypersensitivity induced by intracolonic administration of IBS-D mucosal supernatant is mediated by COX2
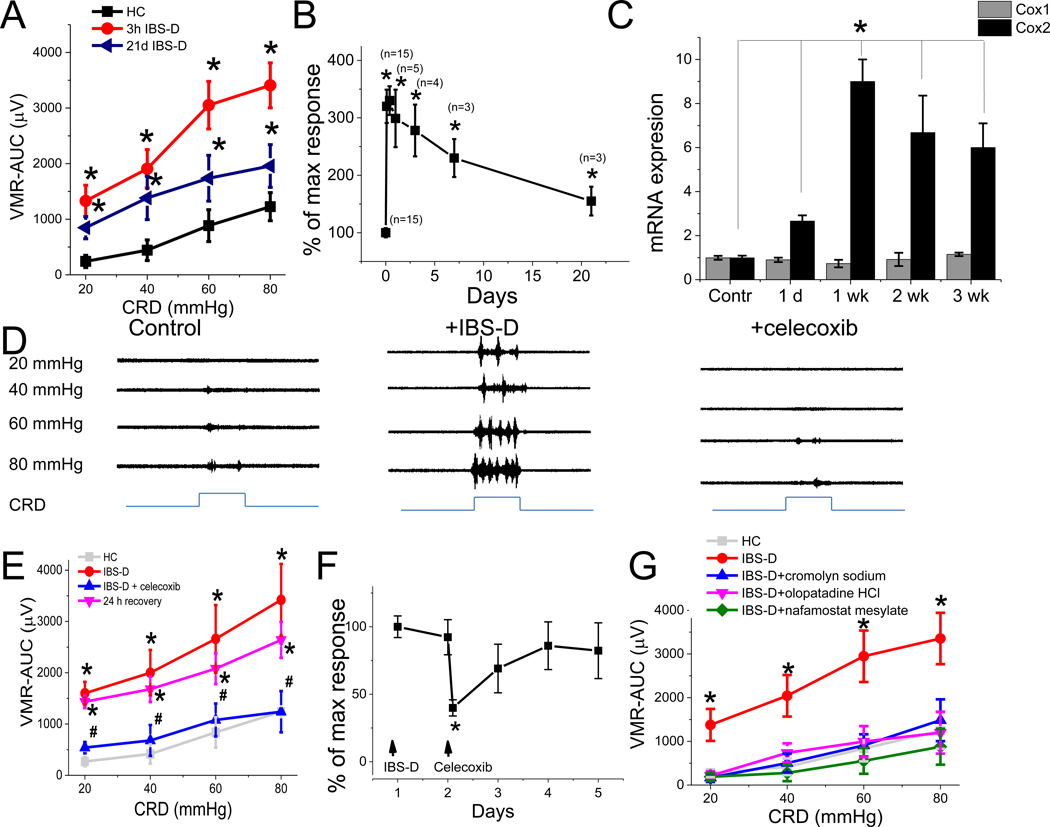
Intracolonic administration of mucosal supernatant from IBS-D patient colonic biopsies induced higher intensity of abdominal contractions (i.e., visceromotor response [VMR]) compared to VMR recorded after administration of mucosal supernatants from HC (Figure 2A). Allodynia and hypersensitivity were produced by supernatants from IBS-D patients (n = 15). Immunohistological study revealed that this increase in colonic sensitivity was not associated with an increase in the number of colonic MC (Supplementary Figure 1). Conversely, only 1 of 16 HC mucosal supernatants produced a heightened VMR to colorectal distension (CRD) and the remaining 15 HC mucosal supernatants did not generate elevated responses compared to responses observed in rats treated with physiological saline. This sample was excluded from analysis. Note that exaggerated VMR recorded in rats treated with IBS-D mucosal supernatant was observed with both innocuous (20–40 mm Hg) and noxious (60–80 mm Hg) stimuli at 3–5, and 24 h; 3, 7, and 21 days after intracolonic administration of IBS-D mucosal supernatant, and this increase correlated with increased levels of colonic COX2 but not COX1 mRNA (Figure 2B and C). Conversely, no increase in COX2 mRNA expression or PGE2 release was observed in L6-S2 DRGs (Supplementary Figure 2). No VH was observed earlier than 3 h (Supplementary Figure 3). Administration of IBS-D supernatant, cromolyn sodium, or celecoxib did not affect the colonic pressure–volume relationship during phasic CRD (20–80 mm Hg) compared to controls (HC), indicating that these substances do not affect colorectal compliance in rats (Supplementary Figure 4).
Figure 2. Visceral sensitivity induced by intracolonic administration of IBS-D colonic biopsy supernatant is mast cell and COX2 dependent.
(A) VMR–AUC to CRD in rats 3 h and 21 days after intracolonic administration of IBS-D and HC biopsy supernatants (n = 6 each group). *P < 0.05 from HC. (B) Changes in VMR (% maximal response to 80 mm Hg CRD) over time in response to single intracolonic administration of IBS-D biopsy supernatant (n = 3– 15 for each time point). *P < 0.05 from HC. (C) RT–PCR of COX1 (grey) and COX2 (black) gene expression in rat colonic mucosa measured 1 day; 1, 2, 3 weeks after single intracolonic injection of IBS-D supernatant compared to control (n = 3–9 each group). * P < 0.05 from control. (D) Representative recordings of VMR to CRD before (control, left) and 3 h after intracolonic administration of supernatant from IBS-D patients (middle), and 30 min after administration of celecoxib (right). (E) VMR to CRD in rats shows that hypersensitivity induced by intracolonic IBS-D supernatant (red, n = 8) was abolished by celecoxib (blue, n = 8). The effect of celecoxib was reversible; VH reappeared 24 h after celecoxib injection (magenta, n = 8). Results are expressed as mean ± SEM, *P < 0.05 from HC, #P < 0.05 from IBS-D. (F) Changes in VMR (% maximal response to 80 mm Hg CRD) over time in rats treated with intracolonic administration of IBS-D supernatant followed by IP celecoxib 30 min later (n = 6 for each time point). *P < 0.05 from response recorded on day 1. (G) VMR to CRD shows that VH evoked by intracolonic IBS-D supernatant was abolished by cromolyn sodium (n = 5, blue), olopatadine HCl (n = 5, magenta), or nafamostat mesylate (n = 5, green). *P < 0.05 from HC, #P < 0.05 from IBS-D. A, B, E, F, and G: Results are expressed as mean ± SEM; ANOVA followed with Bonferroni post-hoc test; C: Student t test.
To demonstrate that VH evoked by intracolonic administration of IBS-D mucosal supernatant is mediated by the COX2–PEG2 pathway, we showed that pretreatment of the naïve rat with the specific COX2 inhibitor celecoxib (n = 8) reversed VH evoked by IBS-D supernatant (Figure 2D and E). The inhibitory effect of celecoxib was transient, as VH reappeared within 24 h after drug administration (Figure 2F).
To elucidate the role of MC in the development of VH, we showed that MC stabilizer cromolyn sodium (30 mg/kg, intraperitoneal [IP], n = 6) completely abolished VH evoked by IBS-D mucosal supernatant (Figure 2G). In addition, administration of olopatadine HCl (4 mg/kg, IP, n = 6), a histamine 1 receptor antagonist, and nafamostat mesylate (1 mg/kg, IP, n = 5), a serine protease inhibitor, also prevented the development of VH evoked by IBS-D mucosal supernatant (Figure 2G). Taken together, our findings suggest that MC degranulation products such as PGE2, histamine, and tryptase are each capable of inducing VH in an IBS rat model, although the mechanisms and site(s) of action remain to be elucidated.
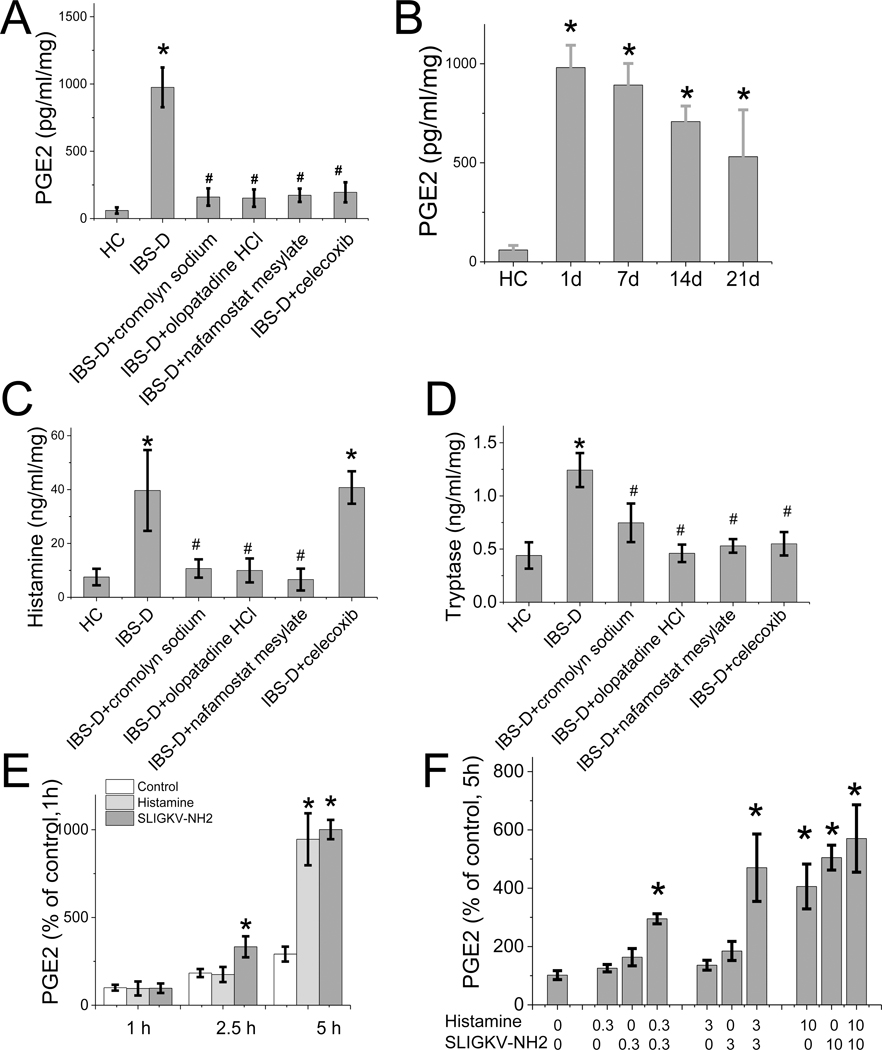
Increased levels of rat colonic tissue PGE2, histamine, and tryptase evoked by intracolonic administration of IBS-D mucosal supernatant
In response to intracolonic administration of IBS-D mucosal supernatant, the level of PGE2 in the rat colonic tissue was increased to 975 ± 147 pg/mL/mg compared to 60 ± 23 pg/mL/mg after administration of mucosal supernatant obtained from HC (n = 23, P < 0.05) (Figure 3A). Colonic PGE2 level remained elevated 1, 7, 14, and 21 days after single intracolonic administration of IBS-D mucosal supernatant (Figure 3B). In separate studies, we showed that this increase in colonic PGE2 was abolished by the MC stabilizer cromolyn sodium, the COX2 inhibitor celecoxib, and the histamine 1 receptor antagonist olopatadine HCl, as well as the protease inhibitor nafamostat mesylate (Figure 3A).
Figure 3. Proinflammatory mediators released from rat colonic tissue after intracolonic administration of IBS-D or HC mucosal supernatants.
(A) The release of PGE2 from colonic mucosa of rats treated with HC or IBS-D mucosal supernatant with and without pretreatment (30 min before colonic infusion) with cromolyn sodium (n = 5), olopatadine HCl (n = 4), nafamostat mesylate (n = 4), or celecoxib (n = 5). Results are expressed as mean ± SEM, *P < 0.05 from HC, #P < 0.05 from IBS-D, ANOVA followed with Bonferroni post-hoc test. (B) Release of PGE2 from rat colonic tissue 1, 7, 14, and 21 days after single intracolonic administration of mucosal supernatant from IBS-D patients or HC (n = 3–6 for each data point). Results are expressed as mean ± SEM, *P < 0.05 from HC, ANOVA followed with Bonferroni post-hoc test. (C) Release of histamine from rat colonic tissue treated with intracolonic infusion of IBS-D or HC colonic supernatant with and without pretreatment (30 min before colonic infusion) with cromolyn sodium (n = 4), olopatadine HCl (n = 4), nafamostat mesylate (n = 4), and celecoxib (n = 5), *P < 0.05 from HC, #P < 0.05 from IBS-D, ANOVA followed with Bonferroni post-hoc test. (D) Release of tryptase from rat colonic mucosa after similar treatment outlined in (C). Results are expressed as mean ± SEM, *P < 0.05 from HC, #P < 0.05 from IBS-D, ANOVA followed with Bonferroni post-hoc test. (E) In vitro studies using isolated rat colonic MC showed that the release of PGE2 stimulated by histamine (10 μM, n = 4) and PAR2 receptor agonist SLIGKV-NH2 (10 μg/mL, n = 6) was significantly elevated at 5 h of stimulation, compared to PGE2 levels in non-stimulated conditions (n = 5). Results are expressed as mean ± SEM, *P < 0.05, ANOVA followed with Bonferroni post-hoc test. (F) At 5 h, the release of PGE2 stimulated by low doses of histamine (0.3 and 3 μM, n = 4) and PAR2 receptor agonist SLIGKV-NH2 (0.3 and 3 μg/mL, n = 4) shows the potentiation between histamine and PAR2 receptor agonist on PGE2 release (*P < 0.05 compared to levels of PGE2 in non-stimulated conditions) (n = 4). Results are expressed as mean ± SEM, *P < 0.05, ANOVA followed with Bonferroni post-hoc test.
We also measured rat colonic tissue histamine and tryptase after intracolonic administration of IBS-D mucosal supernatant. Similar to the elevated PGE2 levels, colonic histamine and tryptase were elevated after stimulation by intracolonic instillation of IBS-D biopsy supernatant, and the increased levels were abolished by cromolyn sodium, olopatadine HCl, and nafamostat mesylate. In contrast to PGE2, the elevated level of histamine was not affected by the COX2 inhibitor celecoxib (Figure 3C and D). These findings, together with the observations obtained with the pain behavior studies, suggest that PGE2 is critical for the development of VH, whereas histamine and tryptase released from MC are critical, but not sufficient to induce VH. This conclusion is based on the observations that the COX2 inhibitor celecoxib abolished VH evoked by IBS-D mucosal supernatant but did not inhibit histamine levels stimulated by intracolonic instillation of IBS-D mucosal supernatant (Figure 3C). However, it is intriguing that inhibitors of COX2, tryptase, and histamine receptor 1 all abolished VH evoked by IBS-D biopsy supernatant, suggesting that histamine and tryptase are involved in mediating VH and their actions are dependent on COX2–PGE2 pathways.
MC mediators histamine and proteases stimulate PGE2 production in colonic mast cells
To confirm that histamine and tryptase stimulate the synthesis of PGE2 in MC, we performed in vitro studies using isolated colonic MC from rats. We showed that stimulation with histamine (10 μM, n = 4) and PAR2 receptor agonist SLIGKV-NH2 (10 μg/mL, n = 4) each produced an increase in PGE2 at 5 h (Figure 3E), and these two mediators, at low doses, potentiated the action of each other on PGE2 production (Figure 3F). However, these increases were delayed for 3–5 h after the administration of the agonists. This coincided with the delayed development of VH after intracolonic administration of IBS-D supernatant in the in vivo experiments (Supplementary Figure 2).
Visceral hypersensitivity mediated by histamine and tryptase was inhibited by MC stabilizer and COX2 inhibitor
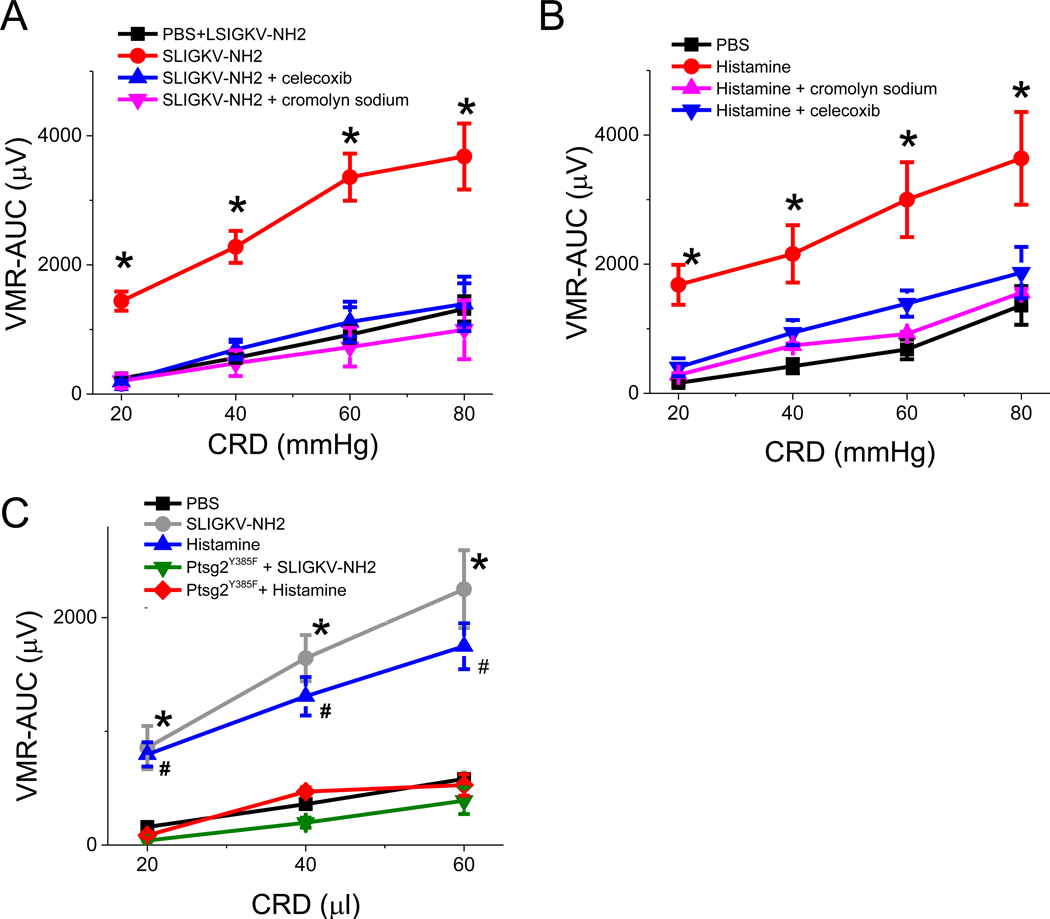
IBS colonic mucosal supernatant contains a host of mediators that may participate in the induction of VH.5,11. These include tryptase, histamine, and PGE2. To confirm the role of histamine and proteases in the induction of VH, we showed that pretreatment of IBS-D supernatant with 1 mU ABP1/AOC1/diamine oxidase (an enzyme that converts histamine to 5-imidazoleacetic acid) or 100 mM serine protease inhibitor nafamostat mesylate markedly attenuated VH induced by intracolonic administration of IBS-D mucosal supernatant (Supplementary Figure 5). These results suggest that both colonic mucosal proteases and histamine may contribute to the induction of VH. We hypothesize that tryptase and histamine act via PGE2 that is synthesized in MC to induce VH. To test this hypothesis, we performed pain behavior studies in response to colorectal balloon distension in rats. Intracolonic administration of PAR2 receptor agonist SLIGKV-NH2 (0.3 mg in 1 mL PBS, n = 6) and histamine (30 μg in 1 mL PBS, n = 6) each generated a delayed response in VMR 3–5 h after intracolonic administration of each agonist (Figure 4A and B), the magnitude of which was similar to the responses generated by intracolonic IBS-D mucosal supernatant. In each case, the VMR response was blocked by MC stabilizer cromolyn sodium or reversed by COX2 inhibitor celecoxib (Figure 4A and B), suggesting mediation by prostaglandin released from MC. In separate studies, we demonstrated that intracolonic administration of histamine or PAR2 agonist SLIGKV-NH2 failed to generate VH in Ptgs2Y385F mutant mice (Figure 4C), thus confirming the above hypothesis.
Figure 4. VMR to CRD in rats treated with intracolonic infusion of SLIGKV-NH2 orhistamine.
(A) Visceral hypersensitivity (VH)–AUC to CRD evoked by intracolonic administration of SLIGKV-NH2 (n = 6) was blocked by pretreatment with celecoxib (n = 6, blue) and cromolyn sodium (n = 6, magenta). Results are expressed as mean ± SEM, *P < 0.05 from PBS (control, black, two-way ANOVA followed with Bonferroni post-hoc test. (B) Similarly, VH to CRD evoked by intracolonic administration of histamine (n = 4, red) was blocked by pretreatment with cromolyn sodium (n = 6, magenta) and celecoxib (n = 6, blue). Results are expressed as mean ± SEM, *P < 0.05 from PBS (control, black, two-way ANOVA followed with Bonferroni post-hoc test. (C) Effects of intracolonic administration of SLIGKV-NH2 and histamine in control (C57BL/6) and Ptgs2Y385F mutant mice, n = 6 in each group. Results are expressed as mean ± SEM, *P < 0.05 from VMR observed in C57BL/6 mice in the SLIGKV-NH2–treated group. # P < 0.05 from VMR observed in C5TBL/6 mice in the histamine-treated group. Two-way ANOVA followed with Bonferroni post-hoc test.
PGE2 released from MC is critical to mediate visceral hypersensitivity
To provide further evidence that PGE2 synthesized and released from MC is critical to mediate VH in our rodent IBS models, we performed pain behavior (i.e., VMR) studies in MC–deficient (W/WV) and COX2 mutant (Ptgs2Y385F) mutant mice. As shown in Figure 5A and B, intracolonic administration of IBS-D supernatant did not generate VH in W/WV and Ptgs2Y385F mutant mice. Similarly, intracolonic infusion of IBS-D supernatant also generated little or no increase in PGE2 in the colonic mucosa of W/WV or Ptgs2Y385F mutant mice (Figure 5C).
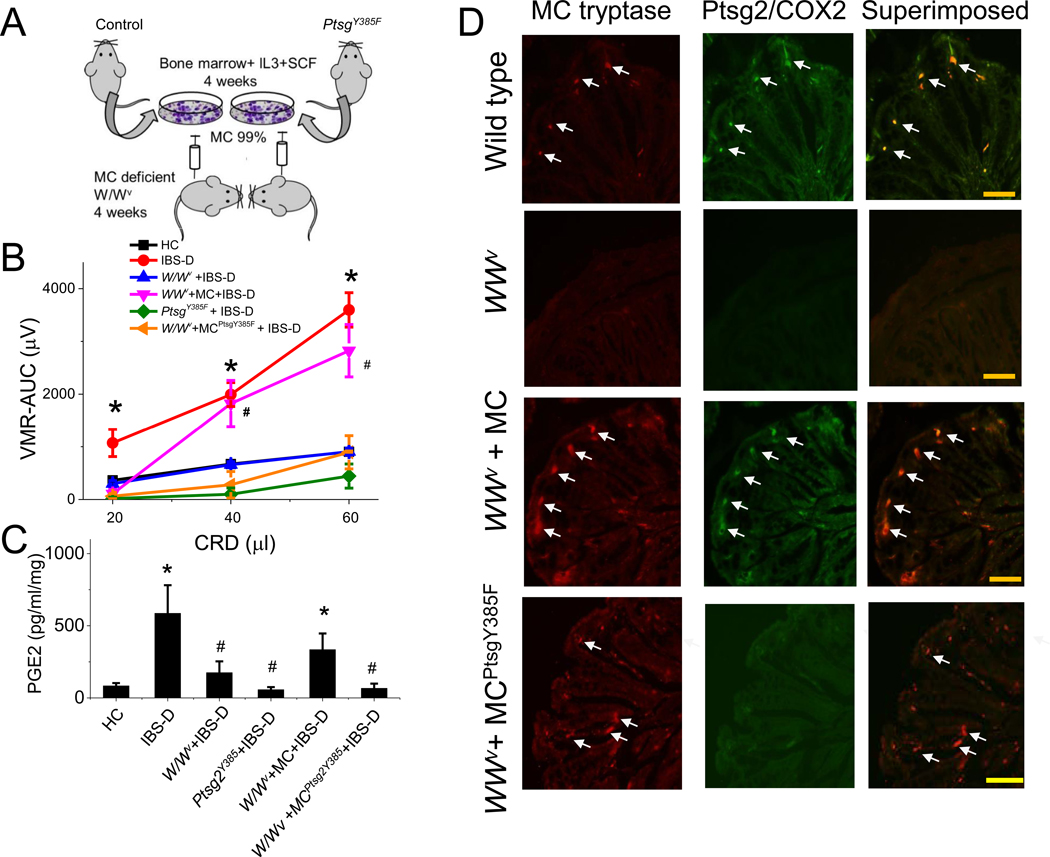
Figure 5. Mast cell reconstitution experiments.
(A) Experimental design of bone marrow–derived MC reconstitution in MC–deficient mice ( W/WV). MC were derived from bone marrow cultured with IL3/stem cell factor for 4 wk. Reconstitution occurred within 4 wk after transfer of these bone marrow–derived MC via tail vein injection. ( B) Visceral sensitivity to intracolonic administration of IBS-D mucosal supernatant was impaired in MC–deficient ( W/WV, n = 4, blue), COX2 mutant (Ptgs2Y385F, n = 4, green), and W/WV mice reconstituted with bone marrow–derived MC from Ptgs2Y385F mutant mice (n = 4, orange). VH to CRD in response to intracolonic IBS-D mucosal supernatant in wild-type (n = 4, red) and in MC–deficient ( W/WV) mice reconstituted with bone marrow–derived MC from wild-type mice ( n = 4, magenta). Results are expressed as mean ± SEM, *P < 0.05 from HC; two-way ANOVA followed with Bonferroni post-hoc test. (C) PGE2 levels in response to intracolonic administration of IBS-D mucosal supernatant in colonic samples from wild-type, W/WV, COX2 mutant (Ptgs2Y385F), and W/WV mice reconstituted with bone marrow–derived MC from wild-type or Ptgs2 Y385F mutant mice. Results are expressed as mean ± SEM, n = 6 in each group, *P < 0.05 from HC; #P < 0.05 from IBS-D, two-way ANOVA followed with Bonferroni post-hoc test. (D) immunohistochemical studies show COX2 and MC tryptase immunoreactivity in colonic mucosa of wild-type mice (control), W/WV mice, and W/WV mice reconstituted with bone marrow–derived MC from wild-type (W/WV + MC) or Ptgs2Y385F mutant (W/WV + MC Ptgs2Y385F) mice (4 wk after injection). Arrows show colocalization of MC tryptase and COX2 immunoreactivity. Scale bar, 200 μm.
Reconstitution of bone marrow–derived MC in W/WV mice, as previously described,15 restored the pain response and VH, as well as increases in mucosal PGE2 induced by intracolonic infusion of IBS-D supernatant (Figure 5A, B, and C). In contrast, reconstitution of MC from mutant Ptgs2Y385F mice failed to restore VH in response to IBS-D supernatant (Figure 5B). Similarly, IBS-D supernatant also failed to stimulate any increase in prostaglandin in the colonic tissue of mice restored with MC from mutant Ptgs2Y385F mice (Figure 5C). This underlies the importance of MC-generated PGE2 in the mediation of VH in our IBS-model.
Immunohistochemical data shown in Figure 5D confirmed that MC tryptase immunoreactivity colocalized with COX2 immunoreactivity in the control mice and in the W/WV mice reconstituted with MC from the wild-type mice. In contrast, there was little or no MC tryptase or COX2 immunoreactivity observed in the colon of W/WV mice.
EP2 receptor mediates visceral hypersensitivity induced by IBS-D biopsy supernatant
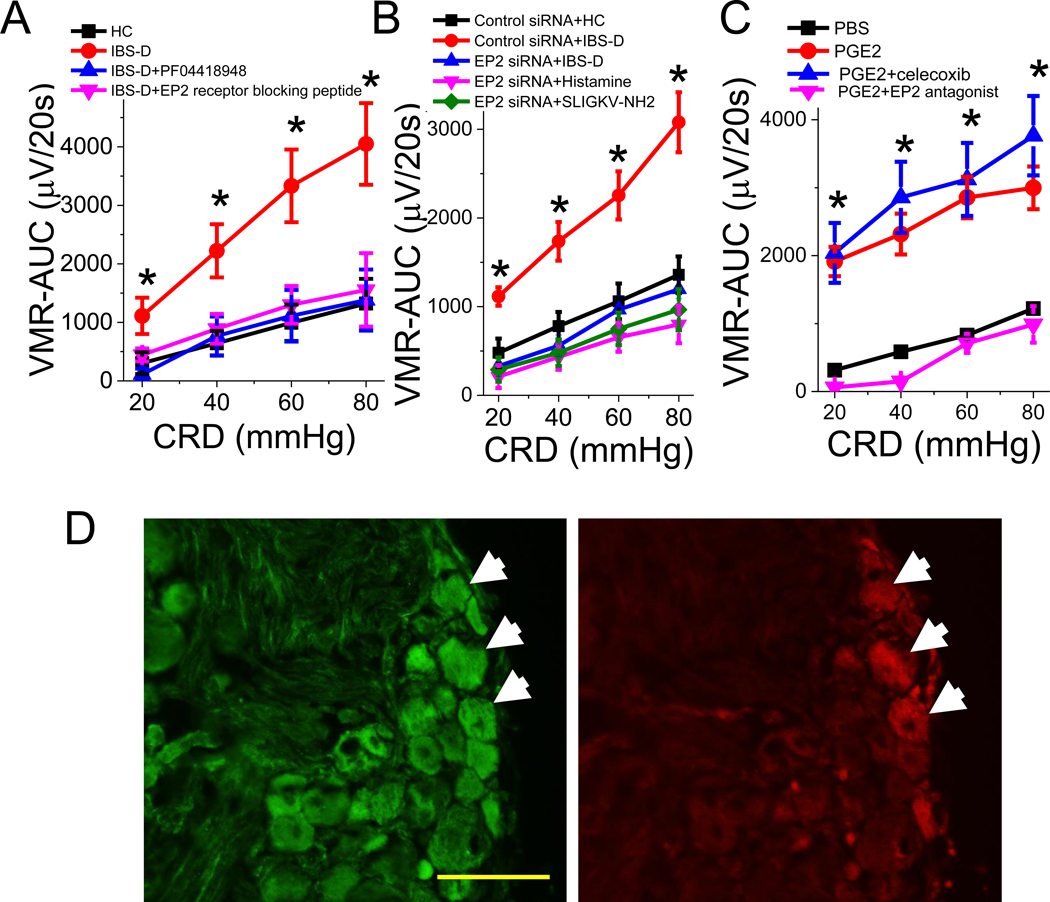
To examine the role of PGE2 receptor in the mediation of VH in our IBS model, we showed that administration of specific EP2 receptor antagonist PF0441894 (10 mg/kg, IP, n = 5) or EP2 receptor blocking peptide (10 μg/kg, IP, n = 4) each reversed the VH induced by intracolonic infusion of IBS-D supernatant (Figure 6A). Similarly, intrathecal administration of EP2 siRNA (n = 5) also blocked the actions of intracolonic administration of IBS-D supernatant, histamine, or PAR2 receptor agonist SLIGKV-NH2 to induce VH in rats (Figure 6B). RT–PCR studies demonstrated that 3 days after intrathecal administration of EP2 siRNA, there was a 75% reduction in EP2 gene expression in the L6–S2 DRG (Supplementary Figure 6). Random siRNA served as controls. In separate studies we showed that the exaggerated pain response evoked by intracolonic infusion of PGE2 (30 μg/mL, 1 mL) was not affected by COX2 inhibitor celecoxib, but was blocked by the EP2 antagonist PF0441894 (Figure 6C). Immunohistochemical staining confirmed EP2 gene expression in the colon-projecting DRG neurons (Figure 6D). However, Q-PCR data indicate that the VH induced by intracolonic administration of IBS-D supernatant was not associated with the increase of EP2 receptor in the L6–S2 DRG or spinal cord (Supplementary Figure 7).
Figure 6. Visceral hypersensitivity induced by intracolonic administration of mucosal supernatant from IBS-D patients is mediated by EP2 receptors on DRG neurons.
(A) Visceral hypersensitivity (VH)–AUC induced by intracolonic administration of IBS-D mucosal supernatant (red) in rat was abolished by EP2 receptor antagonist PF04418948 (n = 5, blue), and by EP2 receptor blocking peptide (n = 4, magenta). Results are expressed as mean ± SEM, *P < 0.05 from HC (black), two-way ANOVA followed with Bonferroni post-hoc test. (B) Intrathecal administration of EP2 siRNA (blue, n = 5) but not control siRNA (red, n = 3) abolished VH evoked by intracolonic administration of IBS-D mucosal supernatant, histamine (magenta, n = 4), and PAR2 receptor agonist SLIGKV-NH2 (green, n = 4). Results are expressed as mean ± SEM, *P < 0.05 from control siRNA, two-way ANOVA followed with Bonferroni post-hoc test. (C) VH evoked by intracolonic administration of PGE2 (n = 10, red) was abolished by EP2 receptor antagonist PF04418948 (magenta, n = 5), but not by celecoxib (blue, n = 5). (D) Immunohistochemical demonstration that CM-DiI retrogradely labeled colon-projecting DRG neurons (red) colocalized with EP2 receptor immunoreactivity (green), as indicated by arrows. Note that EP2 receptors are also expressed by non–DRG neurons. Scale bar, 100 μm.
Discussion
VH to mechanical stimulation of the colon is a common finding among IBS patients.17 However, the mechanisms responsible for the development of VH remain poorly understood. Our study shows for the first time, to our knowledge, that PGE2 produced by the colonic MC is critical to induce VH in an experimental model of IBS. This new observation is supported by the following findings: 1) colonic biopsies from IBS-D patients contain high levels of PGE2 accompanied by a 2–3-fold increase in COX2 gene expression; 2) immunohistochemical studies demonstrated that COX2 immunoreactivity in the mucosa is almost exclusively localized in the MC; 3) intracolonic administration of IBS-D mucosal supernatant in naïve rats caused marked increases in PGE2, histamine, and tryptase in the colonic tissue, which were prevented by cromolyn sodium, a MC stabilizer, and by celecoxib, a COX2 inhibitor; 4) pain behavior studies showed exaggerated increases in VMR to CRD in response to intracolonic infusion of IBS-D mucosal supernatant compared to responses to supernatant from HC. Similar enhanced pain responses were observed with intracolonic administration of histamine or tryptase. These pronociceptive effects were blocked by cromolyn sodium and celecoxib. 5) Intracolonic infusion of IBS-D supernatant, histamine, or tryptase failed to induce VH or a rise in colonic PGE2 in MC–deficient and COX2 mutant mice. The pronociceptive effects of IBS-D supernatant were restored by reconstitution of MC generated from wild-type mice but not from Ptgs2Y385F mutant mice and 6) intrathecal silencing of the expression of PGE2 receptor EP2 in L6–S2 DRG abolished VH in rats receiving intracolonic infusion of IBS-D supernatant, histamine, or tryptase. These findings support our hypothesis that MC products such as histamine and tryptase activate COX2 in the MC to increase the synthesis of PGE2, and this in turn stimulates EP2 receptors in the DRG to induce VH to mechanical distension.
Animal models and clinical studies support a mechanistic role of colonic MC in the pathogenesis of VH in IBS.5,8 However, other studies question the role of MC in this disorder.18–20 MC are strategically located beneath the mucosa so that they can rapidly react to any environmental changes in the lumen and transmit sensory information to DRG neurons and beyond. Multiple factors such as bacteria, food products, toxins, and endogenous peptides can activate MC and promote degranulation. This results in the immediate release of preformed mediators such as tryptase and histamine or the de nova synthesis of agents like prostaglandins.21,22 Our demonstration that COX2 is almost exclusively localized in the MC suggests that most of the PGE2 in the mucosa is synthesized and released from colonic MC.
Proteases, histamine, and PGE2 each have been implicated in causing VH in IBS. Cenac et al11 showed that the proteases such as tryptase and trypsin, both of which can activate PAR2, are upregulated in IBS. Rodent studies have shown that the pronociceptive effects of IBS biopsy supernatants, when introduced intracolonically, can largely be abolished by the serine protease inhibitor FUT-175 (i.e., nafamostat mesylate).11 In this study, we confirmed these observations by showing that nafamostat mesylate was able to block the enhanced pain response evoked by intracolonic infusion of mucosal supernatant from IBS-D patients. This is somewhat surprising as other MC mediators, such as histamine and prostaglandins that are present in the biopsy supernatant, also have also been shown to induce VH in IBS.23,24 To investigate this further, we showed that histamine 1 receptor blockade with olopatadine HCl25 was able to prevent VH in response to intracolonic infusion of biopsy supernatant from the same group of IBS-D patients. To confirm that both proteases and histamine are able to induce VH in our experimental model, we showed that intracolonic infusion of PAR2 receptor agonist SLIGKV-NH2 or histamine each generated an exaggerated pain response in the naïve rats. However, it should be noted that similar to the pain response we observed in rats receiving intracolonic infusion of IBS-D biopsy supernatant, the heightened VMR response was delayed 3–5 h after intracolonic administration of PAR2 receptor agonist or histamine. This delayed response was also observed by other investigators using the same experimental model11 and should be contrasted with in vitro signaling studies in DRG neurons, in which the response was observed within seconds,6,11 and raising the question whether the exaggerated responses were mediated by generation of secondary mediators in response to histamine or proteases.
In this study, we provide strong experimental evidence that the pronociceptive effects of histamine and proteases are mediated by the generation of prostaglandins in the MC, and the newly synthesized prostaglandins are released into the mucosa to activate EP2 receptors in the neighboring sensory neurons to induce VH to mechanical stimulation of the colon. Furthermore, we demonstrate a synergistic interaction between histamine and tryptase on PGE2 synthesis. These findings may help to explain how inhibitors of tryptase and histamine 1 receptor can each block the development of VH evoked by intracolonic administration of IBS-D mucosal supernatant.
Our studies showed that the pronociceptive effects of histamine and proteases were prevented by celecoxib and cromolyn sodium (Figure 4), suggesting mediation by MC–dependent prostaglandin synthesis. Further support for this possibility was provided by our studies showing that intracolonic administration of histamine or tryptase caused a delayed increase in colonic PGE2 (Figure 3E), which coincided with the development of VH. These events could be blocked by the MC stabilizer and the COX2 inhibitor (Figure 4). MC-deficient and Ptgs2Y385F mutant mice provided conclusive evidence by showing failure of intracolonic infusion of histamine or tryptase, but not PGE2, to elicit VH in these mutant mice (Figure 5).
Prostaglandins, in particular PGE2, play important roles in the initiation of inflammation and pain.26 Mucosal PGE2 levels are increased in IBS patients, as reported in our study and by others.6,9 Prostaglandins are known to sensitize sensory neurons, including those innervating the gastrointestinal tract.27 Hence, it is an attractive candidate as a common mediator for VH in IBS. However, in vitro studies have shown that EP1–EP2 antagonist has no effect on the activation of [Ca2+]in in DRG neurons exposed to IBS supernatants.6 This is not unexpected as prostaglandins are well known to sensitize gastrointestinal fibers to proinflammatory agents, but they do not activate sensory neurons alone. For example, Kim et al28 showed that PGE2 (1 μm) alone did not evoke any response; however, it enhanced serotonin-evoked currents by 167% in gut-innervating afferent sensory neurons. Similarly, Stucky et al29 reported that pretreatment of isolated DRG neurons with PGE2 increased the proportion of intermediate-size neurons that responded to bradykinin. These observations support our hypothesis that PGE2 can sensitize gastrointestinal afferent fibers to inflammatory mediators such as histamine and proteases. Our observation that the pronociceptive effects of IBS-D biopsy supernatant were blocked by EP2 antagonist or intrathecal silencing of EP2 receptor suggest that the stimulation is mediated by PGE2 acting via DRG neuron EP2 receptors. We further observed that the exaggerated pain responses evoked by intracolonic instillation of histamine and tryptase were prevented by silencing the EP2 receptors in DRG neurons. These findings support our hypothesis that increased histamine and proteolytic activity in the colonic mucosa of IBS patients stimulate PGE2 production through histamine or PAR2 receptor in MC; PGE2 in turn acts on DRG neurons to induce VH.
A limited number of clinical studies evaluate the use of MC stabilizers in IBS.30 Preliminary clinical data showed that a 6-month treatment with disodium cromoglycate significantly reduced release of tryptase from jejunal biopsies and increased clinical improvement of bowel function in IBS-D patients.31 A placebo-controlled trial involving 60 IBS-D patients showed that ketotifen, a MC stabilizer with H1 receptor antagonism properties, increased the threshold for discomfort in IBS patients with VH, reduced IBS symptoms, and improved health-related quality of life.20 However, it is not clear whether this beneficial effect is secondary to the MC stabilizing properties of ketotifen or H1 receptor antagonism. In addition, a study of 120 IBS-D patients showed that dietary exclusion, along with oral disodium cromoglycate, helped to provide long-term symptomatic relief.31 Similarly, there is little information regarding the use of COX2 inhibitors in IBS patients. In a clinical study involving 61 IBS-D patients meeting Rome IV criteria, a 30-day treatment with mesalazine, a drug with anti-inflammatory properties including cyclooxygenase and prostaglandin inhibition, effectively reduced key symptoms, such as stool frequency, abdominal pain, and bloating.32 All of these studies, however, have limitations such as small sample size, imperfect experimental design, and selection bias. Additional double-blind controlled studies involving larger groups of well-defined IBS patients are needed to confirm those observations.
Based on our findings, we would like to propose the following working model to explain the mechanism responsible for the development of VH in IBS (Figure 7). Fecal material in the colon of IBS patients contains increased amounts of tryptase, histamine, and other bioactive substances.7,9,11,30 Upon entry to the lamina propria, these proinflammatory mediators together with tryptase and histamine released endogenously from mucosal MC can upregulate COX2 gene expression in the mucosal MC, resulting in the increased synthesis and release of PGE2. PGE2, in turn, activates EP2 receptors in the submucosal sensory nerve fibers from the DRG neurons, which transmit nociceptive signals to the spinal cord. Based on our observations that inhibitors of tryptase and histamine 1 receptors can each block the development of VH evoked by intracolonic infusion of IBS-D biopsy supernatant, and that intracolonic administration of histamine and tryptase fail to elicit VH in MC–deficient and COX2 mutant mice, we propose that histamine and tryptase are critical mediators of VH. The synergistic interaction between histamine and tryptase on PGE2 synthesis may explain the observation that inhibitors of tryptase and histamine 1 receptors can each block the development of VH evoked by intracolonic administration of IBS-D mucosal supernatant; however, their pronociceptive actions are mediated by MC–dependent prostaglandin synthesis. In this manner, MC PGE2 serves as the final common mediator to induce VH in IBS.
Figure 7. The role of PGE2 produced by colonic mast cells in the pathogenesis of visceral hypersensitivity in IBS.
Proinflammatory mediators in the mucosa such as histamine, tryptase, among others, are increased in IBS. These mediators, together with histamine and tryptase from MC, activate G protein–coupled receptors (GPCRs) on MC, resulting in degranulation of numerous vesicular mediators (histamine, tryptase, PGE2, etc.) and induction of transcriptional activation of COX2, which increases the synthesis of prostaglandins, including PGE2. MC located close to sensory nerve fibers in the submucosa release PGE2, which acts on sensory fiber EP2 receptors and potentiates the action of pronociceptive mediators released by mechanical or chemical stimulation, leading to the development of VH.
Supplementary Material
What You Need to Know.
Background and Context
Patients with irritable bowel syndrome with diarrhea (IBS-D) frequently have visceral hypersensitivity. Molecules that are part of the inflammatory response, such as histamine and proteases, produced by mast cells, might promote visceral hypersensitivity.
New Findings
Colon biopsies from patients with IBS had increased levels of prostaglandin E2 and the enzyme that synthesizes it. Blocking these molecules prevented visceral hypersensitivity in mice.
Limitations
This study was performed using colon biopsies and rodents. Further studies are needed in patients.
Impact
Abnormal synthesis of PGE2 by colonic mast cells appears to induce visceral hypersensitivity in patients with IBS-D. Agents that block these molecules might be used to treat patients with IBS-D.
Lay summary
We identified molecules that are overproduced by colon tissues of patients with IBS-D. These molecules induced abdominal sensitivity in mice and agents that block these molecules reduced this hypersensitivity.
Acknowledgments
Grant Support: The studies were supported by the National Institutes of Health grants R01DK110436 and P30DK34933.
Abbreviations used in this paper:
- AUC
area under the curve
- CRD
colorectal distension
- DRG
dorsal root ganglia
- EP2
PGE2 receptor type EP2
- HC
healthy controls
- IBS
irritable bowel syndrome
- PAR2
protease-activated receptor 2
- PGE2
prostaglandin E2
- VH
visceral hypersensitivity
- VMR
visceromotor response
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel symptom. Gastroenterology. 1995;109(1):40–52. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie J Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14(2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–1192. [DOI] [PubMed] [Google Scholar]
- 4.Price DD, Zhou Q, Moshiree B, et al. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7(8):529–535. [DOI] [PubMed] [Google Scholar]
- 5.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. [DOI] [PubMed] [Google Scholar]
- 6.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. [DOI] [PubMed] [Google Scholar]
- 7.Weston AP, Biddle WL, Bhatia PS, et al. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38(9):1590–1595. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Rhee PL, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21(1 Pt 1):71–78. [DOI] [PubMed] [Google Scholar]
- 9.Cenac N, Bautzova T, Le Faouder P, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology. 2015;149(2):433–44. [DOI] [PubMed] [Google Scholar]
- 10.Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150(4):875–887. [DOI] [PubMed] [Google Scholar]
- 11.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117(3):636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho AM, Vergnolle N, Guiard B, et al. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122(4):1035–1047. [DOI] [PubMed] [Google Scholar]
- 13.Grabauskas G, Xiaoyin W, Turgeon DK, et al. Marked elevation in mucosal proinflammatory PGE2 is responsible for pain in diarrhea-predominant IBS (IBS-D) patients. Gastroenterology. 2015;148(4) Suppl 1:S-775. [Google Scholar]
- 14.Zhou SY, Gillilland M 3rd, Wu X, et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest. 2018;128(1):267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rijnierse A, Koster AS, Nijkamp FP, et al. Critical role for mast cells in the pathogenesis of 2,4-dinitrobenzene-induced murine colonic hypersensitivity reaction. J Immunol. 2006;176(7):4375–4384. [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119(3):229–240. [DOI] [PubMed] [Google Scholar]
- 17.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there any relationship? Am J Gastroenterol. 2012;107(5):715–726. [DOI] [PubMed] [Google Scholar]
- 19.Boyer J, Saint-Paul MC, Dadone B, et al. Inflammatory cell distribution in colon mucosa as a new tool for diagnosis of irritable bowel syndrome: A promising pilot study. Neurogastroenterol Motil. 2018;30(1):e13223. [DOI] [PubMed] [Google Scholar]
- 20.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59(9):1213–1221. [DOI] [PubMed] [Google Scholar]
- 21.Galli SJ, Maurer M, Lantz CS, et al. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11(1):53–59. [DOI] [PubMed] [Google Scholar]
- 22.Austen KF. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989;10(11):381–386. [DOI] [PubMed] [Google Scholar]
- 23.Bueno L, Fioramonti J. Visceral perception: inflammatory and non-inflammatory mediators. Gut. 2002;51 Suppl 1:i19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng B, La JH, Schwartz ES, et al. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302(10):G1085–G1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmori K, Hayashi K, Kaise T, et al. Pharmacological, pharmacokinetic and clinical properties of olopatadine hydrochloride, a new antiallergic drug. Jpn J Pharmacol. 2002;88(4):379–397. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Shaffer A, Portanova J, et al. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J Pharm Exp Ther. 1997;283(3):1069–1075. [PubMed] [Google Scholar]
- 27.Gold MS, Zhang L, Wrigley DL, et al. Prostaglandin E(2) modulates TTX-R I(Na) in rat colonic sensory neurons. J Neurophysiol. 2002;88(3):1512–1522. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Jin Z, Lee G, et al. Prostaglandin potentiates 5-HT responses in stomach and ileum innervating visceral afferent sensory neurons. Biochem Biophys Res Commun. 2015;456(1):167–172. [DOI] [PubMed] [Google Scholar]
- 29.Stucky CL, Thayer SA, Seybold VS. Prostaglandin E2 increases the proportion of neonatal rat dorsal root ganglion neurons that respond to bradykinin. Neuroscience. 1996;74(4):1111–1123. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Song J, Hou X. Mast cells and irritable bowel syndrome: From the bench to the bedside. J Neurogastroenterol Motil. 2016;22(2):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2013;14:1151–1160. [DOI] [PubMed] [Google Scholar]
- 32.Bafutto M, Almeida JR, Leite NV, et al. Treatment of postinfectious irritable bowel syndrome and noninfective irritable bowel syndrome with mesalazine. Arq Gastroenterol. 2011;48(1):36–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.