Abstract
Background
Atopic dermatitis skin lesions demonstrate increased expression of IL-25 by keratinocytes and increased numbers of type 2 innate lymphoid cells (ILC2s) that express high levels of IL-25 receptor (IL-25R). IL-13 is expressed in atopic dermatitis skin lesions and plays an important role in pathogenesis of the disease.
Objective
Our aim was to determine the role of IL-25 and ILC2s in a mouse model of antigen-driven allergic skin inflammation.
Methods
Wild-type mice; mice that express an Il13-driven enhanced green fluorescent protein; and mice that lack IL-25R, IL-25 in keratinocytes, or IL-13 or IL-25R in ILC2s were subjected to acute or chronic epicutaneous sensitization with ovalbumin. Sensitized skin was examined by histology for epidermal thickening. Cellular infiltrates were analyzed for surface markers and intracellular expression of enhanced green fluorescent protein by flow cytometry. Gene expression was quantitated by RT quantitative PCR.
Result
In both acute and chronic antigen-driven allergic skin inflammation, signaling by keratinocyte-derived IL-25 in ILC2s is important for epidermal hyperplasia, dermal infiltration by CD4+ T cells, and cutaneous expression of Il13 and the IL-13–dependent TH2-cell–attracting chemokines Cc17 and Ccl22. ILCs are the major source of IL-13 in acutely sensitized mouse skin, whereas T cells are its major source in chronically sensitized mouse skin.
Conclusion
ILC2 activation by IL-25 is essential for IL-13 expression at sites of allergic skin inflammation.
Key words: Atopic dermatitis, IL-25, IL-13, ILC2
Abbreviations used: AD, Atopic dermatitis; eGFP, Enhanced green fluorescent protein; IL-25R, IL-25 receptor; ILC, Innate lymphoid cell; ILC2, Type 2 innate lymphoid cell; MC, Mast cell; TEWL, Transepidermal water loss; TSLP, Thymic stromal lymphopoietin; WT, Wild-type
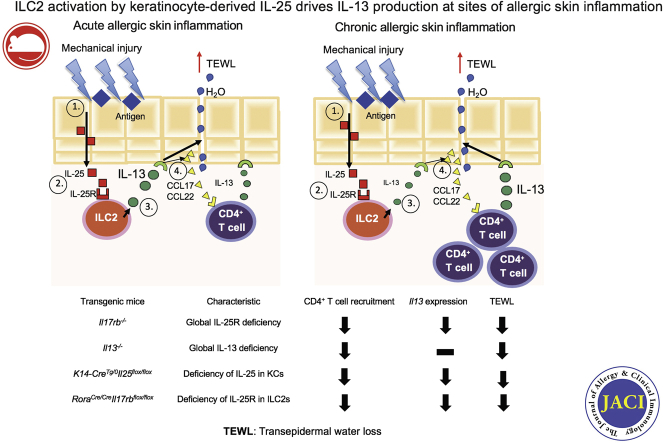
Graphical abstract
The hallmarks of atopic dermatitis (AD) are defective skin barrier, type-2–dominated cutaneous inflammation, and epidermal hyperplasia.1 IL-13 is expressed in AD skin lesions and plays an important role in pathogenesis of the disease.2,3 IL-13 upregulates expression of the TH2-cell–attracting chemokines CCL17 and CCL22 by human keratinocytes.4 IL-13 also suppresses keratinocyte expression of genes important for epidermal integrity, including filaggrin, claudins, and desmoglein-1, which are downregulated in AD skin lesions.5, 6, 7, 8 Transgenic cutaneous expression of Il13 drives epidermal hyperplasia, increased expression of TH2-cell–attracting chemokines, and recruitment of CD4+ T cells.9
IL-25 is a member of IL-17 cytokine family produced by epithelial cells, macrophages, eosinophils, mast cells (MCs), and basophils.10, 11, 12 The IL-25 receptor (IL-25R) is a heterodimer of the IL-17RA chain (shared by receptors for other IL-17 family members) and the IL-17 receptor B chain (specific for IL-25R).10, 11, 12 Both nonimmune and immune cells express IL-25R; they include epithelial cells, type 2 innate lymphoid cells (ILC2s), eosinophils, and CD4+ T cells.10, 11, 12
Innate lymphoid cells (ILCs) are lymphoid cells that lack lineage markers and antigen-specific surface receptors and are enriched at the interfaces between the body and the environment.13 ILC2s express the transcription factor RAR-related orphan receptor alpha, which is essential for their development14; the type 2 cytokines IL-5 and IL-13; and receptors for the epithelial-derived cytokines IL-25, thymic stromal lymphopoietin (TSLP), and IL-33.13,15 IL-25, IL-33, and TSLP, alone or in combination, promote IL-13 production by ILC2s in vitro.13,16
AD skin lesions demonstrate increased expression of IL-25 by keratinocytes16, 17, 18, 19, 20 and increased numbers of ILC2s that express high levels of receptors for IL-25, TSLP, and IL-33.16,21 IL-25 alone, or in combination with type 2 cytokines, impairs skin barrier function.18,19,22,23 TSLP and IL-33 are important in allergic skin inflammation24, 25, 26; however, the role of IL-25 is not completely understood. We show that ILC2 activation by IL-25 is essential for IL-13 production at sites of allergic skin inflammation.
Methods
Mice
Il17rb–/–, Il13–/–, Il13eGFP/+ mice on a BALB/c background were previously described.27,28 Il17rbfl/fl mice on a BALB/c background were obtained from Dr Ziegler, and RoraCre/Cre mice on a C57Bl/6 background were obtained from Dr O’Leary29 and crossed on a BALB/c background. IL-25–floxed mice on a C57Bl/6 background were obtained from Dr Dong.30 K14-CreTg/0 mice on a C57Bl/6 background were obtained from Jackson Laboratories. BALB/c and C57Bl/6 mice were purchased from Charles River Laboratory. All mice were kept in a pathogen-free environment and fed an ovalbumin-free diet. All procedures were performed in accordance with the Animal Care and Use Committee of the Children's Hospital Boston.
Epicutaneous sensitization
Female mice (aged 6 to 8 weeks) were epicutaneously sensitized for 10 days or 7 weeks, as described previously.25,31 Analyses were done at day 12 or day 49.
Histology and measurement of epidermal thickness
Skin specimens were fixed in 4% paraformaldehyde embedded in paraffin and analyzed as previously described.32
Mouse skin cell preparation and flow cytometry
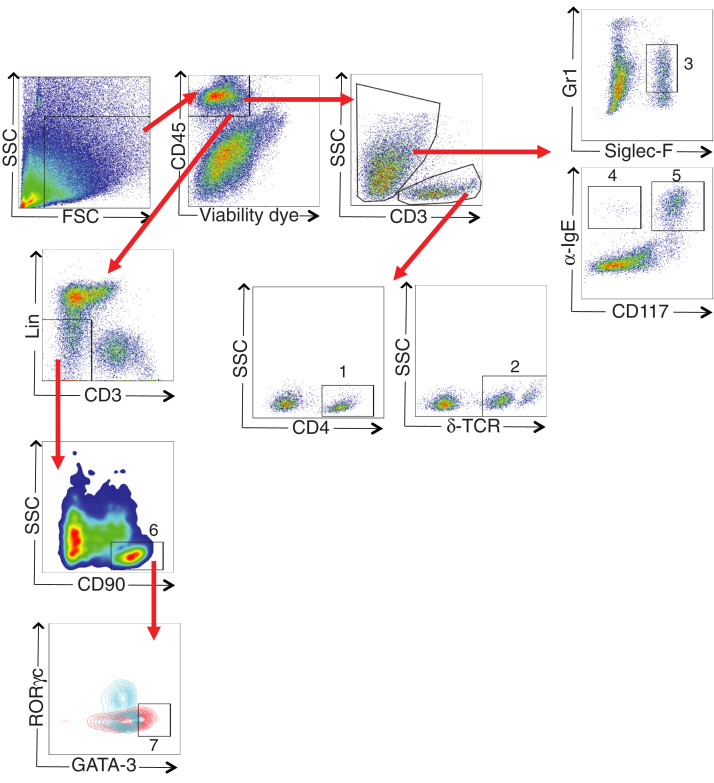
Cell isolation from the back skin was performed as previously described.33 Cells were preincubated with FcγR-specific blocking mAb (2.4G2) and washed before staining with the following mAbs: B220 (RA3-6B2), CD3 (17A2), CD4 (GK1.5), CD11c (N418), CD19 (1D3), CD45 (30F11), CD90.2 (53-2.1), Gr1 (RB6-8C5), and δ TCR (ebioGL3) from eBioscience (San Diego, Calif); CD11b (M1/70), F4/80 (BM8), and CD117 (2B8) from Biolegend (San Diego, Calif); and anti-IgE (R35-72) from BD Biosciences (San Jose, Calif). BV605 streptavidin from Biolegend was used to detect biotinylated antibodies. Cells were analyzed by flow cytometry by using an LSRFortessa machine (BD Biosciences). The data were analyzed with FlowJo software. CD4+ T cells (CD45+CD3+CD4+), γδ TCR+ T cells (CD45+CD3+δTCR+), eosinophils (CD45+CD3–GR1+SiglecF+), basophils (CD45+CD3–α–IgE+CD117–), MCs (CD45+CD3–α–IgE+CD117+), ILCs (CD45+CD3–Lin–CD90+), and ILC2s (CD45+CD3–Lin– CD90+GATA3+) in the skin were identified as shown in Fig E1 (in the Online Repository available at www.jacionline.org).
Fig E1.
Gating strategy. Representative flow cytometry plots showing gating strategy for CD4+ T cells (1), γδ TCR+ T cells (2), eosinophils (3), basophils (4), MCs (5), ILCs (6), and ILC2s (7). To gate GATA3+ cells in the ILC population (red), we used γδ TCR+ T cells (blue) as a negative control. FSC, Forward scatter; SSC, side scatter.
mRNA expression analyses
Total skin RNA extraction and measurement of cytokines were performed and analyzed as previously described.34
TEWL
Transepidermal water loss (TEWL) was measured by using a Dermalab instrument DermaLab universal serial bus module (Cortex Technology, Hadsund, Denmark). TEWL was assessed on the epicutaneously sensitized skin, and readings were recorded for 1 minute. The probe was removed from the skin and replaced, after which a second measurement was taken. An average of the 2 readings was used as the TEWL for each mouse.
Cell culture and in vitro cytokine expression
Single-cell suspensions of skin-draining lymph nodes and splenocytes were cultured and stimulated with ovalbumin, and their supernatants analyzed for cytokines by ELISA as previously described.34
Statistical analysis
Statistical significance was determined by a 2-tailed Student t test. A P value less than .05 was considered statistically significant.
Results
IL-25 signaling is required for acute allergic skin inflammation
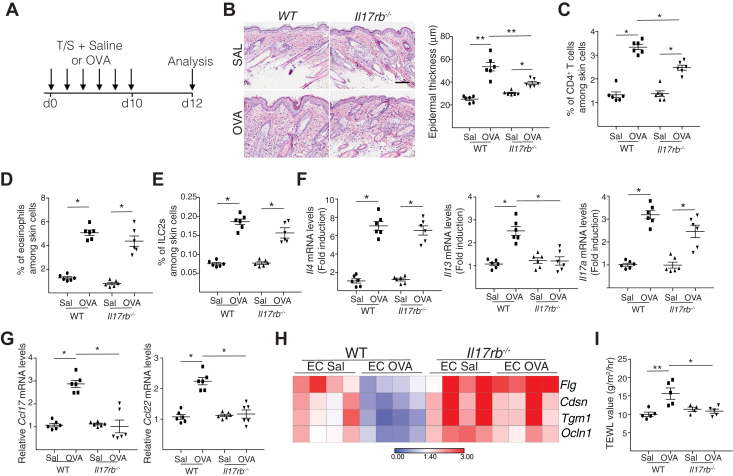
To determine the role of IL-25 in acute allergic skin inflammation, we examined the response of Il17rb–/– mice to epicutaneous sensitization with ovalbumin over a 12-day period (Fig 1, A). Il17rb–/– mice epicutaneously sensitized with ovalbumin exhibited significantly diminished epidermal thickening and significantly less dermal infiltration by CD4+ T cells compared with wild-type (WT) controls (Fig 1, B and C). Dermal infiltration by eosinophils, basophils, and MCs was comparable in the 2 groups (Fig 1, D and see Fig E2, A in the Online Repository available at www.jacionline.org). The percentage of CD3–Lin–CD90+GATA3+ ILC2s increased to a comparable extent in ovalbumin-sensitized skin of the 2 strains (Fig 1, E). These findings demonstrate that IL-25 signaling is important for epidermal thickening and optimal accumulation of CD4+ T cells at sites of acute allergic skin inflammation.
Fig 1.
IL-25 signaling is required for acute allergic skin inflammation. A, Experimental protocol. B-I, Representative hematoxylin and eosin staining (B [left]) and epidermal thickness measurement (B [right]); percentages of CD4+ T cells (C), eosinophils (D), and ILC2s (E); mRNA levels of Il4, Il13, Il17a (F), and Ccl17 and Ccl22 (G) expressed relative to the mean of saline (SAL)-sensitized WT controls; heat map of mRNA levels in epidermal sheets (H); and TEWL (I) in saline-sensitized and ovalbumin (OVA)-sensitized skin of Il17rb–/– mice and WT controls. Results in B to I are representative of 2 independent experiments with 4 or 5 mice per group. ∗P < .05; ∗∗P < .005. EC, Epicutaneous; T/S, tape stripping.
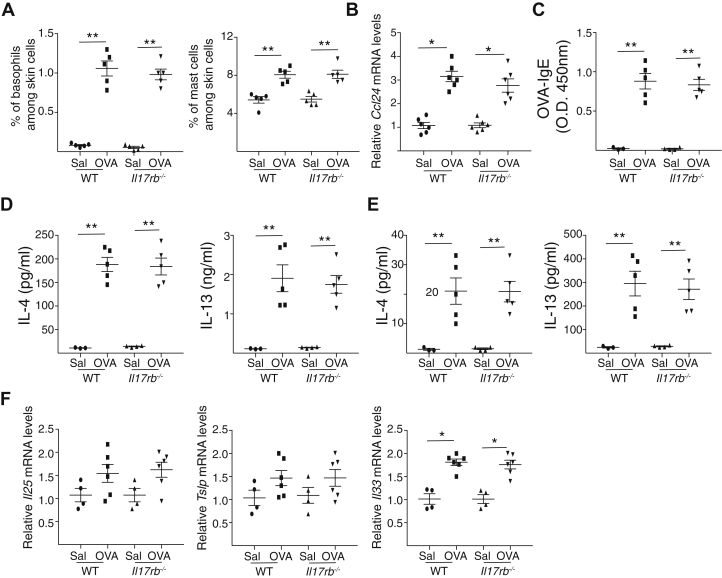
Fig E2.
IL-25 signaling is dispensable for accumulation of basophils and MCs at sites of allergic skin inflammation and for local and systemic TH2 response induced by epicutaneous sensitization. A-D, Percentage of skin basophils and MCs (A), serum ovalbumin (OVA)-specific IgE levels (B), IL-4 and IL-13 secretion by skin-draining lymph nodes (C) and splenocytes (D) and Il25, Tslp, and Il33 mRNA levels in OVA- and saline (SAL)-sensitized Il17rb–/– mice and WT BALB/c controls. Results are representative of 2 independent experiments with 4 or 5 mice per group. Horizontal lines represent means ± SEMs. ∗P < .05; ∗∗P < .005. Calculations performed by using a 2-tailed Student t test.
Type 2 cytokines are important for epidermal thickening and cutaneous expression by keratinocytes of Ccl17 and Ccl224,9 (chemokine ligands for CCR4), which is important for the accumulation of T cells in ovalbumin-sensitized mouse skin.35 Epicutaneous sensitization of WT BALB/c mice with ovalbumin resulted in local upregulation of Il4, Il13, and Il17a but not Ifng expression (Fig 1, F and data not shown). It also resulted in local upregulation of Ccl17 and Ccl22 (Fig 1, G), as well as Ccl24, which encodes eotaxin 2 (see Fig E2, B). Upregulation of Il13, Ccl17, and Ccl22 expression was virtually abolished in ovalbumin-sensitized skin of Il17rb–/– mice compared with in WT controls, whereas upregulation of Il4, Il17a, and Ccl24 expression was unaffected and Ifng expression remained unchanged (Fig 1, F-G and see also Fig E2, B and data not shown). These results indicate that IL-25R signaling is essential for Il13 expression at sites of acute allergic skin inflammation.
The systemic TH2 immune response to epicutaneous sensitization with ovalbumin was comparable to that in Il17rb–/– mice and WT controls. This was evidenced by comparable serum levels of ovalbumin-specific IgE and secretion of IL-4 and IL-13 by skin-draining lymph node cells and splenocytes in response to in vitro stimulation with ovalbumin (Fig E2, C-E). Thus, defective systemic TH2 immune response to epicutaneous sensitization cannot explain the failure of Il17rb–/– mice to upregulate Il13 expression in the ovalbumin-sensitized skin.
Upregulation of Il33, Il25, and Tslp mRNA expression in the skin following ovalbumin sensitization was comparable in Il17rb–/– mice and WT controls (Fig E2, F). Expression by epidermal sheets of epidermal barrier integrity and the tight junction genes Flg, Cdsn, Tgm1, and Ocln1 was downregulated in ovalbumin-sensitized skin compared with in saline-sensitized skin in WT mice but not in Il17rb–/– mice (Fig 1, H). Consistent with this finding, TEWL increased in ovalbumin-sensitized skin compared with in saline-sensitized skin in WT mice but not in Il17rb–/– mice (Fig 1, I). These results demonstrate that IL-25R signaling disrupts epidermal barrier integrity in epicutaneously sensitized skin.
Keratinocyte-derived IL-25 is required for acute allergic skin inflammation
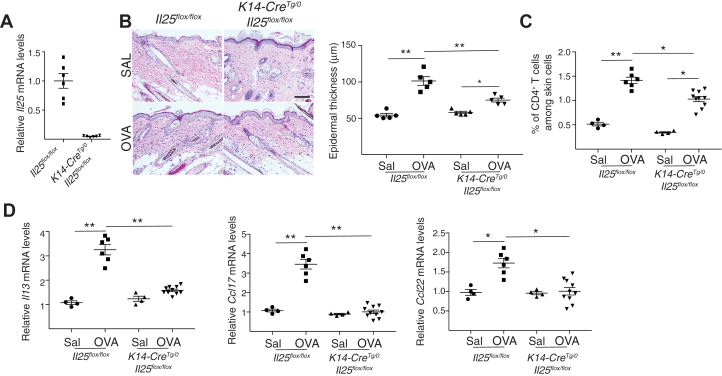
We used K14-CreTg/0Il25flox/flox mice, which have selective IL-25 deficiency in keratinocytes, to investigate the role of keratinocyte-derived IL-25 in acute allergic skin inflammation. Il25 expression in the skin was virtually abolished in K14-CreTg/0Il25flox/flox mice compared with in Il25flox/flox controls (see Fig E3, A in the Online Repository available at www.jacionline.org). Ovalbumin-sensitized skin from K14-CreTg/0Il25flox/flox mice demonstrated significantly reduced epidermal thickening, infiltration by CD4+ T cells, and expression of Il13, Ccl17, and Ccl22 mRNA compared with in Il25flox/flox controls (Fig E3, B-D). Infiltration with eosinophils and Il4 expression were comparable in the 2 strains (data not shown). These results implicate keratinocytes as the source of IL-25, which promotes acute allergic skin inflammation.
Fig E3.
Keratinocyte-derived IL-25 is required for acute allergic skin inflammation. A.Il25 mRNA levels in skin from u K14-CreTg/0Il25flox/flox mice and Il25flox/flox controls expressed relative to the mean of Il25flox/flox controls. B-D, Representative hematoxylin and eosin staining (B [left]) and epidermal thickness measurement (B [right]); quantitation of percentage of CD4+ T cells (C); and mRNA levels of Il13, Ccl17, and Ccl22 expressed relative to the mean of saline (SAL)-sensitized controls (D) in ovalbumin (OVA)- and SAL-sensitized skin of K14-CreTg/0Il25flox/flox mice and Il25flox/flox controls. Results in A to D are representative of 2 independent experiments with 4 or 5 mice per group. ∗P < .05; ∗∗P < .005.
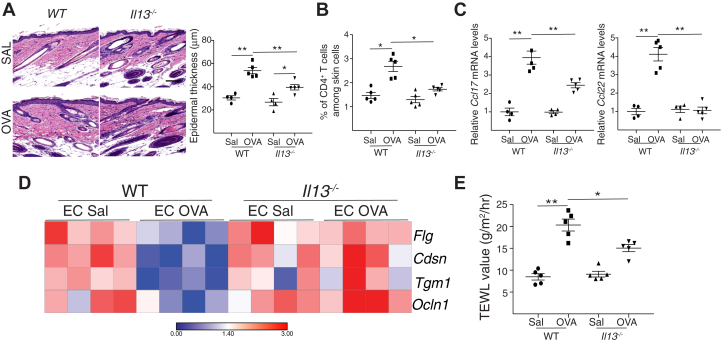
The phenotype of acute allergic skin inflammation in IL-13–deficient mice recapitulates that of IL-25R–deficient mice
Epidermal thickening, accumulation of CD4+ T cells, and expression of Ccl17 and Ccl22 were all significantly diminished in ovalbumin-sensitized skin of Il13–/– mice compared with in WT controls (Fig 2, A-C). Importantly, epidermal sheets from ovalbumin-sensitized skin of Il13–/– mice exhibited increased mRNA levels of Flg, Cdsn, Tgm1, and Ocln1 compared with the levels in their WT counterparts (Fig 2, D, E). TEWL in ovalbumin-sensitized skin was significantly lower in Il13–/– mice than in WT controls (Fig 2, E). The similarity of the phenotype of ovalbumin-sensitized skin in Il13–/– and Il17rb–/– mice suggests that IL-13 mediates the ability of IL-25 to promote acute allergic skin inflammation.
Fig 2.
Acute allergic skin inflammation in IL-13–deficient mice recapitulates that in IL-25R–deficient mice. A-E, Representative hematoxylin and eosin staining (A [left]) and epidermal thickness measurement (A [right]), quantitation of percentage of CD4+ T cells (B) and mRNA levels of Ccl17 and Ccl22 expressed relative to the mean of WT controls, and (C) heat map of mRNA levels in epidermal sheets (D) and TEWL (E) in ovalbumin (OVA)- and saline (SAL)-sensitized skin of Il13–/– mice and WT controls Results in A to E are representative of 2 independent experiments with 4 or 5 mice per group. ∗P < .05; ∗∗P < .005. EC, Epicutaneous.
ILCs are the major source of IL-13 in acute allergic skin inflammation
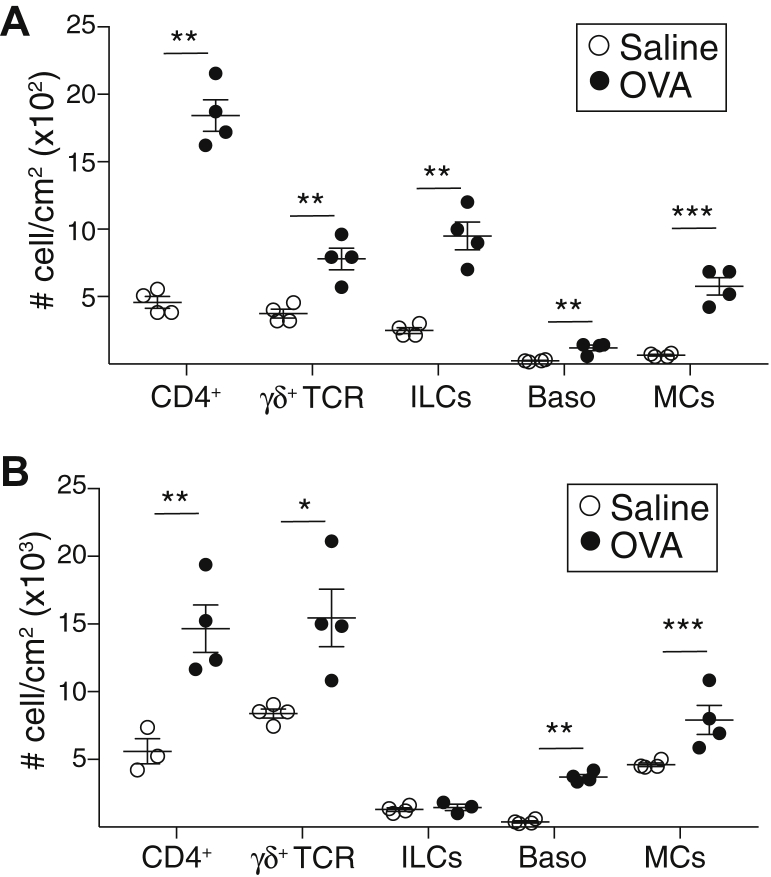
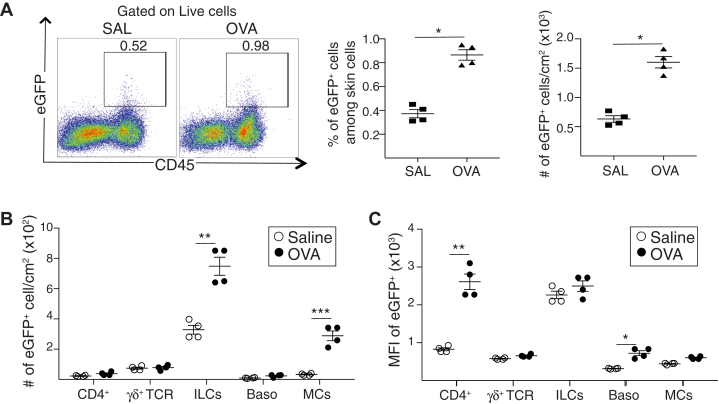
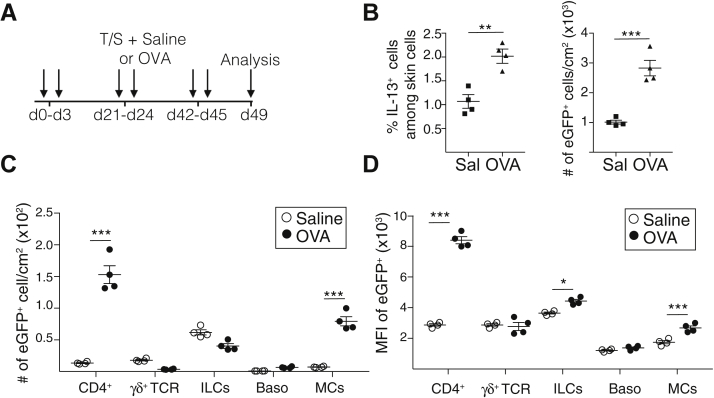
To identify the cellular sources of IL-13 in acute allergic skin inflammation, we used Il13eGFP reporter mice, in which the IL-13 promoter drives the expression of enhanced green fluorescent protein (eGFP).28 Flow cytometry analysis revealed that acute epicutaneous sensitization with ovalbumin resulted in a significant increase in the number of CD4+ T cells, TCRγδ+ cells, ILCs, basophils, and MCs (see Fig E4, A in the Online Repository available at www.jacionline.org). In addition, epicutaneous sensitization with ovalbumin caused an approximately 2-fold increase in the percentage and number of CD45+eGFP+(IL-13+) cells in the skin of Il13eGFP mice compared with in the controls (Fig 3, A). ILCs were the most abundant eGFP+ cells and had the highest expression of eGFP (IL-13) in saline-sensitized skin (Fig 3, B and C). CD4+ cells, TCRγδ+ cells, basophils, and cKIT+IgE+ MCs each accounted for a small fraction of eGFP+(IL-13+) cells in saline-sensitized skin and expressed eGFP (IL-13) at levels lower by 3-fold or more compared with those in ILCs (Fig 3, B and C). Ovalbumin sensitization caused a significant increase in the numbers of eGFP+ (IL-13+) ILCs and MCs but not in the numbers of eGFP+ (IL-13+) CD4+ T cells, TCRγδ+ cells, or basophils (Fig 3, B). It did not alter eGFP (IL-13) expression in ILCs, TCRγδ+ cells, or MCs, but it did cause a 3-fold increase in eGFP (IL-13) expression in CD4+ T cells that reached the level of eGFP (IL-13) expression in ILCs (Fig 3, C). Ovalbumin sensitization slightly increased eGFP (IL-13) expression in basophils (Fig 3, C). These results indicate that ILCs are the major IL-13+ cell population at sites of acute allergic skin inflammation.
Fig E4.
Acute and chronic epicutaneous sensitization with ovalbumin (OVA) induced an increase in CD4+ T cells, TCRγδ+ cells, basophils (Baso), and MCs. A and B, Number of cells for CD4+ T cells, TCRγδ+ T cells, ILCs, Baso, and MCs in skin of Il13egpf/+ mice following acute (A) and chronic (B) epicutaneous sensitization. Results are representative of 2 independent experiments with 4 mice per group. Horizontal lines represent means ± SEMs. ∗∗P < .005; ∗∗∗ P < .001. Calculations performed by using a 2-tailed Student t test.
Fig 3.
ILCs are the major source of IL-13 in acute allergic skin inflammation. A-C, Representative flow cytometry plot (left), percentage (center), and number (right) of eGFP+ cells (A); numbers of eGFP+ cells (B); and mean fluorescence intensity (MFI) of eGFP expression (C) for CD4+ T cells, TCRγδ+ T cells, ILCs, basophils (Baso), and MCs in epicutaneously sensitized skin of Il13egpf/+ mice. Results in A to C are representative of 2 independent experiments with 4 or 5 mice per group. ∗P < .05; ∗∗P < .005. SAL, Saline; OVA, ovalbumin.
IL-25 acts directly on ILC2s to drive acute allergic skin inflammation
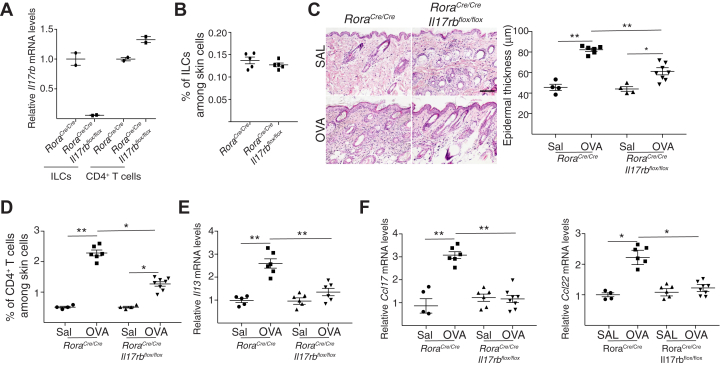
To investigate whether IL-25 acts directly on ILC2s to drive acute allergic skin inflammation, we examined RoraCre/CreIl17rbflox/flox mice, which selectively lack IL-25R expression in ILC2s. Il17rb mRNA expression was virtually abolished in CD45+Lin–CD3–CD90+ ILCs, but it was preserved in CD4+ cells sorted from the skin of RoraCre/CreIl17rbflox/flox mice compared with in RoraCre/Cre controls (Fig 4, A). The percentage of CD45+Lin–CD3–CD90+GATA3+ ILC2s in the skin was comparable between the 2 strains (Fig. 4, B). Ovalbumin-sensitized skin of RoraCre/CreIl17rbflox/flox mice revealed significantly decreased epidermal hyperplasia, infiltration with CD4+ T cells, and expression of Il13, Ccl17, and Ccl22 mRNA compared with in RoraCre/Cre controls (Fig 4, C-F). Infiltration with eosinophils and Il4 expression were comparable in the 2 strains (data not shown). These results suggest that IL-25 acts directly on ILC2s at sites of acute allergic skin inflammation to promote their production of IL-13.
Fig 4.
IL-25 acts directly on ILC2s to drive acute allergic skin inflammation. A and B.Il17rb mRNA levels in sorted skin ILCs and skin CD4+ T cells (A) and percentage of skin ILC2s (B) in RoraCre/CreIl17rbflox/flox mice and RoraCre/Cre controls. C-F, Representative hematoxylin and eosin staining (C [left]) and epidermal thickness measurement (C [right]); quantitation of percentage of CD4+ T cells (D); and mRNA levels of Il13 (E), Ccl17, and Ccl22 (F) in acutely epicutaneously sensitized skin of RoraCre/CreIl17rbflox/flox mice and RoraCre/Cre controls. Values in D and E are expressed relative to the mean of saline (SAL)-sensitized controls. Results in A to F are representative of 2 independent experiments with 4 or 5 mice per group. ∗ P < .05; ∗∗ P < .005. OVA, Ovalbumin.
T cells are the major source of IL-13 in chronic allergic skin inflammation in mice
Adult patients with AD have chronic disease, and T cells are the major source of IL-13 in lesional skin.36 This observation prompted us to investigate the cellular source of IL-13 in a mouse model of chronic allergic skin inflammation elicited by repeated application of ovalbumin to tape-stripped skin of Il13eGFP mice over a 7-week period (Fig 5, A). We previously showed that Il13 expression is significantly increased in ovalbumin-sensitized skin in this model of chronic sensitization.37 Flow cytometry analysis revealed that chronic epicutaneous sensitization with ovalbumin resulted in a significant increase in the number of CD4+ T cells, TCRγδ+ cells, ILCs, basophils, and MCs (Fig E4, B). In addition, chronic epicutaneous sensitization with ovalbumin caused a significant (approximately 2-fold) increase in the percentage and number of CD45+eGFP+(IL-13+) cells in the skin (Fig 5, B). The majority were CD4+ T cells (55%), followed by MCs (28%), ILCs (15%), γδ T cells (<5%), and basophils (<5%) (Fig 5, C). CD4+ T cells had the highest mean fluorescence intensity of IL-13 expression, followed by ILCs and MCs (Fig 5, D). The number of CD4+IL-13+ cells was 10-fold higher and their IL-13 mean fluorescence intensity of IL-13 expression was 4-fold higher in skin chronically sensitized with ovalbumin than in acutely sensitized skin. These results indicate that T cells are the major source of IL-13 in antigen-driven chronic allergic skin inflammation in mice.
Fig 5.
T cells are the major source of IL-13 in chronic allergic skin inflammation in mice. A, Experimental protocol for induction of chronic allergic skin inflammation in mice. B-D, Percentage (B [left]) and number (B [right]) of eGFP+ cells; numbers of eGFP+ cells (C); and mean fluorescence intensity (MFI) of eGFP expression (D) for CD4+ T cells, TCRγδ+ T cells, ILCs, basophils (Baso), and MCs in the skin of Il13egpf/+ mice subjected to chronic epicutaneous sensitization with ovalbumin (OVA). Results in B-D are representative of 2 independent experiments with 4 or 5 mice per group. Horizontal lines in B-D represent means. ∗P < .05; ∗∗P < .005; ∗∗∗P < .001. SAL, Saline.
Cutaneous accumulation of CD4+ T cells and Il13 expression in chronic allergic skin inflammation is dependent on ILC2 activation by IL-25
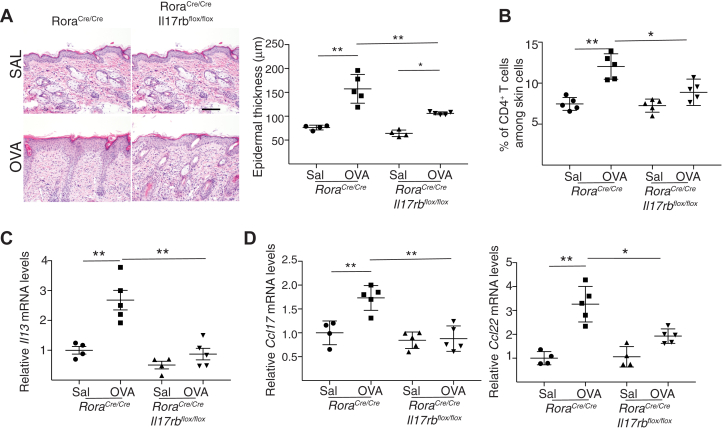
We asked whether IL25 activation of ILC2s is important for the accumulation of CD4+ T cells at sites of chronic allergic skin inflammation. Analysis of the skin of mice subjected to chronic epicutaneous sensitization with ovalbumin revealed that epidermal thickening, skin infiltration by CD4+ T cells, and upregulation of expression of Il13, Ccl17, and Ccl22 mRNA were significantly reduced or virtually abolished in RoraCre/CreIl17rbflox/flox mice compared with in RoraCre/Cre controls (Fig 6, A-D). Infiltration with eosinophils and Il4 expression were comparable in the 2 strains (data not shown). Because T cells were by far the major source of IL-13 in skin chronically sensitized with ovalbumin, the lack of upregulation of Il13 expression in RoraCre/CreIl17rbflox/flox mice suggests that IL-25 activation of ILC2s is necessary for the accumulation of CD4+IL-13+ T cells at sites of chronic allergic skin inflammation in mice.
Fig 6.
IL-17 receptor B (IL-17rb) expression by ILC2s is required to drive chronic allergic skin inflammation. A-D, Representative hematoxylin and eosin staining (A [left]) and epidermal thickness measurement (A [right]); quantitation of percentage of CD4+ T cells (B); and mRNA levels of Il13 (C), Ccl17, and Ccl22 (D) in skin of RoraCre/CreIl17rbflox/flox mice and RoraCre/Cre controls mice subjected to chronic epicutaneous sensitization with ovalbumin (OVA). Values in C and D are expressed relative to the mean of saline (SAL)-sensitized controls. Results in A-D are representative of 2 independent experiments with 4 or 5 mice per group. ∗P < .05; ∗∗ P < .005. N.D., Not detected.
Discussion
We demonstrate that keratinocyte-derived IL-25 activates ILC2s directly to promote IL-13 production, thereby also activating epidermal hyperplasia and CD4+ T-cell accumulation in acute as well as chronic antigen-driven allergic skin inflammation.
Our findings in Il17rb–/– mice demonstrate that IL-25 signaling is essential for the upregulation of Il13 expression at sites of acute allergic skin inflammation caused by short-term (12 days) epicutaneous sensitization of mouse skin with ovalbumin. Consistent with the decreased expression of Il13, IL-13–dependent epidermal hyperplasia and expression of the T-cell–attracting chemokines Ccl17 and Ccl22, as well as the recruitment of CD4+ T cells, were all decreased in the ovalbumin-sensitized skin of Il17rb–/– mice. Expression of the epidermal integrity genes Flg, Cdsn, Tgm1, and Ocln, which are known to be downregulated by IL-13, decreased in WT mice but remained unaltered in Il17rb–/– mice. In parallel, TEWL increased in WT mice but remained unaltered in Il17rb–/– mice. Ovalbumin-sensitized skin of Il13–/– mice phenocopied that of Il17rb–/– mice, strongly suggesting that the changes observed in Il17rb–/– mice were due to failure to upregulate Il13 expression in epicutaneously sensitized skin. Il17rb–/– mice showed a greater reduction in epidermal hyperplasia and TEWL than did Il13–/– mice, suggesting that factors in addition to IL-13 may mediate the effect of IL-25 in antigen-driven acute allergic skin inflammation. A recent report indicates that IL-25 plays an important role in keratinocytes in a mouse model of psoriatic inflammation mediated by a type 17 immune response.23 We have shown that IL-22 and IL-17A play an important role in promoting epidermal hyperplasia induced by epicutaneous sensitization.32 We do not exclude the possibility that IL-25 could promote epidermal hyperplasia and skin barrier defects in our model by IL-13–independent mechanisms.
Previous studies in vitro indicate that IL-25 plays a role in the generation and maintenance of TH2 cells.10,38 Our findings in Il17rb–/– mice demonstrate that IL-25 signaling is dispensable for generation of the local and systemic TH2 response induced by epicutaneous sensitization with ovalbumin. In agreement with our results, IL-25 was found to not be important for in vivo TH2 polarization induced by intradermal or subcutaneous Nippostrongylus brasiliensis infection.39 Collectively, these observations suggest that the role of IL-25 in TH2 polarization is bypassed by multiple nonredundant mechanisms generated in vivo during parasite infection and allergic inflammation.
IL-25 signaling was not important for the expansion of ILC2s, nor was it important for accumulation of eosinophils, basophils, and MCs or upregulation of Il4 expression in ovalbumin-sensitized skin. ILC2s expand in response to cytokines that, in addition to including IL-25, also include IL-33, TSLP, and IL-2. Normal upregulation of Il33 and Tslp expression may have been sufficient to support expansion of ILC2s in the ovalbumin-sensitized skin of Il17rb–/– mice. We found that basophils are the major source of Il4 mRNA in our mouse model of acute AD, as evidenced by RT quantitative PCR analysis of sorted skin cells (unpublished observations), which likely explains the normal upregulation of Il4 expression and the IL-4 downstream target Ccl2440 in ovalbumin-sensitized skin of acutely sensitized Il17rb–/– mice. Normal upregulation of Il4 and Ccl24 expression may explain the normal eosinophil skin infiltration in ovalbumin-sensitized skin of Il17rb–/– mice.
The phenotype of ovalbumin-sensitized skin of mice with selective deficiency of Il25 in keratinocytes, or selective deficiency of Il17rb in ILC2s, recapitulated that in Il17rb–/– mice, indicating that keratinocyte-derived IL-25 acts directly on ILC2s to upregulate Il13 production in ovalbumin-sensitized skin. Importantly, we demonstrated that ILCs are the major source of cutaneous IL-13 in mouse acute allergic skin inflammation. Consistent with our results, ILC2s are also the major source of IL-13 in the lungs of mice undergoing acute airway inflammation induced by papain, house dust mite, or ovalbumin.28,41,42 Skin ILC2s express receptors for TSLP, IL-25, and IL-33, and they produce IL-13 in response to in vitro stimulation with these cytokines.43 Moreover, lack of ILC2s attenuates the skin inflammation elicited in mice by transgenic expression IL-33 in keratinocytes,44 or by application of MC903,16,21 which promotes keratinocyte-derived TSLP overproduction.25 Our results showed that Il13 expression is virtually abolished in the acutely epicutaneously sensitized skin of Il17rb–/– mice despite normal upregulation of Tslp and Il33 expression, suggesting that IL-25 plays a nonredundant role in driving Il13 expression by skin ILC2s.
T cells, rather than ILC2s, were the major source of IL-13 in the skin of mice epicutaneously sensitized with ovalbumin chronically for 7 weeks. Nevertheless, IL-25 activation of ILC2s was critical for the accumulation of CD4+ cells and upregulation of Il13 expression, and thereby for epidermal hyperplasia in chronically sensitized mouse skin, because all of these events were reduced or abolished in RoraCre/CreIl17rbflox/flox mice. In addition to its expression by ILC2s, Rora is expressed by a subpopulation of TH2 cells and regulatory T cells.45,46 However, the systemic TH2 response to epicutaneous sensitization was unaffected in Il17rb–/– mice with global IL-25R deficiency. Thus, IL-25R deficiency in cells other than ILC2s is unlikely to explain the defect in Il13 expression at sites of chronic EC sensitization in RoraCre/CreIl17rbflox/flox mice. Rather, the results suggest that IL-25 activation of ILC2s plays an important role in chronic allergic skin inflammation.
Our results provide evidence for a crosstalk in allergic skin inflammation between keratinocytes producing IL-25 and ILC2s to promote the production of IL-13, which in turn acts back on keratinocytes to promote their proliferation and production of T-cell–attracting chemokines. A similar ILC2-epithelial cell crosstalk has been demonstrated during helminthic infection in the small intestine.47, 48, 49 Collectively, these observations support a paradigm in which a crosstalk between ILC2s and epithelial cells promotes immunity at mucosal barriers.
Clinical implications.
ILC2 activation by IL-25 drives cutaneous IL-13 production in allergic skin inflammation. IL-25 blockade may be beneficial in patients with AD.
Acknowledgments
We thank Dr Dennis O’Leary for his gift of Roracre/cre mice; Dr Hans Oettgen, Dr Hazel Wilkie, and Dr Talal Chatila for reading the manuscript and useful discussions; and Daniel Wong for technical help.
Footnotes
Funded by the National Institutes of Health/National Institute of Allergy and Infectious Diseases Atopic Dermatitis Research Network (grant U19AI117673); the National Institutes of Health (grant AI113294-01A1); and the National Institute of Allergy and Infectious Diseases (T32 training grant 5T32AI007512-32).
Disclosure of potential conflict of interest: J. M. Leyva-Castillo was supported by a postdoctoral fellowship from Consejo Nacional de Ciencia y Tecnología (Mexico) and a Boston Children’s Hospital Office of Faculty Development/Basic/Translational Research Executive Committee (BTREC) and the Clinical and Translational Research Executive Committee (CTREC) Faculty Career Development Fellowship. The rest of the authors declare that they have no relevant conflicts of interest.
Contributor Information
Juan Manuel Leyva-Castillo, Email: Manuel.LeyvaCastillo@childrens.harvard.edu.
Raif S. Geha, Email: raif.geha@childrens.harvard.edu.
Appendix
References
- 1.Brunner P.M., Guttman-Yassky E., Leung D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:S65–76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson E.L., Flohr C., Eichenfield L.F., Bieber T., Sofen H., Taieb A. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE) J Am Acad Dermatol. 2018;78:863–871.e11. doi: 10.1016/j.jaad.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Tsoi L.C., Rodriguez E., Degenhardt F., Baurecht H., Wehkamp U., Volks N. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139:1480–1489. doi: 10.1016/j.jid.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purwar R., Werfel T., Wittmann M. IL-13-stimulated human keratinocytes preferentially attract CD4+CCR4+ T cells: possible role in atopic dermatitis. J Invest Dermatol. 2006;126:1043–1051. doi: 10.1038/sj.jid.5700085. [DOI] [PubMed] [Google Scholar]
- 5.De Benedetto A., Rafaels N.M., McGirt L.Y., Ivanov A.I., Georas S.N., Cheadle C. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786.e1-7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B.E., Leung D.Y., Boguniewicz M., Howell M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honzke S., Wallmeyer L., Ostrowski A., Radbruch M., Mundhenk L., Schafer-Korting M. Influence of Th2 cytokines on the cornified envelope, tight junction proteins, and ss-defensins in filaggrin-deficient skin equivalents. J Invest Dermatol. 2016;136:631–639. doi: 10.1016/j.jid.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Gruber R., Bornchen C., Rose K., Daubmann A., Volksdorf T., Wladykowski E. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am J Pathol. 2015;185:2777–2789. doi: 10.1016/j.ajpath.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Zheng T., Oh M.H., Oh S.Y., Schroeder J.T., Glick A.B., Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129:742–751. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angkasekwinai P., Park H., Wang Y.H., Wang Y.H., Chang S.H., Corry D.B. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds J.M., Angkasekwinai P., Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M., Dong C. IL-25 in allergic inflammation. Immunol Rev. 2017;278:185–191. doi: 10.1111/imr.12558. [DOI] [PubMed] [Google Scholar]
- 13.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 14.Wong S.H., Walker J.A., Jolin H.E., Drynan L.F., Hams E., Camelo A. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saenz S.A., Taylor B.C., Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hvid M., Vestergaard C., Kemp K., Christensen G.B., Deleuran B., Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 18.Kim B.E., Bin L., Ye Y.M., Ramamoorthy P., Leung D.Y.M. IL-25 enhances HSV-1 replication by inhibiting filaggrin expression, and acts synergistically with Th2 cytokines to enhance HSV-1 replication. J Invest Dermatol. 2013;133:2678–2685. doi: 10.1038/jid.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deleuran M., Hvid M., Kemp K., Christensen G.B., Deleuran B., Vestergaard C. IL-25 induces both inflammation and skin barrier dysfunction in atopic dermatitis. Chem Immunol Allergy. 2012;96:45–49. doi: 10.1159/000331871. [DOI] [PubMed] [Google Scholar]
- 20.Jang Y.H., Choi J.K., Jin M., Choi Y.A., Ryoo Z.Y., Lee H.S. House dust mite increases pro-Th2 cytokines IL-25 and IL-33 via the activation of TLR1/6 signaling. J Invest Dermatol. 2017;137:2354–2361. doi: 10.1016/j.jid.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Kim B.S., Siracusa M.C., Saenz S.A., Noti M., Monticelli L.A., Sonnenberg G.F. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vuyst E., Giltaire S., Lambert de Rouvroit C., Malaisse J., Mound A., Bourtembourg M. Methyl-beta-cyclodextrin concurs with interleukin (IL)-4, IL-13 and IL-25 to induce alterations reminiscent of atopic dermatitis in reconstructed human epidermis. Exp Dermatol. 2018;27:435–437. doi: 10.1111/exd.13113. [DOI] [PubMed] [Google Scholar]
- 23.Xu M., Lu H., Lee Y.H., Wu Y., Liu K., Shi Y. An Interleukin-25-Mediated autoregulatory circuit in keratinocytes plays a pivotal role in psoriatic skin inflammation. Immunity. 2018;48:787–798.e4. doi: 10.1016/j.immuni.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 24.He R., Oyoshi M.K., Garibyan L., Kumar L., Ziegler S.F., Geha R.S. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leyva-Castillo J.M., Hener P., Jiang H., Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133:154–163. doi: 10.1038/jid.2012.239. [DOI] [PubMed] [Google Scholar]
- 26.Savinko T., Matikainen S., Saarialho-Kere U., Lehto M., Wang G., Lehtimaki S. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie G.J., Bancroft A., Grencis R.K., McKenzie A.N. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 28.Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou S.J., Babot Z., Leingartner A., Studer M., Nakagawa Y., O'Leary D.D. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angkasekwinai P., Chang S.H., Thapa M., Watarai H., Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spergel J.M., Mizoguchi E., Brewer J.P., Martin T.R., Bhan A.K., Geha R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon J., Leyva-Castillo J.M., Wang G., Galand C., Oyoshi M.K., Kumar L. IL-23 induced in keratinocytes by endogenous TLR4 ligands polarizes dendritic cells to drive IL-22 responses to skin immunization. J Exp Med. 2016;213:2147–2166. doi: 10.1084/jem.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leisten S., Oyoshi M.K., Galand C., Hornick J.L., Gurish M.F., Geha R.S. Development of skin lesions in filaggrin-deficient mice is dependent on adaptive immunity. J Allergy Clin Immunol. 2013;131:1247–1250. doi: 10.1016/j.jaci.2012.12.1576. 50 e1. [DOI] [PubMed] [Google Scholar]
- 34.He R., Oyoshi M.K., Jin H., Geha R.S. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci U S A. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo K., Nagakubo D., Komori Y., Fujisato S., Takeda N., Kitamatsu M. CCR4 Is critically involved in skin allergic inflammation of BALB/c mice. J Invest Dermatol. 2018;138:1764–1773. doi: 10.1016/j.jid.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Obara W., Kawa Y., Ra C., Nishioka K., Soma Y., Mizoguchi M. T cells and mast cells as a major source of interleukin-13 in atopic dermatitis. Dermatology. 2002;205:11–17. doi: 10.1159/000063145. [DOI] [PubMed] [Google Scholar]
- 37.Spergel J.M., Mizoguchi E., Oettgen H., Bhan A.K., Geha R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y.H., Angkasekwinai P., Lu N., Voo K.S., Arima K., Hanabuchi S. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mearns H., Forbes-Blom E.E., Camberis M., Tang S.C., Kyle R., Harvie M. IL-25 exhibits disparate roles during Th2-cell differentiation versus effector function. Eur J Immunol. 2014;44:1976–1980. doi: 10.1002/eji.201344400. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki M., Bartels J., Mallet A.I., Christophers E., Schroder J.M. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol. 1998;160:60–68. [PubMed] [Google Scholar]
- 41.Klein Wolterink R.G., Kleinjan A., van Nimwegen M., Bergen I., de Bruijn M., Levani Y. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 42.Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mjosberg J.M., Trifari S., Crellin N.K., Peters C.P., van Drunen C.M., Piet B. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 44.Imai Y., Yasuda K., Nagai M., Kusakabe M., Kubo M., Nakanishi K. IL-33-induced atopic dermatitis-like inflammation in mice is mediated by group 2 innate lymphoid cells in concert with basophils. J Invest Dermatol. 2019;139:2185–2194.e3. doi: 10.1016/j.jid.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra N., Leyva-Castillo J.M., Jadhav U., Barreiro O., Kam C., O'Neill N.K. RORalpha-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aao6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Dyken S.J., Nussbaum J.C., Lee J., Molofsky A.B., Liang H.E., Pollack J.L. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerbe F., Sidot E., Smyth D.J., Ohmoto M., Matsumoto I., Dardalhon V. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howitt M.R., Lavoie S., Michaud M., Blum A.M., Tran S.V., Weinstock J.V. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Moltke J., Ji M., Liang H.E., Locksley R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]