Abstract
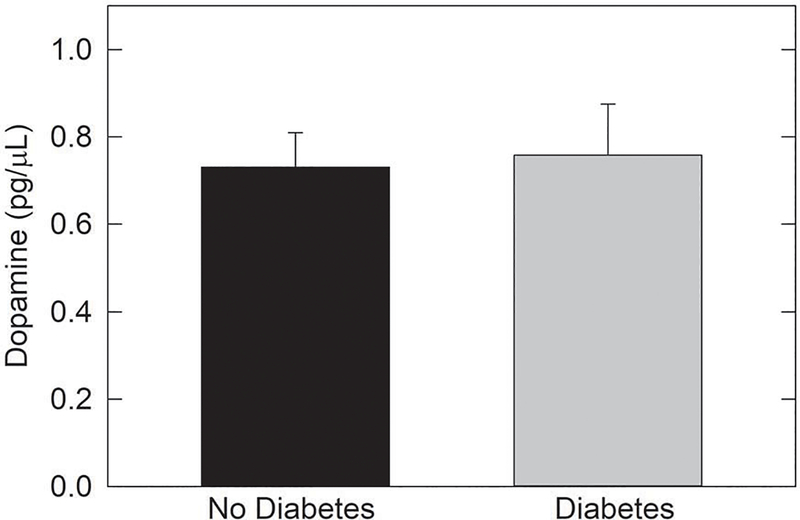
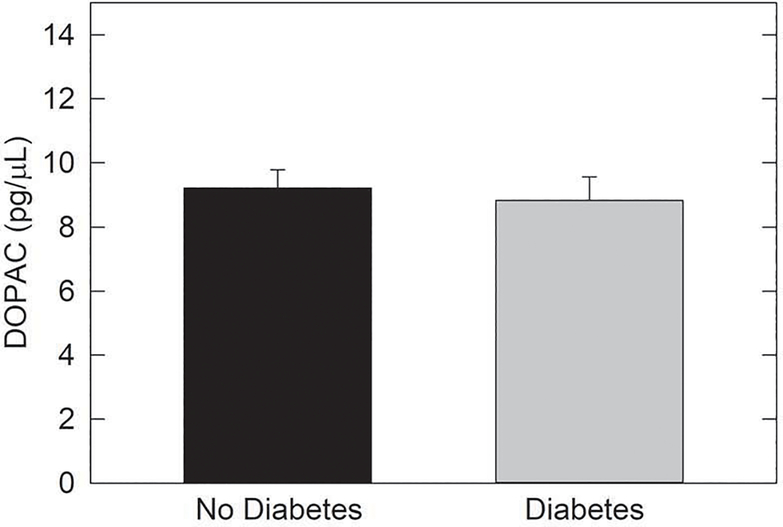
Animal studies suggest that the retinal dysfunction in diabetic subjects that precedes overt clinical vasculopathy may be due to a retinal dopamine deficit. We analyzed levels of dopamine (DA) and its primary metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), in the vitreous of diabetic and non-diabetic human subjects. Adult patients undergoing pars plana vitrectomy for non-hemorrhagic indications were prospectively recruited from the Emory Eye Center in Atlanta, GA. Vitreous samples were analyzed using high performance liquid chromatography (HPLC) to measure levels of DOPAC and DA in the vitreous specimens. Vitreous samples from 9 diabetic patients and 20 from non-diabetic patients were analyzed. No eyes had apparent diabetic retinopathy. Mean normalized DA concentration in vitreous of diabetic subjects was 0.76 ± 0.12 pg/pL vs. 0.73 ± 0.08 pg/pL in non-diabetic vitreous (p=0.849). DOPAC concentration was 8.84 ± 0.74 pg/pL in vitreous of diabetic subjects vs. 9.22 ± 0.56 pg/pL in vitreous of non-diabetic subjects (p=0.691). No difference was observed in the concentrations of DA and DOPAC in the vitreous of people without diabetes compared to those with diabetes without retinopathy.
Keywords: dopamine, diabetes, diabetic retinopathy, vitreous
Short Communication (WC<4000)
Diabetic retinopathy (DR) is a complication of diabetes mellitus and a leading cause of blindness in working age adults; affecting millions of people in the United States (Zhang et al., 2010). Standard treatment for initial stages of diabetic retinopathy involves rigorous management of hyperglycemia (UKPDS, 1998), but at later stages laser photocoagulation, intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors (Gross et al., 2018), and surgery can become necessary. Prevention of blinding complications can be effective under ideal circumstances (Liew et al., 2014), but many patients are unaware or unable to comply with screening and management guidelines (Bressler et al., 2014; Obeid et al., 2019). A broad opportunity exists to treat diabetes and diabetic retinopathy to specifically curtail stages prior to the development of visually threatening disease.
Dopamine (DA) is an endogenous catecholamine neurotransmitter commonly associated with Parkinson’s disease pathophysiology and psychopathology (Brandies and Yehuda, 2008). However, the role of DA in obesity, addiction, circadian rhythms, and diabetes mellitus (DM) has become more evident within the past several years. In fact, bromocriptine, a dopaminergic (D2 receptor) agonist, is FDA approved for treatment of type 2 DM (Gaziano et al., 2010; Gaziano et al., 2012).
Abnormalities in dopaminergic neurotransmission in the retina may have an association with the pathogenesis of DR, but this relationship has not been investigated previously in humans. In diabetic rats and mice, DA levels are reduced in the retina 1 month after DM induction (Nishimura and Kuriyama, 1985). In a mouse model of type 1 DM where DA is reduced, treatment with 3,4-L-dihydroxyphenylalanine (L-DOPA, a DA precursor) improved metrics of visual function using electroretinography and the optomotor reflex (Aung et al., 2014). Dopamine and its metabolites may serve as a biomarker for DR and may play a role in its pathogenesis, and thus present a novel pathway for treatment at earlier stages than in current use. This study compares vitreous levels of dopamine in people with and without type II diabetes.
This study was approved by the Emory University Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. All patients provided signed informed consent prior to participation in the study. Subjects were 18 years of age or older and already consented to undergo pars plana vitrectomy for non-hemorrhagic indications for surgery such as macular holes and epiretinal membranes. Subjects were excluded if vitreous hemorrhage was present. Subjects were also excluded with a history of psychosis, Parkinson’s disease, or with use of medications that may alter systemic dopamine levels (e.g., bromocriptine, levodopa, antipsychotic medications). A total of 31 individuals were recruited, 9 with diabetes and 21 without. One sample of the non-diabetic cohort was excluded because the dopamine and DHBA (internal standard) peaks were undetectable and DOPAC levels were extremely high (~2 times the group mean). At the start of each case, an undiluted sample of vitreous was obtained by their surgeons and samples were immediately frozen on dry ice. Samples were analyzed for dopamine and a metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), using HPLC with electrochemical detection (Stone et al., 2016). Statistical analysis was performed using Microsoft Excel (Office 365; Microsoft Corp.) and Sigma Stat 3.5 (Aspire Software International). Results were normalized to volume of vitreous sample and compared using unpaired Student’s t-test to analyze for between group differences per metabolite in vitreous specimens. All analyses were performed with a priori significance set at a<0.05.
Thirty samples were analyzed, 9 from eyes of people with diabetes and 21 from non-diabetic eyes. Individuals with diabetes did not have clinically detectable diabetic retinopathy. The average sample was 0.52 grams of undiluted vitreous. Mean normalized DA concentration in diabetic vitreous was 0.76 pg/pL (SEM 0.12) vs. 0.73 pg/pL (SEM 0.08) in non-diabetic vitreous (p=0.85). DOPAC concentration was 8.84 pg/pL (SEM 0.74) in diabetic vitreous vs. 9.22 pg/pL (SEM 0.56) in non-diabetic vitreous (p=0.69). Results are presented in Figures 1 and 2.
Figure 1.
Dopamine levels in vitreous of human subjects with or without type II
DM. The levels of dopamine were compared in vitreous from type II DM subjects without DR and vitreous of subjects without diabetes. No diffence was found (t27=− 0.192, p=0.849; N=20 non-DM samples and N=9 DM samples).
Figure 2.
DOPAC levels in vitreous of human subjects with or without type II DM.
The levels of dopamine were compared in vitreous from type II DM subjects without DR and vitreous of subjects without diabetes. No diffence was found (t27=0.401, p=0.691; N=20 non-DM samples and N=9 DM samples).
This study did not detect a difference in the vitreous levels of DA and DOPAC in people with and without type II diabetes. Low dopamine levels are associated with development of diabetic retinopathy in rodent models of type 1 diabetes (Akimov and Renteria, 2012; Aung et al., 2013; Aung et al., 2014; Kirwin et al., 2011; Muir et al., 2012). A literature search did not reveal prior published research on this topic in humans.
Dopamine may play an early and even causative role in some of the visual defects associated with diabetes, such as color vision abnormalities, impaired contrast sensitivity (Jackson and Barber, 2010), and ERG abnormalities (Ghirlanda et al., 1997; Shirao and Kawasaki, 1998). These consequences are likely time-dependent but occur prior to manifestations of overt clinical diabetic retinopathy. It is possible that our study did not find a difference in vitreous levels of dopamine due to testing before dopamine abnormalities occur, as none of our subjects had diabetic retinopathy. Perhaps differences exist in the pathophysiology in rodent models of type 1 diabetes that are dissimilar to human type 2 diabetes. Further study will be necessary to test these possibilities.
Much interest exists in reducing blinding consequences of diabetes at earlier stages. At the low levels of diabetic retinopathy severity, the current practice involves systemic optimization. Fenofibrate has not been widely adopted despite demonstrating effectively reduced progression of retinopathy severity (Chew et al., 2014; Keech et al., 2007). Trials are underway to evaluate the effectiveness of serial anti-VEGF injections in patients with categorically severe (ETDRS level 43/57) non-proliferative diabetic retinopathy (ClinicalTrials.gov Identifier: NCT02718326, NCT02634333). The dopaminergic pathway is a compelling consideration for treatment and further study is necessary to determine optimal conditions for the therapeutic potential.
Highlights.
Animal models of diabetes mellitus (DM) indicate that deficits in retinal dopamine precede vascular dysfunction and contribute to compromised visual function.
Levels of dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) in vitreous of human subjects were analyzed.
No statistically significant differences in dopamine or DOPAC levels were found between samples from type II DM subjects and non-diabetic subjects.
Acknowledgments
Funding: This project was supported by an unrestricted departmental grant from Research to Prevent Blindness to the Emory Eye Center; and grants from the National Institutes of Health P30 EY006360, R01 EY004864 and R01 EY027711. The sponsors had no role in the design or conduct of this research.
Abbreviations
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- L-DOPA
3,4-L- dihydroxyphenylalanine
- HPLC
high performance liquid chromatography
- DM
diabetes mellitus
- DR
diabetic retinopathy
- OU
both eyes
- OD
right eye
- OS
left eye
Footnotes
A preliminary report of this study was presented at the Association for Research in Vision and Ophthalmology (ARVO) meeting in May of 2018
No conflicting interest exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimov NP, Renteria RC, 2012. Spatial frequency threshold and contrast sensitivity of an optomotor behavior are impaired in the Ins2Akita mouse model of diabetes. Behav Brain Res 226, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MH, Kim MK, Olson DE, Thule PM, Pardue MT, 2013. Early visual deficits in streptozotocin-induced diabetic long evans rats. Investigative ophthalmology & visual science 54, 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, luvone PM, Pardue MT, 2014. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci 34, 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandies R, Yehuda S, 2008. The possible role of retinal dopaminergic system in visual performance. Neurosci Biobehav Rev 32, 611–656. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Varma R, Doan QV, Gleeson M, Danese M, Bower JK, Selvin E, Dolan C, Fine J, Colman S, Turpcu A, 2014. Underuse of the health care system by persons with diabetes mellitus and diabetic macular edema in the United States. JAMA ophthalmology 132, 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT, Action to Control Cardiovascular Risk in Diabetes Eye Study Research, G., 2014. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 121, 2443–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Cincotta AH, O’Connor CM, Ezrokhi M, Rutty D, Ma ZJ, Scranton RE, 2010. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes care 33, 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R, 2012. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 1, e002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda G, Di Leo MA, Caputo S, Cercone S, Greco AV, 1997. From functional to microvascular abnormalities in early diabetic retinopathy. Diabetes Metab Rev 13, 15–35. [DOI] [PubMed] [Google Scholar]
- Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL 3rd, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW, Diabetic Retinopathy Clinical Research, N., 2018. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA ophthalmology 136, 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group UKPDS, 1998. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317, 703–713. [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Barber AJ, 2010. Visual dysfunction associated with diabetic retinopathy. Current diabetes reports 10, 380–384. [DOI] [PubMed] [Google Scholar]
- Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG, 2007. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370, 1687–1697. [DOI] [PubMed] [Google Scholar]
- Kirwin SJ, Kanaly ST, Hansen CR, Cairns BJ, Ren M, Edelman JL, 2011. Retinal gene expression and visually evoked behavior in diabetic long evans rats. Investigative ophthalmology & visual science 52, 7654–7663. [DOI] [PubMed] [Google Scholar]
- Liew G, Michaelides M, Bunce C, 2014. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 4, e004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir ER, Rentena RC, Duong TQ, 2012. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Investigative ophthalmology & visual science 53, 6488–6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C, Kuriyama K, 1985. Alterations in the retinal dopaminergic neuronal system in rats with streptozotocin-induced diabetes. Journal of neurochemistry 45, 448–455. [DOI] [PubMed] [Google Scholar]
- Obeid A, Su D, Patel SN, Uhr JH, Borkar D, Gao X, Fineman MS, Regillo CD, Maguire JI, Garg SJ, Hsu J, 2019. Outcomes of Eyes Lost to Follow-up with Proliferative Diabetic Retinopathy That Received Panretinal Photocoagulation versus Intravitreal Anti-Vascular Endothelial Growth Factor. Ophthalmology 126, 407–413. [DOI] [PubMed] [Google Scholar]
- Shirao Y, Kawasaki K, 1998. Electrical responses from diabetic retina. Progress in retinal and eye research 17, 59–76. [DOI] [PubMed] [Google Scholar]
- Stone RA, Cohen Y, McGlinn AM, Davison S, Casavant S, Shaffer J, Khurana TS, Pardue MT, Iuvone PM, 2016. Development of Experimental Myopia in Chicks in a Natural Environment. Investigative ophthalmology & visual science 57, 4779–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R, 2010. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 304, 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]