Capsule Summary:
Serum IL-6 was associated with asthma exacerbation risk but not with symptoms or lung function in urban children; further studies to evaluate the role of IL-6 in the life course of asthma are needed.
Keywords: Asthma, children, IL-6, exacerbations, obesity
To the Editor:
Peripheral blood IL-6 was recently identified as a potential biomarker in adult asthma. Peters et al. associated systemic inflammation, as demonstrated by elevated IL-6 levels, with other markers of metabolic dysfunction and greater asthma severity in both lean and obese adults in the Severe Asthma Research Program.(1) Individuals with elevated IL-6 levels had both reduced lung function and enhanced exacerbation risk. Further, epithelial IL-6 trans-signaling was recently identified as a potential mechanism underlying a phenotype of asthma with enhanced airway inflammation.(2) However, IL-6 has not been evaluated as a biomarker in children with asthma, and it is currently unknown whether systemic inflammation and metabolic dysfunction play a role in asthma severity and exacerbation risk in children and adolescents.
The Asthma Phenotypes in the Inner City (APIC) study, performed within the NIH/NIAID-sponsored Inner City Asthma Consortium, enrolled 717 participants 6–17 years of age and managed their asthma for 1 year according to a guideline-based algorithm as previously described.(3) The overall goal of APIC was to identify factors that differentiated difficult-to-control vs. easy-to-control asthma in urban children. A total of 702 children had baseline serum samples available for IL-6 measurement and were included in this post hoc analysis. Serum IL-6 was measured with a high sensitivity assay with a lower limit of detection of 0.18 pg/ml (Millipore Milliplex MAP kit HSTCMAG-28SK). To compare the relationships of serum IL-6 with demographics and other baseline blood biomarkers, Kruskal Wallis Rank Test and trend test were used for categorical variables, and Pearson correlations were used for continuous variables. Asthma characteristics and outcomes were estimated by mixed models for longitudinal data, odds ratio and rate ratio for binary and count exacerbation outcomes respectively.
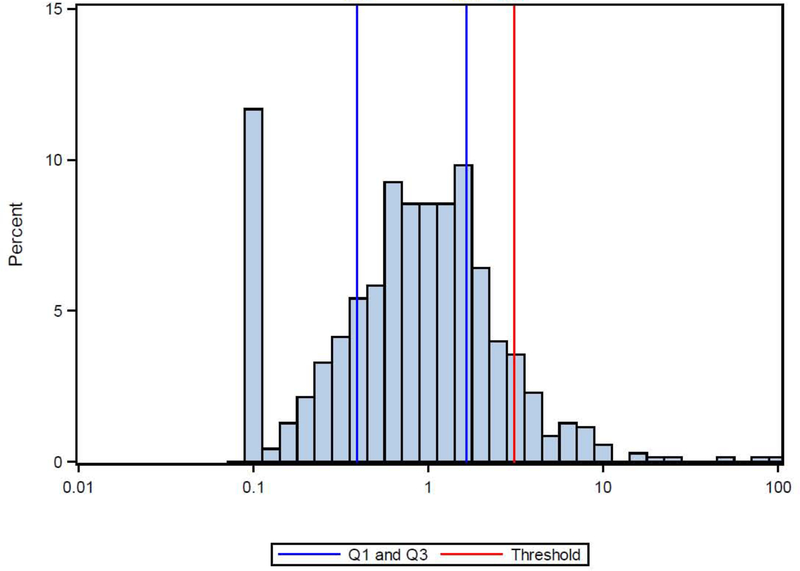
The distribution of serum IL-6 levels is displayed in Figure E1. The median value was 0.85 pg/ml (IQR 0.39–1.65 pg/ml). A relatively small proportion of children (8.4%) had IL-6 levels above the cut-off for “IL-6 high” of 3.1 pg/ml identified by Peters et al in adults. Considering this and the lack of normative IL-6 values for children, we evaluated IL-6 as a continuous variable rather than a dichotomous (high/low) variable. Serum IL-6 significantly increased with age and BMI percentile (Table E1). IL-6 levels were also significantly higher in females versus males and significantly lower in black children versus other study participants (Table E1).
We examined associations between serum IL-6 and other baseline laboratory measures (Table 1). IL-6 was significantly correlated with total white blood cells (R 0.18, 95% confidence interval (CI) (0.11, 0.25); p<0.01), total blood neutrophils (0.20 95% CI (0.12, 0.27); p<0.01), and serum C-reactive protein (CRP) (0.10 95% CI (0.03, 0.18); p<0.01). In contrast, IL-6 was not associated with any markers of type-2 inflammation, including total blood eosinophils, total IgE, or number of allergen sensitizations (Table 1).
Table 1.
Associations of Serum IL-6 and Other Biomarkers at Baseline
| Characteristic | N | Correlation (95% CI) | P-value | Correlation (95% CI) | P-value |
|---|---|---|---|---|---|
| Total White Blood Cell | 692 | 0.18 (0.11, 0.25) | <0.01 | 0.14 (0.07, 0.22) | <0.01 |
| Total Monocyte - log10 | 691 | −0.01 (−0.09, 0.06) | 0.71 | −0.01 (−0.09, 0.06) | 0.74 |
| Total Neutrophils - per ul (log10) | 690 | 0.20 (0.12, 0.27) | <0.01 | 0.14 (0.07, 0.22) | <0.01 |
| Total Eosinophils - per ul (log10) | 690 | −0.01 (−0.08, 0.07) | 0.85 | 0.01 (−0.06, 0.09) | 0.71 |
| Total IgE (kUA/L) - log10 | 694 | 0.01 (−0.06, 0.09) | 0.71 | 0.02 (−0.05, 0.09) | 0.60 |
| CRP ng/mL - log10 | 699 | 0.10 (0.03, 0.18) | <0.01 | 0.06 (−0.02, 0.13) | 0.14 |
| Blood pressure - Systolic | 697 | 0.05 (−0.03, 0.12) | 0.21 | −0.01 (−0.08, 0.07) | 0.87 |
| Blood pressure - Diastolic | 697 | 0.06 (−0.02, 0.13) | 0.13 | 0.04 (−0.04, 0.11) | 0.35 |
| Number of Allergen Sensitizations | 699 | −0.01 (−0.08, 0.07) | 0.82 | 0.01 (−0.07, 0.08) | 0.87 |
Pearson Correlation
Models adjusted for gender, race, age, and BMI
While the observed associations of IL-6 with BMI percentiles and markers of metabolic dysfunction were similar to that seen previously in adults, the relationships between IL-6 and measures of asthma severity were not. At study baseline, IL-6 was not significantly associated with the Composite Asthma Severity Index (CASI),(4) asthma treatment step, symptom days in the past 14 days, lung function, or exhaled nitric oxide (Table 2). Similarly, during the one-year longitudinal characterization period when study participants were managed by standardized guidelines-based asthma and rhinitis management, baseline IL-6 was not associated with these same measures of asthma severity and control or with previously described asthma phenotypes.(5) However, a significant association was observed between baseline IL-6 and the probability of experiencing an asthma exacerbation treated with systemic corticosteroids during the 1-year study. The odds of experiencing at least 1 exacerbation during the study increased by 24% for each quartile increase in serum IL-6 (unadjusted Odds Ratio (OR) 1.24 (95% CI 1.02, 1.51), p=0.03; adjusted OR 1.32 (1.08, 1.62), p<0.01). A similar significant relationship was observed for number of exacerbations during the study (adjusted Risk Ratio 1.27 (1.07, 1.50), p<0.01; Table 2). After adjusting for other asthma exacerbation risk factors, baseline IL-6 remained a significant predictor of subsequent asthma exacerbation risk (Table E2).
Table 2.
Associations Between Serum IL-6 and Asthma Characteristics
| Unadjusted | Adjusted‡ | ||||
|---|---|---|---|---|---|
| Characteristic | N | Statistic (95% CI) | P-value | Statistic (95% CI) | P-value |
| Cross-sectional Estimate | |||||
| CASI Score (0–20) at Screening | 698 | 0.15 (−0.15, 0.44) | 0.32 | 0.17 (−0.13, 0.47) | 0.27 |
| Asthma Treatment Step at Screening | 699 | 0.03 (−0.15, 0.21) | 0.78 | 0.01 (−0.18, 0.19) | 0.92 |
| Max Symptom Days in last 14 Days at Screening | 699 | 0.30 (−0.07, 0.68) | 0.11 | 0.31 (−0.07, 0.70) | 0.11 |
| FEV1 (% predicted) at Screening | 699 | −0.04 (−1.63, 1.54) | 0.96 | −0.31 (−1.93, 1.30) | 0.70 |
| FEV1/FVC at Screening | 688 | 0.10 (−0.80, 1.00) | 0.82 | 0.14 (−0.76, 1.04) | 0.76 |
| FEV1 Bronchodilator Response | 689 | −0.87 (−2.17, 0.42) | 0.19 | −0.64 (−1.96, 0.68) | 0.34 |
| eNO (ppb) at Baseline - log10 | 641 | 0.02 (−0.01, 0.05) | 0.24 | 0.02 (−0.01, 0.05) | 0.28 |
| Longitudinal Estimate | |||||
| CASI Score (0–20) | 699 | 0.02 (−0.16, 0.20) | 0.80 | 0.06 (−0.12, 0.24) | 0.50 |
| Asthma Treatment Step | 699 | −0.01 (−0.18, 0.16) | 0.94 | 0.00 (−0.17, 0.18) | 0.96 |
| Max Symptom Days in last 14 Days | 699 | 0.01 (−0.13, 0.15) | 0.89 | −0.00 (−0.15, 0.14) | 0.98 |
| FEV1 (% predicted) | 699 | 0.22 (−0.91, 1.35) | 0.71 | −0.04 (−1.18, 1.10) | 0.94 |
| FEV1/FVC | 698 | 0.31 (−0.33, 0.95) | 0.34 | 0.23 (−0.40, 0.86) | 0.48 |
| eNO (ppb) - log10 | 681 | 0.01 (−0.02, 0.04) | 0.43 | 0.00 (−0.02, 0.03) | 0.75 |
| Odds Ratio | |||||
| Exacerbation between Baseline and end of study* | 699 | 1.24 (1.02, 1.51) | 0.03 | 1.32 (1.08, 1.62) | <0.01 |
| Asthma Treatment Class (Difficult vs. Easy)† | 473 | 0.97 (0.78, 1.22) | 0.81 | 0.97 (0.77, 1.23) | 0.81 |
| Rate Ratio | |||||
| Exacerbation Count between Baseline and end of study | 699 | 1.22 (1.04, 1.44) | 0.02 | 1.27 (1.07, 1.50) | <0.01 |
The probability of having at least one exacerbation increases significantly with one interquartile range increase in IL-6
The probability of being in the Easy-to-Control Asthma group decreases with an increase in IL-6
Models adjusted for gender, race, age, and BMI. Visit was additionally adjusted for the longitudinal model
In this study of urban children with persistent asthma over a wide range of disease severity, we observed significant correlations between serum IL-6 and BMI and markers of metabolic dysfunction similar to what has previously been reported in adults. However, despite the very large proportion of our study population with obesity (BMI >95%ile), we observed fewer asthmatic children with IL-6 levels >3.1 pg/ml compared to what was previously reported for asthmatic adults.(1) Furthermore, we did not observe associations between IL-6 and markers of asthma severity such as the CASI, lung function, and symptoms. Interestingly, we did observe an increased risk of asthma exacerbations, a major source of morbidity and mortality, as serum IL-6 levels increased.
These findings suggest differential impact of systemic inflammation, as reflected by serum IL-6 levels, on asthma in children/adolescents versus adults. It is interesting that IL-6 was associated with greater exacerbation risk in children, but not lung function or other markers of severity as previously reported in adults. Given the association between recurrent exacerbations and loss of lung function over time,(6, 7) it is interesting to speculate that the children with higher serum IL-6 and more frequent exacerbations could eventually develop the more severe phenotype seen in adults with high IL-6 levels.
IL-6 was not associated with any markers of type 2 inflammation in our study, but was independently associated with increased exacerbation risk regardless of T2 biomarker status in the cohort. Further studies are needed to identify mechanisms that may link systemic inflammation as assessed by serum IL-6 and propensity to asthma exacerbation.
Strengths of our study include a large sample of very well characterized children managed with a standardized, guideline-based approach. Furthermore, APIC included primarily minority children who are often underrepresented in clinical studies. Limitations include lack of normative IL-6 values in children, utilization of serum rather than plasma and a different assay for the measurement of IL-6 compared to the study by Peters et al.(1), making direct comparisons with the adult data difficult. By analyzing relationships with IL-6 as a continuous variable, we confirmed the expected relationships between IL-6 and measures of metabolic dysfunction, suggesting this was a valid approach.
In summary, in contrast to prior work in adults with asthma, serum IL-6 levels were not associated with measures of disease severity or control such as symptoms and lung function. However, we observed a significant association between IL-6 and risk of asthma exacerbations. Further studies such as interventional trials with anti-IL6 therapy are needed to define the potential role of IL-6 and systemic inflammation in the life course of asthma.
Acknowledgments
Disclosure Statement:
Dr. Jackson reports receiving grants from NIH and GlaxoSmithKline, personal fees for DSMB from Pfizer and for consulting from Novartis, Sanofi-Regeneron, Vifor Pharma and Astra Zeneca.
Dr. Bacharier reports personal fees from GlaxoSmithKline, Genentech/Novartis, Merck, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura, and Circassia.
Dr. Wood receives grant support from the NIH, Astellas, Aimmune, DBV, Sanofi, and Regeneron, and royalties from Up To Date
Dr. Busse reports grants from NIH-NIAID, during the conduct of the study; personal fees from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Novartis, personal fees from Sanofi, personal fees from Boston Scientific, personal fees from Regeneron, personal fees from Arrowhead, personal fees from resTORbio, personal fees from Medscape, personal fees from Gossamer Bio, personal fees from Genentech, personal fees from Elsevier, outside the submitted work
A. Calatroni, Dr. Gill, J. Hu, Dr. Liu, Dr. Wheatley, Dr. Gern, Dr. Gruchalla, Dr. Hershey, Dr. Kattan, Dr. Kercsmar, Dr. Kim, Dr. O’Connor, Dr. Patel, and Dr. Pongracic report no conflicts of interest
Figure E1.
Distribution of serum IL6 (N=702) with 25th and 75th percentiles (blue lines) and threshold for high and low IL6 (red line).0.18 pg/ml is the lower limit of detection.
Table E1.
Serum IL-6 and Baseline Demographics
| Characteristic | Overall | N | Median [Q1, Q3] | P-value |
|---|---|---|---|---|
| Age† | 6–8 | 206 | 0.67 [0.26, 1.29] | <0.01 |
| 9–11 | 218 | 0.97 [0.43, 1.70] | ||
| 12–14 | 181 | 0.97 [0.46, 1.83] | ||
| 15–17 | 97 | 0.87 [0.46, 1.73] | ||
| Gender* | Male | 404 | 0.75 [0.36, 1.56] | 0.03 |
| Female | 298 | 0.94 [0.43, 1.75] | ||
| Race* | Black | 451 | 0.75 [0.33, 1.59] | <0.01 |
| Other | 248 | 1.05 [0.49, 1.69] | ||
| BMI† | <85 | 345 | 0.75 [0.34, 1.44] | <0.01 |
| 85–95 | 116 | 0.83 [0.33, 1.83] | ||
| >95 | 241 | 1.04 [0.49, 1.73] | ||
Kruskal Wallis Rank test
Trend test
Table E2.
Multivariable Models Assessing Associations Between IL-6 and Risk of Exacerbation and Exacerbation Count Adjusting for other Risk Factors for Exacerbation
| Exacerbation* | Exacerbation Count | |||
|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | P-value | Rate Ratio (95% CI) | P-value |
| IL-6 | 1.24 (1.02, 1.51) | 0.03 | 1.21 (1.02, 1.43) | 0.03 |
| IL6+Demographics(age, BMI, gender, and race) | 1.31 (1.07, 1.61) | 0.01 | 1.24 (1.08, 1.43) | <0.01 |
| IL6+Demographics+WBC+Neutrophil+CRP | 1.26 (1.02, 1.55) | 0.03 | 1.20 (1.03, 1.39) | 0.02 |
| IL6+Demographics+WBC+Neutrophil+CRP+Total IgE | 1.26 (1.02, 1.55) | 0.03 | 1.17 (1.01, 1.35) | 0.03 |
| IL6+Demographics+WBC+Neutrophil+CRP+Total IgE+Eosinophil | 1.26 (1.02, 1.55) | 0.03 | 1.17 (1.01, 1.35) | 0.03 |
| Stepwise model selection | 1.31 (1.07, 1.61) | <0.01† | 1.21 (1.02, 1.44) | 0.03‡ |
The probability of having at least one exacerbation
Age, Total IgE, and IL-6 were used in the model
Age, Neutrophil, Total IgE, and IL-6 were used in the model
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel J Jackson, University of Wisconsin School of Medicine and Public Health.
Leonard B Bacharier, Washington University School of Medicine.
Agustin Calatroni, Rho Inc..
Michelle A Gill, University of Texas Southwestern Medical Center.
Jack Hu, Rho Inc..
Andrew H Liu, Children’s Hospital of Colorado and University of Colorado School of Medicine.
Lisa M Wheatley, National Institute of Allergy and Infectious Diseases.
James E Gern, University of Wisconsin School of Medicine and Public Health.
Rebecca S Gruchalla, University of Texas Southwestern Medical Center.
Gurjit K Khurana Hershey, Cincinnati Children’s Hospital.
Meyer Kattan, Columbia University College of Physicians and Surgeons.
Carolyn M Kercsmar, Cincinnati Children’s Hospital.
Haejin Kim, Henry Ford Health System.
George T O’Connor, Boston University School of Medicine.
Shilpa Patel, Children’s National Health System.
Jacqueline A Pongracic, Ann Robert H Lurie Children’s Hospital of Chicago.
Robert A Wood, Johns Hopkins University School of Medicine.
William W Busse, University of Wisconsin School of Medicine and Public Health.
References
- 1.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. The Lancet Respiratory medicine. 2016;4(7):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jevnikar Z, Ostling J, Ax E, Calven J, Thorn K, Israelsson E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016;138(4):1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index-an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129(3):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138(4):1016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. [DOI] [PubMed] [Google Scholar]
- 7.Ortega H, Yancey SW, Keene ON, Gunsoy NB, Albers FC, Howarth PH. Asthma Exacerbations Associated with Lung Function Decline in Patients with Severe Eosinophilic Asthma. The journal of allergy and clinical immunology in practice. 2018. [DOI] [PubMed] [Google Scholar]