Abstract
Introduction:
We evaluated visual acuity (VA) over 5 years in a subspecialty non-infectious uveitis population.
Methods:
Retrospective data from 5,530 non-infectious uveitis patients with anterior, intermediate, posterior or panuveitis were abstracted by expert reviewers. Mean VA was calculated using inverse probability of censoring weighting to account for losses to follow-up.
Results:
Patients were a median of 41 years old, 65% female, and 73% white. Initial mean VA was worse among panuveitis (20/84) than posterior (20/64), intermediate (20/47), and anterior (20/37) uveitides. On average, mean VA improved by 0.62, 0.51, 0.37, and 0.26 logMAR-equivalent lines over 2 years, respectively (each P<0.001), then remained stable, except posterior uveitis mean VA worsened to initial levels.
Conclusion:
Mean VA of uveitic eyes improved and, typically, improvement was sustained under uveitis subspecialty care. Because VA tends to improve under tertiary care, mean VA change appears a better outcome for clinical studies than time-to-loss of VA.
Keywords: Visual Acuity, Uveitis, Inverse Probability of Censoring Weighting
Introduction
Non-infectious uveitis has been estimated to be the 6th leading cause of worldwide blindness1,2. Unlike diseases of the elderly (such as age-related macular degeneration, glaucoma and cataract), uveitis most commonly presents during mid-adulthood with a correspondingly higher potential quality of life and economic impact over time3. As a complex, chronic condition, uveitis also requires a high health professional and patient effort per case with an average of 6 visits per year4.
Uveitis is classified into four broad categories (Anterior, Intermediate, Posterior and Panuveitis) using International Uveitis Study Group (IUSG)/Standardization of Uveitis Nomenclature criteria5. While the group with predominantly anterior involvement represents the majority of cases, the other—generally more severe—types represent about half of uveitis subspecialty center referrals6. Clarification of the expected visual outcome of these groups of patients is needed, in order to better counsel patients and plan for clinical research studies.
In order to better understand the visual outcome of cases of uveitis receiving tertiary care, taking into account both positive and negative changes in visual acuity (VA) over time, here we evaluate the VA outcomes for a large cohort of uveitis cases receiving tertiary care in the United States.
Materials and Methods
The Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study is a large, retrospective cohort study of patients with noninfectious ocular inflammation seen at 5 tertiary centers in the United States between 1978 and 2007. Institutional review board approval for the SITE Cohort Study was obtained and maintained at each center’s governing institutional review board before and throughout the period of data collection. Each institution’s institutional review board approved a waiver of consent and a Health Insurance Portability and Accountability Act exemption for this study because it entailed retrospective chart review. The project was conducted in adherence with the principles of the Declaration of Helsinki and relevant federal and state laws in the United States.
Study Population
The methods of the SITE Cohort Study previously have been described in detail7. One site frequently used a co-management approach wherein most visits were conducted by collaborating physicians when patients were doing well, making estimation of censoring likelihood impracticable, so that center was excluded from this analysis. Patients known to have HIV infection or primarily infectious uveitis had been excluded from the parent cohort. Only eyes with uveitis were included in this analysis, as opposed to healthy eyes of the same patient or eyes with other non-infectious ocular inflammatory diagnoses.
Data Collection
Data collection for the SITE Cohort Study was done by trained, certified, expert reviewers. All available medical records were abstracted for each eye of each patient at every visit, including detailed information on ocular characteristics based on clinical ophthalmological evaluation. Inflammatory disease activity was assessed in a manner similar to the recommendations of the Standardization of Uveitis Nomenclature expert panel5. Sequelae of ocular inflammation were noted when present.
For this analysis, all visits missing visual acuity were excluded, and all missing covariates were carried forward from their last prior observation. Covariates missing in more than 20% of eyes at baseline were not evaluated; for covariates missing in >0% to 20% of eyes a “missing” category was used in order to avoid excluding large numbers of eyes from the analysis. Uveitis that was both intermediate and anterior5 was counted as intermediate uveitis. Eyes missing any other baseline covariates were excluded from the analysis. Visits of eyes within 30 days post-intraocular surgery also were excluded to avoid expected early postoperative fluctuations in visual acuity.
Main Outcome Measure
Visual acuity (VA) had been entered as numerator/denominator when measured using a visual acuity chart, with the method of measurement (with or without correction, pinhole, or unknown) noted. Each 0.1 increase in logarithm of the Minimum Angle of Resolution (logMAR) corresponds to 1 line of VA loss on a logarithmic visual acuity chart (equivalent to 5 letters on an Early Treatment of Diabetic Retinopathy chart8). Our results are summarized either referring to Snellen equivalent values for mean estimates, or logarithmic-equivalent VA chart lines for mean difference estimates. The logMAR was calculated as −log10(VA fraction), up to a value of 2.0 for visual acuity values of 2/200. All eyes with VA measured using a chart but less than 2/200 were assigned a value of 2.1. In order to avoid excluding visits with very poor vision, eyes where no visual acuity fraction was recorded were assigned logMAR values less than 2/200 using a previously described convention: counting fingers (CF, logMAR=2.2), hand motion (HM, 2.3), light perception (LP, 2.4), or no light perception (NLP, 2.5)9.
Visits with VA measured with pinhole or unknown method, and eyes measured this way at baseline, were excluded to avoid biases, because pinhole visual acuity represents potential VA rather than actual VA. The first chart-derived measurement (with or without correction) was included and subsequent measurements without correction were excluded if the first visit’s measurement had been with correction, and vice versa, to avoid counting changes due to use or non-use of refractive correction. All visits with VA of CF or worse were included, given that refractive correction is not expected to affect VA of such low levels.
In order to allow for non-linear changes in VA over time in the regression, visits after baseline were binned. Visits within each bin were represented as repeated observations at a single time point in regressions. With this approach, the average of the all the visits within the time interval bin from one patient exerted the same influence on the mean result as a single visit from another patient where only one visit occurred during the bin interval, thus avoiding undue influence from unusual cases seen very frequently. The bins used were: the initial visit; “Month 1” (all visits after baseline though ≤3 months); “Month 6” (>3 through ≤9 months), and so on through months 12, 18, 24, 30, and 36. The “Month 48” bin consisted of visits from after month 39 through month 54 inclusive, and the “Month 60” consisted of visits from >54 months through ≤66 months. Due to methodological constraints in estimating the likelihood of censoring, visits from any given bin were included only if at least one visit had occurred in every previous bin; follow-up beyond “Month 60” was excluded as the number with ongoing follow-up beyond that point became sparse.
Statistical Analysis
To show the inadequacy with which gain or loss of 10 letters of VA demonstrates actual VA over time, Kaplan-Meier (KM) curves and time-updated proportions were calculated both for the SITE data and for the data provided by Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study10. The MUST Trial was a comparison of systemic corticosteroids plus immunosuppression when indicated versus fluocinolone acetonide implant for 479 eyes with noninfectious intermediate, posterior, or panuveitis10–13. Because MUST used gold standard methods for assessing best-corrected visual acuity at every visit, this comparison also provides a check on the validity of VA measurements for the SITE Cohort Study. Proportions were calculated daily with values imputed linearly per-patient in between visits.
Preliminary inspection of the data indicated that eyes with initially worse VA were more likely than eyes with initially better VA to continue follow-up over time, and that the mean VA difference was maintained over time, therefore skewing the population mean VA of later visits towards the poorer end of the VA spectrum. To overcome informative censoring bias when estimating VA outcomes over time, observations were weighted by their stabilized inverse probability of censoring14. These weights were calculated by estimating the likelihood of an eye being censored after each visit via logistic regression of all potential predictive factors studied, log(1+follow-up time [not binned]), and the total time (not log) since the last visit. (Predictive factors studied are listed in the online supplement, modeled as time-invariant factors, and baseline plus time-varying versions for time-varying factors, as described by Hernán et al15). Taking this approach, a linear mixed model of VA by site of uveitis, binned time, and their interaction—with weights, random intercepts and accounting for the non-independence of eyes of the same patient—was used to produce mean VA estimates for each type of uveitis over time. P-values and 95% confidence intervals were calculated using the standard deviation of the mean estimate bootstrapped 1000 times.
Results
Eligibility criteria were met for 8,799 eyes (of 5,530 patients) at the four participating centers, which were used to inform inverse probability weighting to model censoring, which occurred in 3,897 eyes that had no follow-up after the initial visit, and an additional 1,208 that had no usable follow-up visits (either VA was measured with a different refractive state than initially or there were no visits within the first 3 months). The remaining 3,694 eyes (of 2,698 patients) contributed to follow-up a total of 37,075 visits (median 5, interquartile range [IQR] 3 to 12). The median follow-up was 183 days (IQR 49 to 613), corresponding to Month 6 (IQR month 1 to 18).
The Table lists the frequency of person- and eye-level characteristics at baseline; for time-varying covariates, frequencies subsequently varied over time. Median age was 41 years (IQR 28 to 53), the most common racial/ethnic groups were white (73%) and black (15%), 65% were female, and there were more non-smokers than smokers (never 58%, current 17%, past 11%, missing 14%).
Table 1:
Characteristics of uveitic eyes (E) at entry into the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study.
| Characteristic | Anterior E=4525 |
Intermediate E=1658 |
Posterior E=1605 |
Panuveitis E=1011 |
|
|---|---|---|---|---|---|
| Age | <18 | 603 (13%) | 295 (18%) | 84 (5%) | 122 (12%) |
| 18–25 | 287 (6%) | 206 (12%) | 124 (8%) | 103 (10%) | |
| 26–35 | 741 (16%) | 375 (23%) | 295 (18%) | 185 (18%) | |
| 36–45 | 981 (22%) | 339 (20%) | 346 (22%) | 212 (21%) | |
| 46–55 | 929 (21%) | 228 (14%) | 352 (22%) | 164 (16%) | |
| 56–65 | 570 (13%) | 131 (8%) | 207 (13%) | 100 (10%) | |
| >65 | 414 (9%) | 84 (5%) | 197 (12%) | 125 (12%) | |
| Gender | Male | 1544 (34%) | 613 (37%) | 623 (39%) | 317 (31%) |
| Race/Ethnicity* | White | 3238 (72%) | 1385 (84%) | 1288 (80%) | 546 (54%) |
| Black | 807 (18%) | 110 (7%) | 120 (7%) | 280 (28%) | |
| Hispanic | 139 (3%) | 71 (4%) | 82 (5%) | 88 (9%) | |
| Other | 341 (8%) | 92 (6%) | 115 (7%) | 97 (10%) | |
| Smoking | Never | 3045 (67%) | 802 (48%) | 792 (49%) | 474 (47%) |
| Past | 492 (11%) | 141 (9%) | 173 (11%) | 123 (12%) | |
| Current | 617 (14%) | 383 (23%) | 283 (18%) | 203 (20%) | |
| Unknown† | 371 (8%) | 332 (20%) | 357 (22%) | 211 (21%) | |
| Behçet Disease | 30 (1%) | 26 (2%) | 94 (6%) | 74 (7%) | |
| Sarcoidosis | 294 (6%) | 94 (6%) | 96 (6%) | 117 (12%) | |
| Juvenile Idiopathic Arthritis (JIA) | 370 (8%) | 15 (1%) | 16 (1%) | 10 (1%) | |
| Spondyloarthropathy | 380 (8%) | 13 (1%) | 17 (1%) | 8 (1%) | |
| Inflammatory Bowel Disease (IBD)** | 175 (4%) | 24 (1%) | 15 (1%) | 15 (1%) | |
| Other Systemic Inflammatory Disease | 163 (4%) | 89 (5%) | 81 (5%) | 34 (3%) | |
| Prior Cataract Surgery | Never | 4001 (88%) | 1520 (92%) | 1444 (90%) | 820 (81%) |
| Prior to Baseline | 524 (12%) | 138 (8%) | 161 (10%) | 191 (19%) | |
| During Follow-up***** | n/a | ||||
| Prior Glaucoma Surgery | 109 (2%) | 18 (1%) | 19 (1%) | 27 (3%) | |
| Prior Retinal Detachment Surgery | 28 (1%) | 15 (1%) | 17 (1%) | 18 (2%) | |
| Prior Pars Plana Vitrectomy (not for retinal detachment) | 83 (2%) | 56 (3%) | 48 (3%) | 64 (6%) | |
| Duration of Uveitis Prior to Presentation for Tertiary Uveitis Care |
≤6 months | 1473 (33%) | 541 (33%) | 659 (41%) | 344 (34%) |
| >6 months to 2 years | 985 (22%) | 377 (23%) | 381 (24%) | 266 (26%) | |
| >2 to 5 years | 786 (17%) | 352 (21%) | 284 (18%) | 170 (17%) | |
| ≥5 years | 1281 (28%) | 388 (23%) | 281 (18%) | 231 (23%) | |
| Anterior Chamber Cells | Quiet | 2342 (52%) | 1093 (66%) | 1283 (80%) | 427 (42%) |
| 0.5+ | 768 (17%) | 302 (18%) | 181 (11%) | 219 (22%) | |
| 1+ | 656 (14%) | 167 (10%) | 63 (4%) | 157 (16%) | |
| 2+ or worse | 759 (17%) | 96 (6%) | 78 (5%) | 208 (21%) | |
| Vitreous Cells | Quiet | 3295 (73%) | 460 (28%) | 825 (51%) | 347 (34%) |
| 0.5+ | 488 (11%) | 264 (16%) | 256 (16%) | 182 (18%) | |
| 1+ | 464 (10%) | 440 (27%) | 280 (17%) | 228 (23%) | |
| 2+ or worse | 278 (6%) | 494 (30%) | 244 (15%) | 254 (25%) | |
| Vitreous Haze*** | Quiet | 3943 (87%) | 1055 (64%) | 1259 (78%) | 624 (62%) |
| 1+ | 207 (5%) | 340 (21%) | 201 (13%) | 208 (21%) | |
| 2+ | 89 (2%) | 139 (8%) | 57 (4%) | 65 (6%) | |
| 3+ or worse | 20 (0%) | 36 (2%) | 12 (1%) | 39 (4%) | |
| Missing† | 266 (6%) | 88 (5%) | 76 (5%) | 75 (7%) | |
| Intraocular Pressure | ≤5 mm Hg | 60 (1%) | 3 (0%) | 8 (0%) | 27 (3%) |
| 6–23 mm Hg | 4010 (89%) | 1474 (89%) | 1424 (89%) | 835 (83%) | |
| 24–29 mm Hg | 201 (4%) | 50 (3%) | 46 (3%) | 42 (4%) | |
| ≥30 | 120 (3%) | 24 (1%) | 27 (2%) | 42 (4%) | |
| Missing† | 134 (3%) | 107 (6%) | 100 (6%) | 65 (6%) | |
| Posterior Synechiae, Any | 874 (19%) | 142 (9%) | 71 (4%) | 198 (20%) | |
| Peripheral Anterior Synechiae, Any | 179 (4%) | 32 (2%) | 13 (1%) | 48 (5%) | |
| Band Keratopathy, Any | No | 3481 (77%) | 1390 (84%) | 1393 (87%) | 823 (81%) |
| Yes | 147 (3%) | 37 (2%) | 20 (1%) | 36 (4%) | |
| Missing† | 897 (20%) | 231 (14%) | 192 (12%) | 152 (15%) | |
| Macular Edema | 376 (8%) | 502 (30%) | 256 (16%) | 175 (17%) | |
| Epiretinal Membrane | 164 (4%) | 110 (7%) | 117 (7%) | 87 (9%) | |
| Choroidal Neovascularization | 1 (0%) | 0 (0%) | 62 (4%) | 11 (1%) | |
| Snowbanking**** | 7 (0%) | 376 (23%) | 2 (0%) | 7 (1%) | |
| Retinal Vascular Sheathing | 66 (1%) | 178 (11%) | 321 (20%) | 145 (14%) | |
| Retinal Vascular Occlusion | 1 (0%) | 4 (0%) | 53 (3%) | 4 (0%) | |
Patients with any missing data at baseline were generally not included in the analysis, but band keratopathy (17%), smoking status (14%), intraocular pressure (IOP, 5%), and vitreous haze (6%) were missing enough baseline measurements to warrant respective separate missing categories
Hispanic includes all patients with Hispanic ethnicity
IBD includes ulcerative colitis and Crohn’s disease
0.5+ was not used for vitreous haze due to a database error; it was instead coded as either Quiet or 1+
Snowbanking may have reflected either active exudation or residual fibrosis
By definition no patients could have had cataract surgery during follow-up before beginning follow-up; 206 (2.3%) of the eyes had cataract surgery performed during follow-up
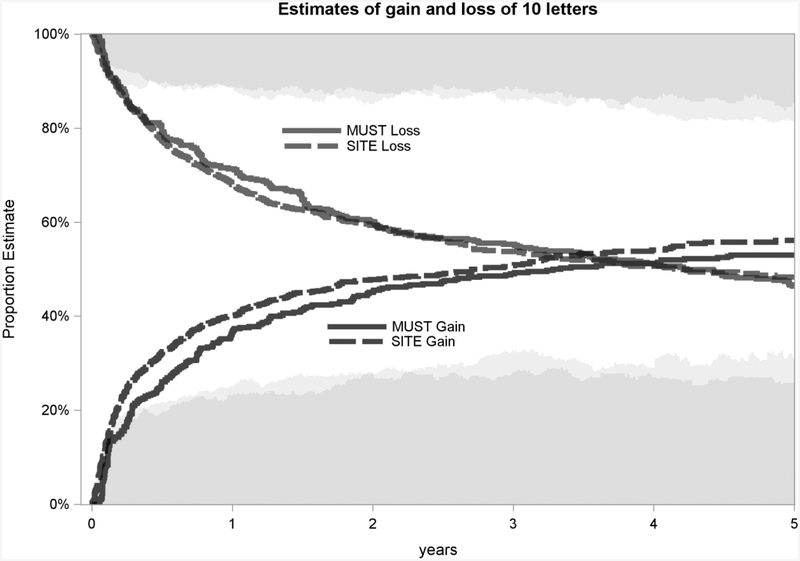
Figure 1 shows the KM curves for gain and loss of 10 letters of VA, for the SITE Cohort Study and the MUST Trial and Follow-up Study. By four years over 50% of eyes had at some point lost 10 letters in both SITE and MUST, but at the 4-year time point, the proportion with VA ≥10 letters worse than baseline was <20% in both studies. Similarly, the KM curve indicates that by 4 years over 50% of patients at some point had gained 10 letters in both SITE and MUST, though the proportion at the 4-year point with VA ≥10 letters better than baseline was less than 30% in both studies. In both studies, gains of visual acuity tended to occur more often than losses of visual acuity while under tertiary management. The visual acuity distributions over time of SITE and MUST were similar to each other.
Figure 1.
Estimates of gain and loss of 10 letters
Comparison of Kaplan-Meier curves for 10 letters of gain or loss, in the observational Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort and in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Cohort. The shading at the top and bottom (light gray for SITE, dark gray for MUST) indicate the percent of remaining patients who are 10 letters below or above, respectively, their baseline visual acuity.
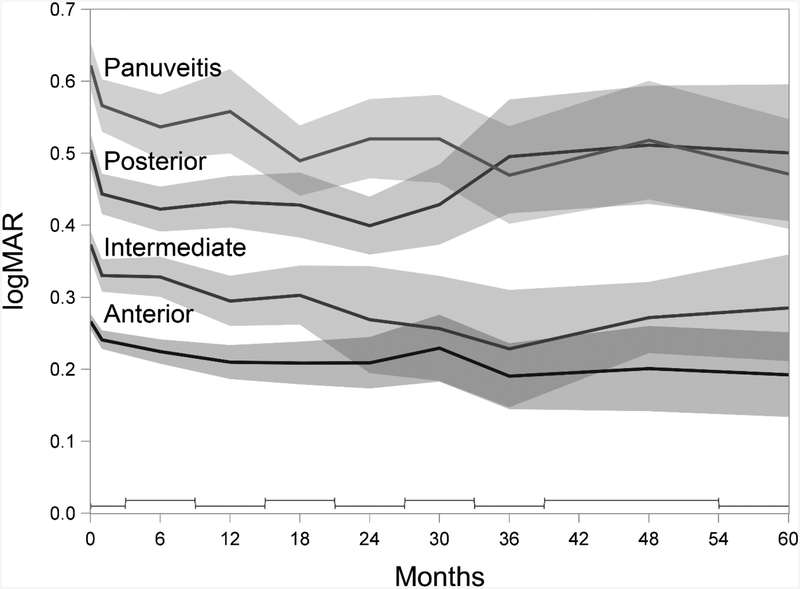
Figure 2 summarizes the modeled mean VA and 95% confidence intervals for anterior, intermediate, posterior and panuveitis cases in SITE over the first 5 years after presentation for tertiary care. Panuveitis cases (mean VA=20/84 [95% CI 20/78, 20/90]) presented with significantly worse mean VA than posterior uveitis cases (20/64 [20/60, 20/68]), which were worse than intermediate uveitis cases (20/47 [20/47, 20/49]), and anterior uveitis cases (20/37 [20/36, 20/38]) (P<0.001). All four groups showed improvement at Month 1 compared to presentation for tertiary care, to 20/74, 20/56, 20/43, and 20/35 respectively (P<0.001 for each change). Each group also showed modest ongoing improvement in mean VA through Month 24, to 20/66, 20/50, 20/37, and 20/32, respectively. By 5 years the mean VA still was more favorable than that at presentation for panuveitis (20/59 [20/50, 20/71], P<0.001), intermediate uveitis (20/38 [20/33, 20/46], P=0.016) and anterior uveitis (20/31 [20/27, 20/36], P=0.014). For posterior uveitis cases, the mean VA had returned to approximately the initial visit’s value (20/64 [20/51, 20/79], P=0.94).
Figure 2.
Mean Visual Acuity (VA) over 5 years after presentation for tertiary care
Mean VA and 95% confidence intervals (in gray) for anterior, intermediate, posterior and panuveitis over the first 5 years after presentation for tertiary care. Visits after baseline within successive bins (staggered bars at the bottom of the graph), were modeled as repeated measurements of a single time interval, represented here as the middle of the bin. Data from the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study.
Discussion
These results indicate that, on average, visual acuity in patients with uveitis initially improves after coming under uveitis subspecialty care, and the mean degree of improvement is of a clinically important degree for the intermediate, posterior and panuveitis groups. After accounting for the propensity of patients with better visual outcomes over time to stop coming for follow-up, visual acuity was maintained at this improved level over the first five years for the average case of anterior, intermediate or panuveitis. After an initial improvement, the average case of posterior uveitis maintained improvement for about two years, but then worsened back to the level of presenting VA. These improvements—in patients treated mostly before the availability of long-lasting implant therapy—are qualitatively similar to the result for the systemic therapy arm of Tomkins-Netzer et al16 and the Multicenter Uveitis Steroid Treatment (MUST) Trial,10,13,17 wherein a similar treatment strategy was used. Thus, the results suggest that the prognosis of the average uveitis case is favorable under tertiary uveitis care. Direct comparison to the MUST Trial and Follow-up Study data, which used gold-standard methods for visual acuity measurement, provides a robust demonstration of the pattern that the average case improves, and validates the SITE results. The larger SITE Cohort Study further detected that posterior uveitis cases tended to have modestly less favorable long-term outcomes; we speculate this may be the result of macular complications such as choroidal neovascularization in this subset.
The changes in VA while receiving tertiary care could be attributed to a variety of positive and negative factors including the development or resolution of cataracts, macular edema, or inflammatory haze. Cataract surgery outcomes in particular have been shown to be improved with better control of uveitis18, so much of the early improvement shown in this analysis could be due to procedures that were postponed until the patients were referred to uveitis subspecialty care. Importantly, the observation that the average case tends to improve initially and then remain stable suggests that analytic approaches evaluating time-to-worsening of visual acuity may not be the best methodological approach for assessing visual outcomes in tertiary uveitis cohorts and uveitis trials19,20. Time-to-event analyses are capable of adequately handling variable follow-up times. However, time-to-event analyses are not designed to handle reversible outcomes to give a clear picture of visual acuity at any given time, as evidenced by the fact that by 4 years the curves from both studies have crossed, paradoxically indicating that more than 100% of people have gained or lost 10 letters. The proportion of eyes ≥10 letters better or worse than their baseline VA gives a better indication of the time-updated state of VA, but does not address loss to follow-up, and can be difficult to model as a three-level ordinal outcome. Our observation that the likelihood of patients discontinuing follow-up was strongly related to presenting visual acuity suggests added analytic difficulties in assessing visual outcomes for studies without active follow-up. Inverse probability weighting is an established approach that can be used when needed to deal with this scenario14.
This analysis did not attempt to quantify the effects of specific treatment decisions and risk factors on mean VA. While marginal structural models using inverse probability of treatment weighting and similar methods are promising tools for investigating these effects in observational data14, such approaches require independent investigation of specific hypotheses as opposed to the overview of mean VA, which was beyond the scope of this manuscript.
The strengths of this study include a large sample size and standardized collection of a large amount of information on each case. The participating centers were selected in part based on the favorable quality of documentation available in their medical records. Limitations include VA measurements collected from clinical records with variable techniques and personnel, which may be partially accounted for by the requirement that the same VA measurement technique to have been used for a patient across visits. The pattern of visual acuity change in the SITE Cohort using this approach was similar to that in the MUST study which used gold-standard methods and had favorable follow-up. Losses to follow-up are unavoidable in any study, and tend to be more in studies with passive follow-up as in this study; we used the inverse probability weighting approach to model the impact of censoring on the results. Non-standardized follow-up intervals were a limitation specifically in the cases in which patients missed an entire window and had to be censored because of the constraints of the statistical model; the windows were chosen in this analysis to minimize patient loss while maintaining relevant assessment time-frames. Although an underlying model accounted for the likelihood of censoring, the model is based on available confounders, so other (unknown) reasons for patients not returning could not inform censoring weights. The study population was limited to patients referred for tertiary care, so results may be less generalizable to other patients. Any further improvements in management that have taken place since the time of the study would tend to further improve outcomes, resulting in a qualitatively similar interpretation that patients tend to improve on average. VA is one of many markers of visual function but was the only function routinely collected for all patients at the four centers; therefore other visual function measures could not be included in this analysis. A large number of eyes/patients were lost to follow-up along the way, which is a reality in observational clinical research. However, the inverse probability weighting approach was designed to mitigate this deficiency. It is unlikely that any residual differences amongst those followed and those not followed differed so profoundly as to qualitatively alter the strong patterns of association observed here, especially given the similarity of patterns seen to those in MUST, which had high follow-up through five years. Comparisons to the MUST results suggest that the SITE approach was reasonably robust despite non-standardized VA measurement and more losses to follow-up than MUST.
In summary, our analysis suggests that eyes with uveitis tended to improve shortly after coming under subspecialty uveitis care, and in most cases remained stably improved for up to five years of follow-up. The results imply that progressive visual loss occurred prior to referral (36% to 45% had care for > 2 years prior to referral), suggesting that uveitis cases may benefit from early referral to uveitis subspecialists when equivalent care is not available in another setting. It would be valuable to patients to have such expertise available anywhere that uveitis occurs, which may require increased investment in ophthalmic training (of both uveitis experts and other ophthalmologists) and continuing medical education where needed, especially in less developed countries. The observation that the average uveitis case under tertiary uveitis care improves suggests that time-to-worsening approaches to studying visual acuity as clinical research outcomes have important limitations in studying treatments of uveitis.
Supplementary Material
Acknowledgements
We thank the MUST Research Group for providing visual acuity data through five years from the MUST Trial and Follow-up Study for comparison to the SITE visual acuity data.
Funding Details
This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness, the Paul and Evanina Mackall Foundation, and the Lois Pope Life Foundation. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C previously was supported by and RBN and HNS continue to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. The MUST Trial and Follow-up Study was funded by National Eye Institute Cooperative Agreements (U10EY014656 (MMA), U10EY014660 (JTH), and U10EY014655 (DAJ)). The funding sources had no role in the design, and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Research Group
University of Pennsylvania/Scheie Eye Institute
John H. Kempen, MD, PhD (Principal Investigator, Penn Center Founder)
Nirali P. Bhatt, MD (Penn Center Director)
Kurt A. Dreger, BS (Database Construction and Management)
Marshall M. Joffe, MD, PhD (Statistical Methodologist)
Craig W. Newcomb, MS (Biostatistician)
Maxwell Pistilli, MS (Biostatistician)
Former Members:
Pichaporn Artornsombudh, MD (Post-doctoral Fellow)
Tonetta D. Fitzgerald, BA, COA, CCRC (Project Coordinator)
Sapna S. Gangaputra, MBBS, MPH (Post-doctoral Fellow)
Asaf Hanish, MPH (Biostatistical Programmer)
Kathy J. Helzlsouer, MD, MHS (Cancer Epidemiologist)
Naira Khacharyan, MD, MPH, PhD (Post-doctoral Fellow)
Srishti Kothari, MBBS, DOMS, DNB (Post-doctoral Fellow)
Abhishek Payal, MBBS, MPH (Post-doctoral Fellow)
Johns Hopkins University/Wilmer Eye Institute
Jennifer E. Thorne, MD, PhD (Center Director, 2007-present)
Kurt A. Dreger, BS (Database Construction and Management)
Former Members:
Hosne Begum, MBBS, MPH (Study Coordinator)
Ebenezer Daniel, MBBS, MPH, PhD (Post-doctoral Fellow)
James P. Dunn, MD (Major Clinician)
Sapna S. Gangaputra, MBBS, MPH (Post-doctoral Fellow)
Douglas A. Jabs, MD, MBA (Clinic Founder, Former Clinic Director)
Abhishek Payal, MBBS, MPH (Post-doctoral Fellow)
Icahn School of Medicine at Mount Sinai
Douglas A. Jabs, MD, MBA (Founder of JHU Clinic, JHU Clinic Director 2005–2007)
Massachusetts Eye Research & Surgery Institution/Ocular Immunology &Uveitis Foundation
C. Stephen Foster, MD, FACS, FACR (Center Director and Founder, and Director and Founder of previous Massachusetts Eye & Ear Infirmary Center, 2004–2005)
Naira Khacharyan, MD, MPH, PhD (Post-doctoral Fellow)
Former Members:
Pichaporn Artornsombudh, MD (Post-doctoral Fellow)
R. Oktay Kaçmaz, MD, MPH (Post-doctoral Fellow)
Abhishek Payal, MBBS, MPH (Post-doctoral Fellow)
Siddharth S. Pujari, MBBS, MPH (Post-doctoral Fellow)
Srishti Kothari, MBBS, DOMS, DNB (Post-doctoral Fellow)
National Eye Institute, Laboratory of Immunology
H. Nida Sen, MD, MS (NEI Center Director)
Robert B. Nussenblatt, MD, MPH (Center Founder and Co-Director)
Former Members:
Grace A. Levy-Clarke, MD (Former Center Director)
Sapna S. Gangaputra, MBBS, MPH (Post-doctoral Fellow)
Oregon Health & Sciences University/Casey Eye Institute
Eric B. Suhler, MD, MPH (Center Director)
James T. Rosenbaum, MD, FACR (Center Founder and Co-Director)
Teresa Liesegang, COT, CRC (Project Coordinator)
University of Pittsburgh
Jeanine Buchanich, PhD (Epidemiologist)
Terri L. Washington (Project Coordinator)
Footnotes
Declaration of Interest Statement
Maxwell Pistilli, Marshall M. Joffe, Sapna S. Gangaputra, Siddharth S. Pujari, Douglas A. Jabs, Grace A. Levy-Clarke, Hosne Begum, Tonetta D. Fitzgerald, and Nirali P. Bhatt, report no conflicts of interest. James T. Rosenbaum serves or has served as a consultant for Abbott Laboratories, Amgen, Allergan, Genentech, Novartis, and UCB and has been involved in clinical trials for Abbott Laboratories, Genentech, Lux Biosciences, and Eyegate Pharma. Jennifer E. Thorne serves a consultant for Allergan and XOMA. C. Stephen Foster serves as a lecturer for Alcon, Inspire, Ista, and Centocor; as a Consultant for Sirion; as a consultant and lecturer for Allergan and Bausch & Lomb; and as an equity owner for EyeGate. John H. Kempen serves or has served in the last three years as a consultant for Clearside, Gilead, and Santen and has received grant funding from EyeGate.
References
- 1.ten Doesschate J. Causes of blindness in The Netherlands. Documenta ophthalmologica. Advances in ophthalmology. Jan 29 1982;52(3–4):279–285. [DOI] [PubMed] [Google Scholar]
- 2.National Advisory Eye Council (U.S.). Vision research : a national plan: 1983–1987. Bethesda, Md.: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health; 1983. [Google Scholar]
- 3.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. Jul-Aug 2004;218(4):223–236. [DOI] [PubMed] [Google Scholar]
- 4.Watson SL, Edelsten C, Kanski JJ. The incidence of visual loss from uveitis. Poster presentation presented at Joint European Research Meetings in Ophthalmology and Vision; 1994; Montpellier, France. [Google Scholar]
- 5.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American journal of ophthalmology. Sep 2005;140(3):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempen JH, Daniel E, Dunn JP, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. Bmj. 2009;339:b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic epidemiology. Jan-Feb 2008;15(1):47–55. [DOI] [PubMed] [Google Scholar]
- 8.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. American journal of ophthalmology. Jul 1982;94(1):91–96. [PubMed] [Google Scholar]
- 9.Kempen JH, Jabs DA, Wilson LA, Dunn JP, West SK, Tonascia JA. Risk of vision loss in patients with cytomegalovirus retinitis and the acquired immunodeficiency syndrome. Archives of ophthalmology. Apr 2003;121(4):466–476. [DOI] [PubMed] [Google Scholar]
- 10.Multicenter Uveitis Steroid Treatment Trial Research G, Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. Oct 2011;118(10):1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Multicenter Uveitis Steroid Treatment Trial Research G, Kempen JH, Altaweel MM, et al. Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. Ophthalmology. Oct 2015;122(10):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempen JH, Van Natta ML, Altaweel MM, et al. Factors Predicting Visual Acuity Outcome in Intermediate, Posterior, and Panuveitis: The Multicenter Uveitis Steroid Treatment (MUST) Trial. American journal of ophthalmology. Dec 2015;160(6):1133–1141 e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Writing Committee for the Multicenter Uveitis Steroid Treatment T, Follow-up Study Research G, Kempen JH, et al. Association Between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs Systemic Anti-inflammatory Therapy and Visual Acuity at 7 Years Among Patients With Intermediate, Posterior, or Panuveitis. JAMA : the journal of the American Medical Association. May 16 2017;317(19):1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joffe MM, Pistilli M, Kempen JH. Marginal Structural Models for Comparing Alternative Treatment Strategies in Ophthalmology using Observational Data. Ophthalmic epidemiology. Jul 2 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. Sep 2000;11(5):561–570. [DOI] [PubMed] [Google Scholar]
- 16.Tomkins-Netzer O, Talat L, Bar A, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. Dec 2014;121(12):2387–2392. [DOI] [PubMed] [Google Scholar]
- 17.Kempen JH, Van Natta ML, Altaweel MM, et al. Factors Predicting Visual Acuity Outcome in Intermediate, Posterior, and Panuveitis: The Multicenter Uveitis Steroid Treatment (MUST) Trial. American journal of ophthalmology. Dec 2015;160(6):1133–1141 e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta S, Linton MM, Kempen JH. Outcomes of Cataract Surgery in Patients With Uveitis: a Systematic Review and Meta-Analysis. American journal of ophthalmology. Jun 28 2014. [DOI] [PubMed] [Google Scholar]
- 19.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. Nov 1996;103(11):1846–1853. [DOI] [PubMed] [Google Scholar]
- 20.DiLoreto DA Jr., Bressler NM, Bressler SB, Schachat AP. Use of best and final visual acuity outcomes in ophthalmological research. Archives of ophthalmology. Nov 2003;121(11):1586–1590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.