Abstract
Although the estimated time for development of pancreatic ductal adenocarcinoma (PDA) is more than 20 years, PDAs are usually detected at late, metastatic stages. PDAs develop from duct-like cells through a multistep carcinogenesis process, from low-grade dysplastic lesions to carcinoma in situ and eventually to metastatic disease. This process involves gradual acquisition of mutations in oncogenes and tumor suppressor genes, as well as changes in the pancreatic environment from a pro-inflammatory microenvironment that favors the development of early lesions, to a desmoplastic tumor microenvironment that is highly fibrotic and immune suppressive. This review discusses our current understanding of how PDA originates.
Keywords: pancreatic ductal adenocarcinoma, pancreatic intraepithelial neoplasia, desmoplastic reaction, microenvironment, carcinogenesis
While mutations in ATM, BRCA1, BRCA2, CDKN2A, and others (see ref 1) increase the risk of pancreatic cancer, mutation of the proto-oncogene KRAS is believed to be the most common initiating event2. Tumorigenesis is accelerated by an increase in the mutant KRAS allele dossage3. Besides these genetic factors and age, other major factors are chronic pancreatitis, smoking, obesity, and type 2 diabetes (see refs 4, 5).
Genetically engineered mouse models have confirmed that PDA development can be initiated by the acquisition of an oncogenic Kras mutations and upregulation of epidermal growth factor receptor (EGFR)/wild-type Kras signaling that leads to development of neoplasia6–11. Equally important to the PDA development is chronic inflammation and inflammatory macrophages12–16. Precancerous neoplasms progress to cancer by acquisition of additional mutations, often leading to loss of tumor suppressor function. At this stage, alternatively-activated macrophages (AAM) promote a spiral of signaling that elicits the desmoplastic reaction17, 18, recruits Tregs, and creates a highly immunosuppressive TME19. The high abundance of cancer-associated fibroblasts (CAFs) then leads to the generation of a collagen- and hyaluronan (HA)-rich extracellular matrix (ECM) which promotes blood vessel collapse and increases hypoxia20. Eventually, up to 80% of pancreatic tumor mass can consist of stromal matrix which surrounds islands of cancer cells, acting as a physical barrier for infiltrating lymphocytes and chemotherapy21. Increased matricellular-enriched fibrosis and tissue tension was associated with tumor progression and shorter patient survival22.
Although the estimated timeline of genetic changes that lead to transition from benign pancreatic neoplasia to malignant PDA is more than 20 years23, patients usually receive a diagnosis of PDA after it already has metastasized. Increasing our understanding of how PDA develops and identifying markers of earliest cancerous lesions may allow the detection of this cancer at stages where it can be treated, or its metastasis can be prevented. Also, better understanding how neoplastic and cancer cells interact with the desmoplastic environment may lead to the development of new, more effective combination therapies.
The PDA Cell of Origin
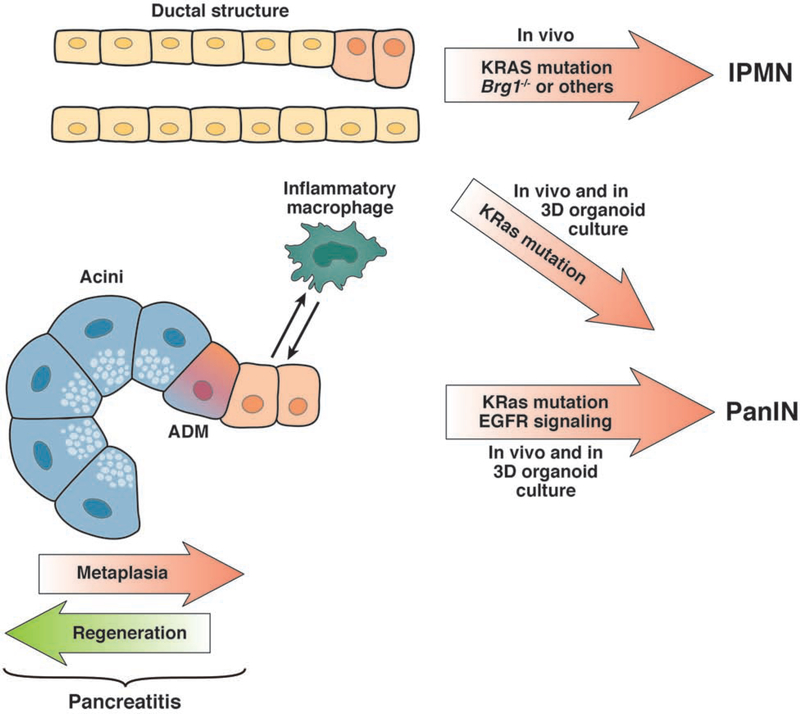
Of the three major epithelial cell types in the pancreas (islet, acinar and duct), the cell(s) capable of giving rise to PDA is controversial. The ductal phenotype of PDA immediately suggests a duct “cell of origin”, but studies in mice have indicated that this might not be the case. The earliest models of pancreatic neoplasia that resembled the human disease were generated by directing Cre recombinase-dependent expression of an oncogenic form of Kras to pancreatic progenitor cells6; in these models, mutant Kras expression is maintained throughout organogenesis and in the adult parenchymal cells thereafter. The pancreata in these mice (designated “KC”) appear to develop normally, with metaplastic and pancreatic intraepithelial neoplasia (PanIN) appearing stochastically when mice are approximately 2 months old. Breeding the KC model with mice with other genetic modifications produces mice with neoplasia that resemble intraductal papillary-mucinous neoplasms (IPMNs)24 or mucinous cystic neoplasms 25. However, this ubiquitous and continuous expression of oncogenic KRAS throughout the pancreata has not helped to identify the PDA cell of origin, or how of the transformation of different cell types might affect the phenotypes of resulting neoplasms (Fig. 1). Use of inducible forms of Cre recombinase to initiate oncogenic Kras expression in specific cell compartments in the adult animal has allowed researchers to explore the consequences of oncogenesis arising from different cell types.
Figure 1. Cells of origin for PDA.
There is evidence that PDAs develop from acinar and/or duct cells. In 3-dimensional organoid culture, duct cells that express oncogenic KRAS can develop into PanIN-like cells; when these are transplanted into mice they form low-grade dysplasias. After additional disruption of Brg1, duct cells with a KRAS mutation can progress to IPMN. Acinar cells are highly plastic and, in crosstalk with inflammatory macrophages, can transdifferentiate to a ductal phenotype (acinar to ductal metaplasia). With an oncogenic KRAS mutation these ADM cells stay locked in a ductal stage, show increased EGFR signaling, and progress to PanIN and PDA.
Ray et al26 used an inducible Cre system (CK19CreERT) to express oncogenic KRAS specifically in pancreatic ducts, resulting in the formation of mucinous, preneoplastic metaplasia with characteristics of low-grade PanIN. When ducts were isolated and placed in 3-dimensional culture, they gave rise to PanIN-like cells27. However, acinar cells in the adult pancreas are highly plastic and can undergo acinar to ductal metaplasia (ADM) under stress conditions to form a duct-like cell type capable of giving rise to PanIN (reviewed in11). In a study intent on resolving the likely PDA cell of origin, Kopp et al28 expressed oncogenic Kras specifically in acinar and duct cells (using Ptf1aCreERT and Sox9CreER mice, respectively) and found that acinar cells were approximately 100-fold more susceptible to transformation than were duct cells, leading to the conclusion that acinar cells are the more likely cell to give rise to PDA in mice. However, it should be noted that in the acinar-specific CreERT2 mice, the CreERT2 transgene is knocked into a Ptf1a allele29, inactivating the locus and making acinar cells inherently more susceptible to transformation30. Although it is unlikely that disruption of a single allele of Ptf1a could account for the entirety of the increased transformation efficiency of acinar cells compared to duct cells, it is likely to have some effects on the outcome.
Consistent with the conclusion that oncogenic Kras is not sufficient to efficiently transform duct cells, studies have found that their transformation potential increases with additional genetic modifications, including those that compromise cell identity (disruption of Brg1 31), increased phosphoinositide 3-kinase signaling within the KRAS pathway (disruption of Pten 32), or with disruption of Tp53 33. Of note, compromising cell identity30, 34, 35 and increasing KRAS activity8 both promote transformation of acinar cells as well. Interestingly, in most models with duct cell-specific expression of oncogenic Kras, the resulting neoplasia either resembled IPMNs or otherwise bypassed low-grade PanINs31–33, 36. This suggests that in some genetic contexts, the pattern of neoplastic progression differs based on what cell type in which oncogenic Kras is expressed.
While acinar cells seem to be the PDA cell of origin in mice, there is some evidence that not all acinar cells are equally capable of undergoing transformation and development into PDA. Westphalen et al37 found DCLK1 to be a marker of a minor duct and acinar cells that act as facultative progenitor cells that act to repair pancreatitis-associated injury. DCLK-positive cells might be a subset of acinar cells that can become tumor-initiating cells, but further studies are needed.
Duct cells also may also be heterogeneous in their capacity to be transformed. Thayer et al identified a subset of duct cells that could be progenitors of IPMNs. Pancreatic duct glands were originally defined as small cystic ducts that branch from the pancreatobiliary gland and the main pancreatic duct38. These structures are analogous to peribiliary glands that act as a reservoir for facultative progenitor cells, which expand in response to injury39–41.
Neoplasia
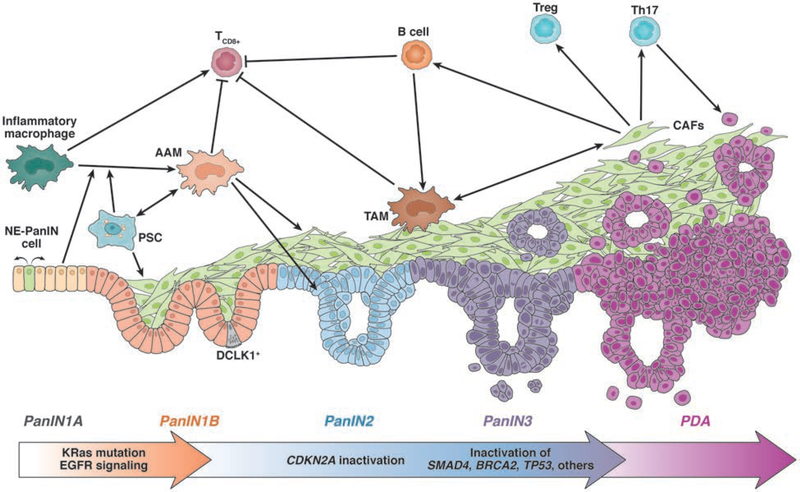
Neoplastic precursors to PDA were defined by histopathological characteristics almost 2 decades ago (Fig. 2). Designating lesions as PanINs, grades 1–3, has been useful in determining the accuracy with which mice genetically engineered to develop pancreatic cancer model the human disease 42. In humans, PanIN1 and PanIN2 are not predictive for development of PDA 43. In contrast, high-grade dysplasia (formerly PanIN3) is rarely found without associated PDA, so this equivalent of carcinoma in situ should be considered a bona fide harbinger of cancer. Detection of PanIN3-associated pathology is paramount to our efforts in early detection and imaging if we hope to make PDA preventable.
Figure 2. Progression of PDA and the associated microenvironment.
PDA develops through different stages of low-grade dysplasia (PanIN1A/B, PanIN2). While PanIN1 lesions develop after acquisition of an oncogenic Kras mutation and upregulated EGFR signaling, further progression to PanIN2 requires additional gene mutations in tumor suppressors such as CDKN2A. NE-PanIN cells respond to neuropeptides and promote expansion of lesions. Increased fibrogenesis accompanies low grade dysplasia formation. This is mediated by AAM, which are attracted by PanIN cells and PSCs. AAM generate a tumor-promoting and immunosuppressive microenvironment by inducing fibrogenesis, inhibiting CD8+ T-cells, and by releasing factors that promote expansion of lesions. A strong desmoplastic reaction can be observed at PanIN3 lesions (carcinoma in situ) and PDA, with different types of cancer-associated fibroblasts (CAFs) that in part originate from activated PSC. CAFs stimulate TAMs and contribute to the presence of CD4+ T cells (such as Treg cells and Th17 cells) and B cells. At this stage, inactivating mutations in tumor suppressor genes (e.g. SMAD4, BRCA2, TP53) are observed.
There is a considerable cellular heterogeneity within pancreatic neoplasms. For example, although tuft cells are rarely found in normal pancreas, metaplastic epithelia and low-grade dysplasias contain a large number of this chemosensory cell type, which mediates inflammatory responses in other glandular tissues44–47,48, 49. Tuft cells express high levels of DCLK150 and often are conflated with the DCLK1-positive putative progenitor cells. However, unlike the DCLK-positive progenitor cells, tuft cells have a distinct actin cytoskeletal structure, terminating in apical microvilli-like tufts and express gustatory sensory pathway components51. In normal glandular tissues, tuft cells act to sense irritants52 and infection44–47 and respond by emitting signals that promote an appropriate inflammatory response, either directly by signaling to inflammatory cells44–46 or indirectly, by stimulating associated neurons47, 52. Studies are underway to determine whether tuft cells in neoplasms serve a similar role in mediating the immune response to transformed cells.
Other cells found in PanIN lesions take on a neuroendocrine (NE) phenotype, though they have transdifferentiated from the transformed exocrine epithelia53, 54. These NE PanIN cells (Fig. 2) respond to neuron signals to promote lesion growth54, but also frequently delaminate and enter the surrounding stroma53. Transdifferentiation to the NE phenotype is promoted by high activity of the oncogene c-Myc. Intriguingly, early dissemination of neoplastic cells in the absence of frank carcinoma is consistent with findings from Rhim et al55 that neoplastic cells not only invade the surrounding stroma before progression to carcinoma, but can be found in the circulation and at secondary sites. The possibility that PDA metastases not only are seeded prior to the detection of cancer, but before progression to cancer, further emphasizes the importance of understanding the cellular plasticity evident at the earliest neoplastic stages of the disease.
Neoplastic and PDA Microenvironments
The microenvironment associated with PDA has complex functions, as it contributes to cancer development, progression to invasion and metastasis, cancer stem cell formation, and generates an ECM-composed barrier to perfusion and treatment56. However, it also seems to restrain tumor cells, since tumors with reduced stroma are undifferentiated and more aggressive57.
The desmoplastic stroma is organized by interactions among cell types including pancreatic stellate cells (PSCs), activated cancer-associated fibroblasts (CAFs), macrophages, other immune cells, precancerous or cancer cells, and the microvasculature (Fig. 2). Stromal cell composition varies among neoplastic and cancerous lesions58, but also among PDA subtypes59, 60.
Macrophage Populations in Tumor Development and Progression
Seminal work from the Barbacid laboratory has shown that besides an oncogenic Kras mutation, pancreatitis (pancreatic inflammation), as an inhibitor of oncogene-induced senescence, is a crucial factor for PDA development12, 13. Adult acinar cells are not very susceptible to transformation by oncogenic Kras alone and ADM initiation requires additional inflammatory signaling events. For example, partial deletion of Nr5a2 (Nr5a2+/− mice), a gene which encodes an orphan nuclear receptor that among other factors maintains acinar cell identity, mediates a transcriptional switch controlling expression of pro-inflammatory genes61, linking the loss of cell identity with an inflammatory response. Oncogenic Kras also drives further pro-inflammatory signaling in precancerous neoplasia through activation of STAT362–65, NF-κB9, 66–68 and GSK3/NFAT signaling69, 70. Moreover, acinar cells that express activated KRAS (with the G12D mutation) induce local inflammation by upregulating chemoattractants for inflammatory macrophages67. Inflammatory macrophages contribute to acinar cell dedifferentiation and ADM and preneoplastic lesion formation by secretion of inflammatory mediators such TNF and CCL5 16, 67. In addition, inflammatory macrophages also upregulate tissue inhibitor of metalloproteinases or matrix metalloproteinases (MMPs), which contribute to re-organization of the acinar microenvironment by promoting ADM16, 71. However, Kras-driven local inflammation is an inefficient driver of oncogenic progression to PDA and requires additional inflammatory insults and genetic alterations12, 13, 72.
Pre-cancerous PanIN1 lesions actively shape the microenvironment by secreting factors such as IL-13 to initiate a macrophage phenotype switch towards alternatively-activated M2 macrophage populations18, which (in mice) are best characterized by YM1, Arg1, FIZZ1 and IL-1ra expression17. IL-13 depletion in KC mice shows that this macrophage population (often labeled as YM1+ macrophages) has a role in suppressing inflammation and promoting lesion growth through secretion of CCL2 and IL-1ra, but also is a crucial driver of fibrogenesis17, 18.
In pancreatic tumors, tumor-associated macrophages (TAMs) comprise inflammatory and alternatively-activated macrophage populations. TAMs regulate immunosuppression through secretion of immunosuppressive cytokines and chemokines, and by promoting Treg recruitment and providing co-stimulatory signals to block T-cell proliferation73–75. In addition, TAMs regulate fibrinogenesis, vascularization and angiogenesis76, 77, and promote epithelial-to-mesenchymal transition (EMT), invasiveness and metastasis 78–80. Moreover, these immunosuppressive populations are expanded in the microenvironment, after epithelial necroptosis in response to chemotherapy 81, or after radiation-induced damage of cancer cells82.
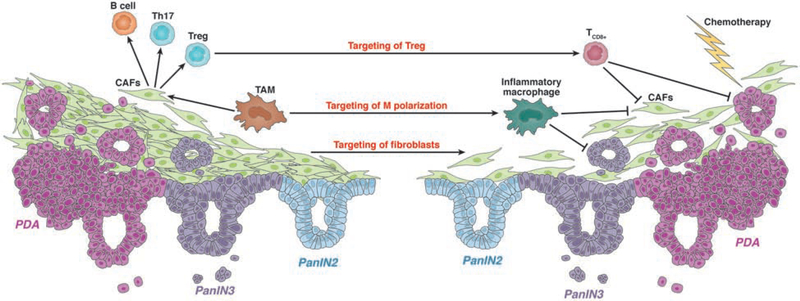
Due to the contributions of macrophages to tumor initiation and progression, strategies to target these cells might reduce fibrosis and increase the anti-tumor T-cell response, which might be effective in combination with chemotherapy 83 (Fig. 3).
Figure 3. Strategies for Therapeutic Targeting of PDA.
Chemotherapy alone is not very effective against PDAs, so strategies have been developed to target cells that support tumor growth and progression. These include targeting CD4+ T cells and strategies to shift macrophage populations to an inflammatory phenotype, to generate an inflammatory microenvironment. Strategies to decrease fibrosis include depletion of AAMs or TAMs, or direct targeting of fibroblasts.
CAFs
The ECM in the stroma of PDAs is mainly produced by CAFs and comprises many components, including collagen I, collagen IV, fibronectin, laminin, hyaluronan, glycosaminoglycan, growth factors, cytokines and chemokines, proteinases and other factors20, 84–86. CAFs can be of different origin and result from pancreatic stellate cells (PSCs), tissue resident fibroblasts, bone marrow-derived mesenchymal cells, adipose tissue and epithelial cells that underwent EMT (discussed in more detail in87).
Major contributors to the desmoplastic reaction in PDA are PSCs88. PSCs in the normal pancreas present as periacinar cells, where they are quiescent89, 90. PSCs are activated during acute or chronic inflammation91–93, changing their morphology into myofibroblast-like cells which express αSMA and are proliferative94. The resulting activated fibroblasts mediate enhanced production of ECM molecules, orchestrate MMP and TIMP-mediated matrix reorganization, and crosstalk with tumor cells via growth factors and cytokines such as EGF, basic fibroblast growth factor (bFGF), and Wnt family members95–97. The high abundance of CAFs and generation of a collagen- and HA-rich ECM promotes constriction of vasculature, creating a hypoxic microenvironment20. This requires tumor cells to adapt their metabolism away from oxidative phosphorylation98, often relying on metabolites produced by other cells within the microenvironment, including CAFs, for biosynthesis and energy99–101.
There are several subtypes of CAFs in the PDA microenvironment, with different functions 102. Myofibroblastic CAFs (myCAFs) express high levels of αSMA and are in close proximity to tumor cells, whereas inflammatory CAFs (iCAFs) express low levels of αSMA and high levels of chemokines and cytokines and are located farther away from cancer cells87, 103. iCAFs contribute to an immune-suppressive environment by excluding CD8+ T cells103–105. A putative immune-modulatory population of antigen presenting CAFs (apCAFs) expresses MHC class II and CD74102. In organoid cultures and in KPC mice, CAF heterogeneity can be induced by factors produced by tumor cells, such as TGFB and IL1. TGFB promotes their differentiation to myCAFs, whereas IL1 promotes generation of iCAFs106. CAF populations are therefore dynamic and convert among phenotypes as lesion- or tumor-associated macrophages102, 106.
In addition to their role in tumor development and progression, CAFs promote resistance to chemo- or radiation therapy through release of exosomes107 and through Hedgehog signaling108, 109. However, CAF and fibrosis depletion for clinical purposes (Fig. 3) is controversial, because it may lead to more aggressive tumors110. This is because iCAFs, which are low in αSMA and IL6, promote tumor growth, whereas myCAFs, which express high levels of αSMA, can function in restraining tumor growth27, 103, 111.
B Cells and T Cells in the TME
In KC mice, early-stage pancreatic lesions have a low abundance of T cells 18, 67, 80. In patients, infiltration of PDAs by CD4+ T cells and decreased infiltration of CD8+ T cells have been associated with shorter survival times 19, 112. CD4+ T-helper 17 (Th17) cells are pro-inflammatory cells that are recruited by CAFs. They produce IL17 and IL23, which contribute to invasion and metastasis as well as microvessel density113. Expansion of Th17 cells is promoted by mast cells114, which are recruited by PDA cells and also contribute to neovascularization and metastasis by releasing VEGF and FGF2115. CD4+, FOXP3+ and CD25+ Treg cells or T- suppressor cells are found in high numbers in the TME and produce TGFB and IL10. Presence of Treg cells correlates with poor survival of patients. Treg cells, along with Th2 cells, which exhibit tumor-promoting functions by secreting IL-13116, can generate an immunosuppressive environment.
CD8+ T cells in the PDA microenvironment are associated with increased survival117, although PDA cells embedded in fibrosis can escape the cytolytic effects of these T cells. CD8+ T cell responses are repressed by increased presence of immunosuppressive macrophages82, which can produce CCL2, the ligand for CCR218. In mice, inhibition of CSF1R or CCR2, reduced numbers of Treg cells and increased numbers of CD8+ T cells, which increased the effects of chemotherapeutic agents and radiation therapy 118–121 (see Fig. 3).
Studies of mice and human tumor PDAs have found evidence for infiltration by B cells at later stages of tumor development and in metastases122, 123. B cell recruitment to the stroma can be mediated through CXCL13, which is expressed by CAFs. Stromal B cells produce IL35 to promote tumor cell proliferation 123. Moreover, B cells in the TME contribute to immune suppression124; and depletion of B cells reduces PDA progression and metastasis in mice122.
Molecular Subtypes and Personalized Treatment
Tumors were historically grouped based on their morphologic characteristics and associated with patterns of progression and patient outcome, based largely on the relative levels of differentiation of the cancer cells. Genome and transcriptome analyses have recently complemented pathology analyses, assigning tumors to molecular subtypes, with the aim to better predict outcomes and select treatment. In PDA, there are four major driver mutations, KRAS, TP53, CDKN2A and SMAD4, all currently undruggable125. Interestingly, metastases largely reflect a subclone of the primary tumor126, suggesting that none of the major driver mutations are determinant of the switch to metastatic behavior. Instead, this transition appears to be strongly promoted by metabolic rewiring and epigenetic changes127, suggesting novel vulnerabilities for metastatic PDA.
Although genomic information might be used to select treatment with in a small population of PDA patients, it has not been robust for molecular subtyping PDAs as a whole. However, transcriptomes have been used to assign PDAs into meaningful categories. Collisson et al128 performed microarray analyses of gene expression profiles of laser-capture microdissected pancreatic cancers, to exclude confounding signals from the fibroinflammatory stroma. Moffitt et al subtyped PDA samples using array hybridization and RNA-seq analyses, and separated tumor cell signatures from parenchymal and stromal cell signatures60. Bailey et al performed RNA-seq analysis of 96 PDAs with high epithelial cellularity59. Thus far, the only consensus subtypes that have been identified by these approaches are the well-differentiated pancreatic progenitor/classical subtype, which retains pancreatic and epithelial identity, and a quasi-mesenchymal, basal-like, squamous subtype, which is undifferentiated, shows downregulation of pancreatic and epithelial identity factors, and is associated with poorest prognosis. In mice treatment with blocking antibodies against CSF1R129 or CXCR2130 can shift the basal-like subtype to a more differentiated phenotype, suggesting the intriguing possibility of inducible plasticity within subtypes.
Tiriac et al performed transcriptome analysis of PDA patient-derived organoids and identified gene-expression signatures associated with responses to specific therapies131. Taking advantage of the in vitro nature of the system, the authors then subtyped the organoids based on their responses to chemotherapeutic agents, which they called pharmacotyping. The authors were then able to identify transcriptomes that associated with specific pharmacotypes. The authors found that these transcriptomes correlated with patient responses to therapy in a retrospective data set. Interestingly, the pharmacotypes did not correlate to their basal and classical signatures. Whether these pharmacotypes can be used to predict patient response to therapy in a prospective study is yet to be determined.
Future Directions
Pancreatic cancer is the deadliest of the common cancers, and survival times have increased only marginally in the past several decades. Our attempts to effectively treat patients with pancreatic cancer have been thwarted by our inability to detect PDA at an early stage, as well as the lack of efficient combination therapy.
Although mutation of KRAS is one of the earliest events in pancreatic tumorigenesis, oncogenic KRAS both activates cell intrinsic carcinogenic pathways while also promoting interactions among epithelial cells, immune cells and fibroblasts that collectively create an immune suppressive fibroinflammatory stroma. Once mutant KRAS expression is blocked rapid stromal remodeling occurs132. Mutant KRAS also induces inflammation, which conversely promotes the plasticity of neoplastic cells at all stages of tumor progression18, 129, 130. Understanding this coevolution of tumor development and the stromal response may be the key to early detection and treatment. For example, early detection has focused largely on detecting unique byproducts of the tumor cells. However, markers of the fibrotic and inflammatory responses might also be used for early detection. Moreover, desmoplasia acts as a physical barrier to drug perfusion108 and the interstitial fluid pressure created by hyaluronan deposition collapses the vasculature necessary for drug delivery20, 133. This all suggests that successful treatment strategies will need to target these stromal compartments.
Taken together, work from recent years emphasizes the importance of dissecting the complex, vicious circle of progression of pancreatic tumors as a whole, not just the cancer cell component. Increasing our understanding of the complex interactions between cells of the stroma and low-grade and high-grade dysplasia cells might lead to new approaches to both detection and therapy.
Acknowledgements
This work was supported by NIH grants CA200572 and CA229560 to PS and U01CA224145 to HCC; support from the Chartrand Foundation and the Funk-Zitiello Foundation to PS; and support from the Sky Foundation to HCC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations
- AAM
alternatively-activated macrophage
- ADM
acinar to ductal metaplasia
- CAFs
cancer-associated fibroblasts
- CCL2
chemokine (C-C motif) ligand 2
- CCR2
C-C chemokine receptor type 2
- CSF1R
colony stimulating factor 1 receptor
- CXCR2
CX-C chemokine receptor type 2
- DCLK1
doublecortin like kinase 1
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial to mesenchymal transition
- IPMN
intraductal papillary-mucinous neoplasms
- MMP
matrix metalloproteinase
- NE
neuroendocrine
- PanIN
pancreatic intraepithelial neoplasia
- PDA
pancreatic ductal adenocarcinoma
- PSCs
pancreatic stellate cells
- SMA
smooth muscle actin
- TAM
tumor-associated macrophages
- TGFB
transforming growth factor beta
- TIMP
tissue inhibitor of metalloproteinases
Footnotes
Conflicts of Interest:
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen GM. Familial Pancreatic Adenocarcinoma. Hematol Oncol Clin North Am 2015;29:641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi C, Hong SM, Lim P, et al. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res 2009;7:230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller S, Engleitner T, Maresch R, et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature 2018;554:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eibl G, Cruz-Monserrate Z, Korc M, et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J Acad Nutr Diet 2018;118:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korc M, Jeon CY, Edderkaoui M, et al. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2017;31:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–50. [DOI] [PubMed] [Google Scholar]
- 7.Ardito CM, Gruner BM, Takeuchi KK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 2012;22:304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology 2009;137:1072–82, 1082 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou GY, Doppler H, DelGiorno KE, et al. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep 2016;14:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navas C, Hernandez-Porras I, Schuhmacher AJ, et al. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell 2012;22:318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storz P Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2017;14:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra C, Collado M, Navas C, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 2011;19:728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007;11:291–302. [DOI] [PubMed] [Google Scholar]
- 14.Liou GY, Doppler H, Braun UB, et al. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun 2015;6:6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013;108:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou GY, Doppler H, Necela B, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. The Journal of cell biology 2013;202:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastea LI, Liou GY, Pandey V, et al. Pomalidomide Alters Pancreatic Macrophage Populations to Generate an Immune-Responsive Environment at Precancerous and Cancerous Lesions. Cancer Res 2019;79:1535–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou GY, Bastea L, Fleming A, et al. The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis. Cell Rep 2017;19:1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67:9518–27. [DOI] [PubMed] [Google Scholar]
- 20.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2008;6:1155–61. [DOI] [PubMed] [Google Scholar]
- 22.Laklai H, Miroshnikova YA, Pickup MW, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 2016;22:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taki K, Ohmuraya M, Tanji E, et al. GNAS(R201H) and Kras(G12D) cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 2016;35:2407–12. [DOI] [PubMed] [Google Scholar]
- 25.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 2007;11:229–43. [DOI] [PubMed] [Google Scholar]
- 26.Ray KC, Bell KM, Yan J, et al. Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One 2011;6:e16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015;160:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp JL, von Figura G, Mayes E, et al. Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012;22:737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan FC, Bankaitis ED, Boyer D, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 2013;140:751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krah NM, De La OJ, Swift GH, et al. The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Figura G, Fukuda A, Roy N, et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat Cell Biol 2014;16:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopp JL, Dubois CL, Schaeffer DF, et al. Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice. Gastroenterology 2018;154:1509–1523 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey JM, Hendley AM, Lafaro KJ, et al. p53 mutations cooperate with oncogenic Kras to promote adenocarcinoma from pancreatic ductal cells. Oncogene 2015. [DOI] [PubMed] [Google Scholar]
- 34.Shi G, Zhu L, Sun Y, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology 2009;136:1368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinelli P, Madriles F, Canamero M, et al. The acinar regulator Gata6 suppresses KrasG12V-driven pancreatic tumorigenesis in mice. Gut 2016;65:476–86. [DOI] [PubMed] [Google Scholar]
- 36.Collet L, Ghurburrun E, Meyers N, et al. Kras and Lkb1 mutations synergistically induce intraductal papillary mucinous neoplasm derived from pancreatic duct cells. Gut 2019. [DOI] [PubMed] [Google Scholar]
- 37.Westphalen CB, Takemoto Y, Tanaka T, et al. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell 2016;18:441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strobel O, Rosow DE, Rakhlin EY, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010;138:1166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpino G, Puca R, Cardinale V, et al. Peribiliary Glands as a Niche of Extrapancreatic Precursors Yielding Insulin-Producing Cells in Experimental and Human Diabetes. Stem Cells 2016;34:1332–42. [DOI] [PubMed] [Google Scholar]
- 40.Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011;54:2159–72. [DOI] [PubMed] [Google Scholar]
- 41.DiPaola F, Shivakumar P, Pfister J, et al. Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury in mice. Hepatology 2013;58:1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res 2006;66:95–106. [DOI] [PubMed] [Google Scholar]
- 43.Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 2015;39:1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016;529:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Moltke J, Ji M, Liang HE, et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016;529:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016;351:1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders CJ, Christensen M, Finger TE, et al. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A 2014;111:6075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delgiorno KE, Hall JC, Takeuchi KK, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology 2014;146:233–244 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey JM, Alsina J, Rasheed ZA, et al. DCLK1 Marks a Morphologically Distinct Subpopulation of Cells With Stem Cell Properties in Preinvasive Pancreatic Cancer. Gastroenterology 2014;146:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerbe F, van Es JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 2011;192:767–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sbarbati A, Osculati F. A new fate for old cells: brush cells and related elements. J Anat 2005;206:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin W, Ogura T, Margolskee RF, et al. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol 2008;99:1451–60. [DOI] [PubMed] [Google Scholar]
- 53.Farrell AS, Joly MM, Allen-Petersen BL, et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat Commun 2017;8:1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinha S, Fu YY, Grimont A, et al. PanIN Neuroendocrine Cells Promote Tumorigenesis via Neuronal Cross-talk. Cancer Res 2017;77:1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012;148:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut 2012;61:1377–9. [DOI] [PubMed] [Google Scholar]
- 57.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korc M Pancreatic cancer-associated stroma production. Am J Surg 2007;194:S84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 60.Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cobo I, Martinelli P, Flandez M, et al. Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature 2018;554:533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loncle C, Bonjoch L, Folch-Puy E, et al. IL17 Functions through the Novel REG3beta-JAK2-STAT3 Inflammatory Pathway to Promote the Transition from Chronic Pancreatitis to Pancreatic Cancer. Cancer Res 2015;75:4852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corcoran RB, Contino G, Deshpande V, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res 2011;71:5020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukuda A, Wang SC, Morris JPt, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011;19:441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011;19:456–69. [DOI] [PubMed] [Google Scholar]
- 66.Liou GY, Doppler H, Necela B, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol 2013;202:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liou GY, Doppler H, Necela B, et al. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov 2015;5:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ling J, Kang Y, Zhao R, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baumgart S, Chen NM, Siveke JT, et al. Inflammation-induced NFATc1-STAT3 transcription complex promotes pancreatic cancer initiation by KrasG12D. Cancer Discov 2014;4:688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumgart S, Chen NM, Zhang JS, et al. GSK-3beta Governs Inflammation-Induced NFATc2 Signaling Hubs to Promote Pancreatic Cancer Progression. Mol Cancer Ther 2016;15:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawey ET, Johnson JA, Crawford HC Matrix metalloproteinase type 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci U S A 2007;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carriere C, Young AL, Gunn JR, et al. Acute pancreatitis accelerates initiation and progression to pancreatic cancer in mice expressing oncogenic Kras in the nestin cell lineage. PLoS One 2011;6:e27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169:2756–61. [DOI] [PubMed] [Google Scholar]
- 74.Plate JM, Harris JE. Immune cell functions in pancreatic cancer. Crit Rev Immunol 2000;20:375–92. [PubMed] [Google Scholar]
- 75.Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 2012;41:409–15. [DOI] [PubMed] [Google Scholar]
- 76.Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013;24:695–709. [DOI] [PubMed] [Google Scholar]
- 77.Gocheva V, Wang HW, Gadea BB, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 2010;24:241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campbell AS, Albo D, Kimsey TF, et al. Macrophage inflammatory protein-3alpha promotes pancreatic cancer cell invasion. J Surg Res 2005;123:96–101. [DOI] [PubMed] [Google Scholar]
- 79.Habtezion A, Edderkaoui M, Pandol SJ. Macrophages and pancreatic ductal adenocarcinoma. Cancer Lett 2016;381:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013;93:844–54. [DOI] [PubMed] [Google Scholar]
- 81.Seifert L, Werba G, Tiwari S, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016;532:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seifert L, Werba G, Tiwari S, et al. Radiation Therapy Induces Macrophages to Suppress T-Cell Responses Against Pancreatic Tumors in Mice. Gastroenterology 2016;150:1659–1672 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neesse A, Algul H, Tuveson DA, et al. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 2015;64:1476–84. [DOI] [PubMed] [Google Scholar]
- 85.Dougan SK. The Pancreatic Cancer Microenvironment. Cancer J 2017;23:321–325. [DOI] [PubMed] [Google Scholar]
- 86.Jiang H, Hegde S, DeNardo DG. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother 2017;66:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 2014;211:1503–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005;128:907–21. [DOI] [PubMed] [Google Scholar]
- 89.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421–32. [DOI] [PubMed] [Google Scholar]
- 91.Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol 2009;7:S48–54. [DOI] [PubMed] [Google Scholar]
- 92.Yamada T, Kuno A, Masuda K, et al. Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther 2003;307:17–23. [DOI] [PubMed] [Google Scholar]
- 93.Yokota T, Denham W, Murayama K, et al. Pancreatic stellate cell activation and MMP production in experimental pancreatic fibrosis. J Surg Res 2002;104:106–11. [DOI] [PubMed] [Google Scholar]
- 94.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013;144:1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res 2010;316:2713–22. [DOI] [PubMed] [Google Scholar]
- 96.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330:827–30. [DOI] [PubMed] [Google Scholar]
- 97.Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 2016;5:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009;8:3984–4001. [DOI] [PubMed] [Google Scholar]
- 100.Sousa CM, Biancur DE, Wang X, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016;536:479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Auciello FR, Bulusu V, Oon C, et al. A Stromal Lysolipid-Autotaxin Signaling Axis Promotes Pancreatic Tumor Progression. Cancer Discov 2019;9:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elyada E, Bolisetty M, Laise P, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res 2014;2:187–93. [DOI] [PubMed] [Google Scholar]
- 106.Biffi G, Oni TE, Spielman B, et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov 2019;9:282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Richards KE, Zeleniak AE, Fishel ML, et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017;36:1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003;425:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Panni RZ, Sanford DE, Belt BA, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother 2014;63:513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang Y, Xu X, Guo S, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One 2014;9:e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He S, Fei M, Wu Y, et al. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci 2011;12:7424–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suurmond J, Habets KL, Dorjee AL, et al. Expansion of Th17 Cells by Human Mast Cells Is Driven by Inflammasome-Independent IL-1beta. J Immunol 2016;197:4473–4481. [DOI] [PubMed] [Google Scholar]
- 115.Ma Y, Hwang RF, Logsdon CD, et al. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res 2013;73:3927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011;60:1419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ademmer K, Ebert M, Muller-Ostermeyer F, et al. Effector T lymphocyte subsets in human pancreatic cancer: detection of CD8+CD18+ cells and CD8+CD103+ cells by multi-epitope imaging. Clin Exp Immunol 1998;112:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gyori D, Lim EL, Grant FM, et al. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kalbasi A, Komar C, Tooker GM, et al. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 2017;23:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018;67:1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee KE, Spata M, Bayne LJ, et al. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov 2016;6:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov 2016;6:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov 2016;6:270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aguirre AJ, Nowak JA, Camarda ND, et al. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov 2018;8:1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Makohon-Moore AP, Zhang M, Reiter JG, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet 2017;49:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McDonald OG, Li X, Saunders T, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 2017;49:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Candido JB, Morton JP, Bailey P, et al. CSF1R(+) Macrophages Sustain Pancreatic Tumor Growth through T Cell Suppression and Maintenance of Key Gene Programs that Define the Squamous Subtype. Cell Rep 2018;23:1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steele CW, Karim SA, Leach JD, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016;29:832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tiriac H, Belleau P, Engle DD, et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov 2018;8:1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Yan W, Mathew E, et al. Epithelial-Myeloid cell crosstalk regulates acinar cell plasticity and pancreatic remodeling in mice. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]