Abstract
Thomas J Dougherty from Roswell Park Cancer Center played a major role in the progress of photodynamic therapy (PDT) from a laboratory science into a real world clinical therapy to treat patients with cancer. Nevertheless over the succeeding 45 years, it is fair to say that the overall progress of clinical PDT for cancer has been somewhat disappointing. The goal of this perspective article is to summarize some of the clinical trials run by various companies using photosensitizers with different structures that have been conducted for different types of cancer. While some have been successful, others have failed, and several are now ongoing. I will attempt to touch on some factors, which have influenced this checkered history, and look forward to the future of clinical PDT for cancer.
Keywords: Thomas J Dougherty, Photodynamic therapy, Cancer, Photofrin, Approved photosensitizers, Clinical trials
Graphical Abstract
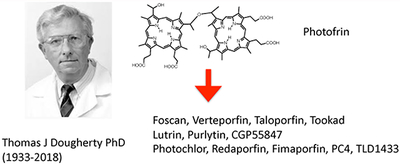
Thomas J Dougherty PhD was instrumental in the first clinical approvals of Photofrin PDT for cancer. Since then progress has been somewhat mixed. Foscan, Verteporfin, Taloporfin, and Tookad have been approved, while Lutrin, Purlytin, CGP55847 have failed. Photochlor, Redaporfin, Fimaporfin, PC4, and TLD1433 are presently undergoing clinical trials.
INTRODUCTION
The phenomenon underlying the scientific basis of photodynamic therapy (PDT) was discovered in 1900 in Munich, Germany by Oscar Raab, a medical student working with Professor Herman von Tappeiner. While investigating the effects of acridine dyes on protozoa, he made the chance discovery during a thunderstorm that the combination of acridine red and light killed Infusoria, a species of paramecium (1). He went on to show that this cytotoxic effect was greater than that of either acridine red alone, light alone or acridine red exposed to light and then added to the paramecium. Raab associated this property of dyes (light-mediated cytotoxicity) with the optical property of fluorescence. He postulated that the effect was caused by the transfer of energy from light to the chemical, similar to the process of photosynthesis seen in plants after the absorption of light by chlorophyll. In a second paper, von Tappeiner discussed the potential future application of fluorescent substances in medicine (2). This discovery led to the first therapeutic medical application of an interaction between a PS and light in which von Tappeiner, together with a dermatologist named Jesionek, used a combination of topically applied eosin and white light to treat skin tumors (3). Together with Jodlbauer, von Tappeiner went on to demonstrate the requirement of oxygen in these photosensitization reactions (4) and in 1907 they introduced the term “photodynamic action” to describe this phenomenon (5).
Starting in 1950 onwards, various investigators studied the property of different porphyrins and porphyrin derivatives to localize in different tumors after intravenous injection (6–8). The overall goal was to use this approach to detect malignancies by exciting red fluorescence using a Woods UVA lamp. In 1955, Schwartz et al. (9) attempted to purify the tumor-localizing fraction from an impure hematoporphyrin (HP) mixture. He used a mixture of acetic and sulfuric acids and neutralized with sodium acetate to produce a substance known as hematoporphyrin derivative (HpD). Lipson and Baldes showed (10, 11) that administration of HpD to patients undergoing bronchoscopy or esophagoscopy for suspected malignant disease could lead to detection of otherwise invisible tumors. Diamond and co-workers (12) were the first to show that injection of HP to mice with subcutaneous brain tumors followed by white light exposure 24 h later produced necrosis in all but the deepest regions of the tumors. Neither HP alone, nor light alone produced any effect.
Dougherty first studied the effects of photoactivated fluorescein as an anticancer therapy (13). In 1975 Dougherty and coworkers (14) at the Roswell Park Cancer Institute in Buffalo reported the first successful complete tumor cures following administration of HpD and activation with red light in the treatment of experimental tumors in mice. In 1976, Kelly and Snell reported the first human study of PDT using HpD in patients with bladder cancer (15). Initially tumor fluorescence was measured, but in one patient, a quartz rod connected to a mercury vapor lamp was used to photoactivate the HpD and induce tumor destruction.
In 1978, Dougherty reported the first large series of patients successfully treated with PDT (16). Twenty-five patients with 113 primary or secondary skin tumors, all of which were refractory or had recurred following conventional treatment, were treated with HpD followed by exposure to red light from a xenon arc lamp at times ranging from 24–168 h after injection. Ninety-eight lesions completely regressed, 13 exhibited a partial response and only two were resistant to treatment. The primary tumor types that responded included squamous cell carcinomas (SCC), basal cell carcinomas (BCC) and malignant melanomas, and the metastatic skin lesions arising from primary tumors of the breast, colon and endometrium. Side effects included erythema, edema and some skin necrosis, although these effects were reduced by increasing the time interval between HpD injection and light exposure to at least 3 days.
This very successful demonstration of the clinical efficacy of PDT encouraged commercial development of Photofrin, chiefly championed by Dougherty over several years (17, 18).
In 2013, I edited a textbook titled “Handbook of Photomedicine” (19) and was fortunate to receive a personal communication from Thomas Dougherty summarizing his role in the development of clinical PDT.
“My role in developing Photodynamic Therapy (PDT) came about as a result of a series of events I could not have expected. For example my knowledge of photochemistry was not from my formal education as this was a new and undeveloped field at that time, but directly from George Hammond, the man known as the ‘father of photochemistry’, who was a consultant at DuPont where I had landed directly after graduate school and where I was assigned a project involving photo-degradation of one of their products. When I decided that industry was not for me I thought I might be able to do more fulfilling research at Roswell Park Cancer Institute, which was in the same city (Buffalo) as was the DuPont Lab where I worked (and also my hometown). I managed to get a starting position at Roswell (working on someone’s grant) with the help of George Hammond who wrote a letter of recommendation for me to a friend he knew there (who happened to have worked with Linus Pauling). When I finished with the grant project, I obtained a more secure position in the Department of Radiation Oncology where I was able to work on my own ideas. I started by studying compounds that would produce oxygen when exposed to ionizing radiation in order to get around the low efficacy of radiation therapy in low oxygenated areas of tumors and can result in tumor regrowth. In testing the cellular toxicity of the compound I synthesized I was warned by a technician that I should do the cell culture test in the dark or the light would kill all the cells (the test was based on fluorescence of fluorescein developed only in viable cells!) I wondered if anyone had tried this approach to cancer treatment, so I exposed cancer cells to sunlight after adding fluorescein. They all died! We then went on to optimization of parameters first in experimental animals and then in patients. I learned how to initiate and treat tumors in animals, design, write and assist in human clinical trials, deal with the FDA, negotiate with pharmaceutical companies (quite an experience) and of course how to write grant applications- which thanks to many members of our group have supported our research since 1974.
As time went on I also learned that I was not the only one who thought of using light –activated drugs therapeutically - the first one was in 1799! Most of this came to me through contacts since I did not have access to journals going that far back. However these were mostly single patient or anecdotal reports, some of which were negative mainly to lack of optimized PDT variables. If I had known this at the time I may not have continued. Sometimes ‘ignorance is bliss’. “
PHOTOSENSITIZERS APPROVED FOR PDT FOR CANCER
The most widely used PDT regimens that have been approved for cancer (and in particular for non-melanoma skin cancer) are undoubtedly based on ALA (5-aminolevulinic acid) and its methyl ester (Metvix), which are generally applied topically to the skin. ALA-PDT has been widely reviewed elsewhere (20, 21) and will not be covered in detail in this paper.
Photofrin
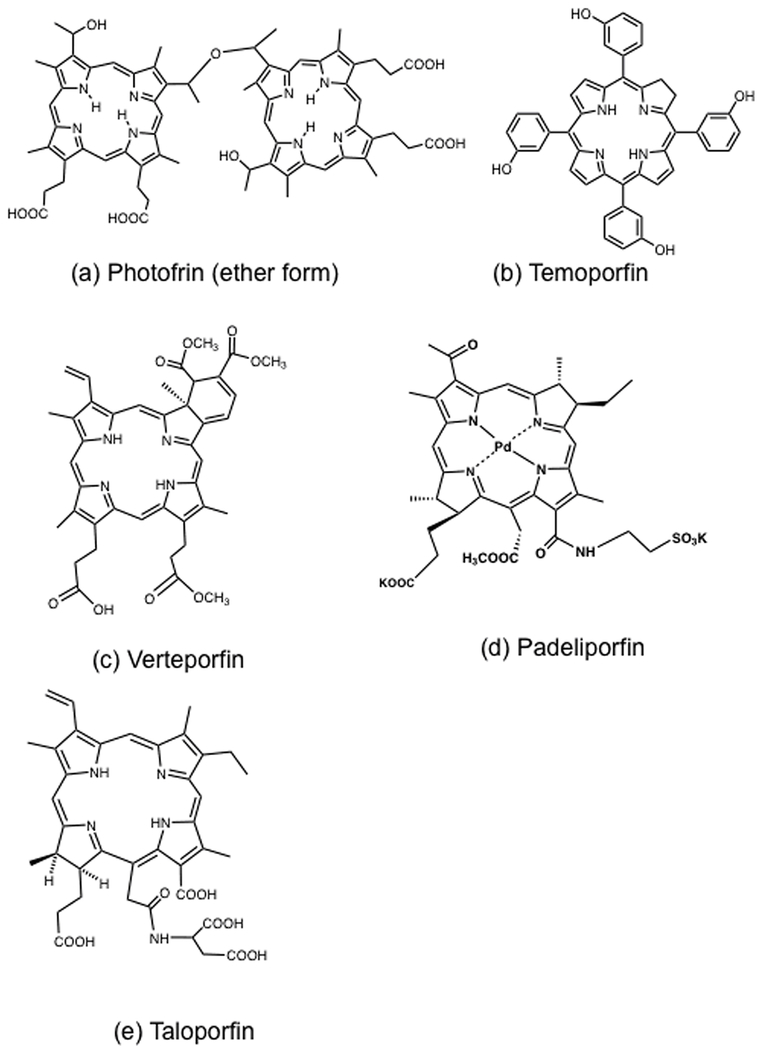
Photofrin is the second-generation optimization of the original hematoporphyrin derivative (HpD) worked on by Dougherty (17). The commercial history of Photofrin was somewhat convoluted (22). In 1980 Dougherty formed the company Oncology Research and Development (ORD) that developed Photofrin from HpD, and in 1984 Johnson & Johnson acquired ORD. In 1987 J&J sold its subsidiary Photomedica to QLT, that then formed a partnership with Lederle Laboratories (part of American Cyanamid). In 2000 QLT sold Photofrin to Axcan Pharma that was in turn acquired by Wyeth Holdings and Pinnacle Biologics. The precise chemical structure of Photofrin and HpD has eluded complete characterization despite numerous efforts in that direction (23–25). The structure contains a mixture of oligomers of hematoporphyrin joined to each other by ether and ester bonds (Figure 1a shows the ether-linked dimer).
Figure 1.
Chemical structures of (a) Photofrin (ether-linked dimer); (b) Temoporfin (Foscan); (c) Verteporfin (Visudyne); (d) Padeliporfin (Tookad soluble); (e) Taloporfin (LS11).
Photofrin (Porfimer sodium) was first approved for the treatment of bladder cancer in Canada in 1993. It was approved in Japan in 1994 (for early stage lung cancer. The first U.S. Food and Drug Administration (FDA) approval came in 1995 for advanced obstructive esophageal cancer, and in 1998, it was approved for the treatment of early stage non-small cell lung cancer. At the present time there are 50 trials concerning Photofrin-mediated PDT listed on the Clinical Trials website (https://clinicaltrials.gov/). Some of the types of cancer that have been investigated include: unresectable hilar cholangiocarcinoma (26); bile duct invasion of hepatocellular carcinoma (27); recurrent high grade glioma (28); locally advanced pancreatic cancer (29); locally advanced or recurrent head and neck cancer (30); epithelioid malignant pleural mesothelioma (31); advanced rectal cancer (32).
The regimen of Photofrin PDT is normally an injection of 2 mg/kg body weight followed after 48 hours by illumination of the tumor with 630 nm light (doses of 120 -180 J/cm2) either delivered by an external spot or by interstitial fibers inserted into the tumor mass. However the absorption peak at 630 nm is the last of the four porphyrin Q-bands and only has a molar absorption coefficient of ~2000 M−1cm−1. One of the main advantages of Photofrin is that as a sodium salt it is water soluble and therefore easy to administer by a simple IV injection. The disadvantages include significant skin photosensitivity that may last as long as four weeks and necessitate avoidance of sunlight or bright indoor lighting (33). Another disadvantage is that when Photofrin-PDT is applied to hollow organs such as the bladder or esophagus, it can cause stenosis or structures leading to obstruction (34). This was proposed to be caused by accumulation of Photofrin in the underlying collagen and smooth muscle layers. This is probably the reason why despite being the first approved indication, Photofrin PDT for bladder cancer has fallen into disuse.
Variations on the theme of hematoporporphyrin derivative have been approved in Europe under the name of Photosan (Seehof Laboratorium F&E GmbH, Wesselburen, Germany) (35), in Russia under the name of Photogem or Photohem (Photogem LLC, Moscow, Russia) (36), and in China under the names of Hiporfin (Huading Modern Bio‐pharmaceutical, Co., Ltd, Chongqing, China) (37) and Deuteporfin (Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd. Shanghai, China) (38).
Foscan
Foscan also known as temoporfin or m-tetrahydroxyphenylchlorin (mTHPC) (Figure 1b) was approved by the European Medicines Agency (EMA) in 2001 (39) for advanced head and neck squamous cell carcinoma. Foscan was submitted to the FDA in 2000 for approval, but this was denied. Trials of Foscan on the Clinical Trials website include inoperable bile duct cancers (40); non-resectable non-small-cell lung cancer (41); and nasopharyngeal carcinoma (42). Foscan was originally manufactured by Scotia Pharmaceuticals in Scotland but was then transferred to Biolitec in Germany. Foscan is administered at a dose of only 0.15 mg/kg and the drug light interval is usually 96 hours. Foscan is not water-soluble but is administered by IV injection dissolved in 40% ethanol and 60% propylene glycol. It is activated by 652 nm light at 100 mW/cm2 and a dose of 20 J/cm2. The advantages of Foscan are considered to be its high potency and a high absorption peak at 652 nm characteristic of a chlorin as opposed to a porphyrin. On the other hand since Foscan is considered to be an exceptionally powerful PS, its disadvantages may include damage to normal tissues surrounding the tumor. Moreover there is a risk of cutaneous burns due to extravasation of the PS at the infusion site (43). Skin photosensitivity lasts a moderately long time (2 weeks) but not as long as Photofrin.
Verteporfin
Verteporfin or Visudyne or was approved by the FDA in 2000 for the treatment of choroidal neovascularization caused by wet age-related macular degeneration (AMD) (44). It is supplied as a freeze-dried liposomal preparation that can be rehydrated in 5% dextrose and injected IV. Its chemical structure is a chlorin despite its rather confusing name of “benzoporphyrin dervivative mono-acid ring A” (Figure 1c), and it has a good absorption peak at 690 nm. It is rapidly cleared from tissue with skin photosensitivity only being present for 2 days after the dose of 6 mg/kg (45). Visudyne PDT for AMD involves a drug-light interval of 15 minutes after the start of the infusion, and a 689 nm laser spot (1000 μm larger than the CNV lesion) with a fluence of 50J/cm2 delivered at 600 mW/cm2 over 83 seconds (46). Visudyne was supplied by QLT of Vancouver Canada until it was transferred to Novartis AG. Visudyne was widely used throughout the world for wet AMD until the introduction of monoclonal antibodies against VEGF gained popularity (47). Visudyne PDT is still used for a range of different chorioretinal conditions (48). Although verteporfin PDT was not much studied as a cancer treatment for some time, in recent years several clinical trials for different types of cancer have emerged. Some trials listed on clinicaltrials.gov are primary breast cancer prior to surgery (49), cutaneous metastases of breast cancer (50), locally advanced pancreatic cancer (51), refractory brain tumors, including astrocytoma, ependymoma, and medulloblastoma (52), multiple basal cell carcinoma of the skin (53), and stage III or stage IV melanoma (54).
Tookad
Tookad is a term that has been applied to two different compounds that were synthesized by Avigdor Scherz and Yoram Salomon at the Weizmann Institute in Israel. The original Tookad termed WST-09 was a Pd-substituted bacteriopheophorbide derivative that was not water-soluble and was formulated in Cremophor EL for IV injection. It has a large Q-band at around 760 nm providing good tissue penetration. Due to the difficulties faced by the use of Cremophor in clinical studies, in 2005 the researchers developed a water-soluble derivative by aminolysis using the amino-acid taurine, that was termed WST-11 or Stakel and then Padeliporfin (Figure 1d) and is manufactured by Steba Biotech based in Luxembourg (55). Both forms of the Tookad compound were found to be very fast acting, and have been shown to be activated within the blood vessels in a largely Type I photochemical process called vascular targeted PDT or VTP. Tookad has been clinically investigated for locally recurrent prostate cancer using interstitial transperineal optical fibers inserted with trans-rectal ultrasound guidance under general anesthesia (56). Tookad is intravenously infused over 10 minutes, after which light delivery is carried out (753 nm delivered at 150 mW/cm of fiber, with an energy of 200 J/cm) (57). Both types of Tookad (WST-09 and WST-11) have been clinically tested for prostate cancer, but WST-11 received EMA approval in 2017, is marketed in Mexico and is under consideration by the FDA. The safety and efficacy of Tookad VTP were demonstrated in a multicenter randomized controlled phase III trial of 413 patients in 10 countries (58). Clinical trials are ongoing in low risk prostate cancer (59), intermediate risk prostate cancer (60) and in renal cancer (61).
Talaporfin sodium
This chlorin(e6) derivative was approved in Japan for PDT of early-stage lung cancer in 2004, and is marketed under the name Laserphyrin® by Meiji Seika Pharma Co., Ltd., Tokyo (62). Its chemical structure is the tetrasodium salt of mono-L-aspartyl chlorin e6, also known as NPe6. In 2007 Smith and co-workers demonstrated that its correct structure was that with the aspartic acid attached to the 152-side chain COOH position (63) (Figure 1e). It is a water-soluble chlorin administered by IV injection at 1 mg/kg, and is eliminated fairly rapidly thus avoiding any problems with skin photosensitivity. Clinically the drug-light interval is 0.25-4 hours. Talaporfin sodium was licensed to Light Sciences Oncology Inc in Bellevue, WA where it is known as Aptocine, LS11 or Litx. This company has designed implantable LED light sources that can be percutaneously inserted into the tumor tissue delivering a total of 200 J of energy. Clinical trials include unresectable hepatocellular carcinoma (64) and liver metastases of colorectal cancer (65).
FAILED PHOTOSENSITIZERS FOR PDT FOR CANCER
Motexafin lutetium
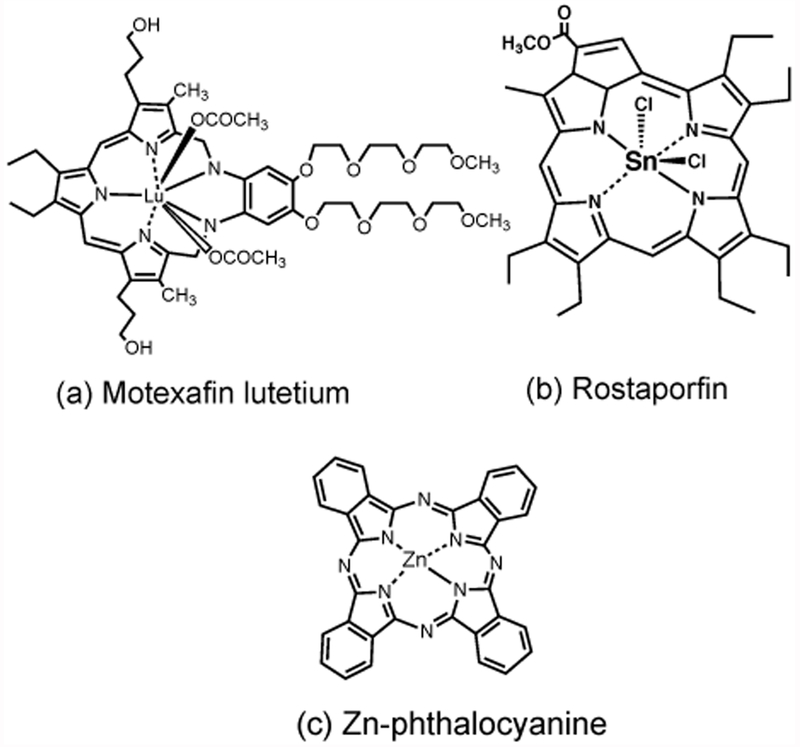
Motexafin lutetium also known as Lutrin or Antrin was developed by Jonathan Sessler at University of Texas at Austin (66) and licensed to Pharmacyclics Inc (67). It is a pentadentate lutetium texaphyrin (Figure 2a) that is water soluble and can be injected as a solution in 5% mannitol (68). It was activated with 732 nm laser that provided deep tissue penetration with a fluence of 150 J/cm2 delivered at 150 mW/cm2 (69). A phase 1 clinical trial using interstitial light delivery with optical fibers placed 1 cm apart for recurrent prostate cancer was carried out in 17 patients (70). Dose ranging used Lutrin (0.5 – 2 mg/kg); fluence (25 - 150 J/cm2); drug light interval (24 - 3 hours). PDT induced a large but transient increase in serum PSA levels. Patients who received a higher PDT dose showed both a greater short-term increase in PSA and also a significantly more durable PSA response (biochemical delay) (68). Use of an integrated PDT dosimetry system revealed substantial heterogeneity both intra-patient and inter-patient in light, photosensitizer, oxygen, and tissue optical properties within the prostate (71). Other trials were for chest-wall recurrence of breast cancer (72), intra-abdominal tumors (ovarian, colon, or stomach) (73, 74) and cervical intraepithelial neoplasia (75). After being discontinued for cancer indications, a phase 1 clinical trial was conducted for atherosclerotic plaque (76), and pre-clinical studies for AMD (77).
Figure 2.
Chemical structures of (a) Motxafin Lutetium (Lutrin); (b) Rostaporfin (SnET2); (c) Zinc phthalocyanine (CGP 55847).
Tin ethyl etiopurpurin
Tin ethyl etiopurpurin (SnET2) (Figure 2b) also known as Purlytin, Rostaporfin or Photrex was synthesized by Alan Morgan at University of Toledo OH (78), and manufactured by Miravant Pharmaceuticals (79). It was tested in a phase II/III clinical trial in 8 patients with 86 lesions of recurrent cutaneous metastatic breast cancer (80). It was formulated in a Cremophor EL micellar preparation and injected at a dose of 1.2 mg/kg followed 24 hours later by 660 nm laser at 150 mW/cm2 for a total light dose of 200 J/cm2. At 6-month follow-up, lesions showed 92% complete response, and 8% partial response. Lesions less than 0.5 cm in diameter had a 100% complete response. There was another trial of SnET2 for cutaneous AIDS-related Kaposi’s sarcoma (81). However, SnET2 was not developed further for cancer, although there was a trial for AMD where it was called PhotoPoint (82).
Liposomal zinc phthalocyanine
Liposomal zinc phthalocyanine (Zn-PC) (Figure 2c) was developed in the 1990s by Ciba Geigy AG (Basel, Switzerland) as CGP 55847. A large-scale production process for liposomes containing monomeric Zn-PC by controlled dilution of organic solvents was described (83). Although no details have been published it was tested in phase I/II clinical trials for solid tumors before being abandoned.
PHOTOSENSITIZERS IN ONGOING CLINICAL TRIALS
2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH)
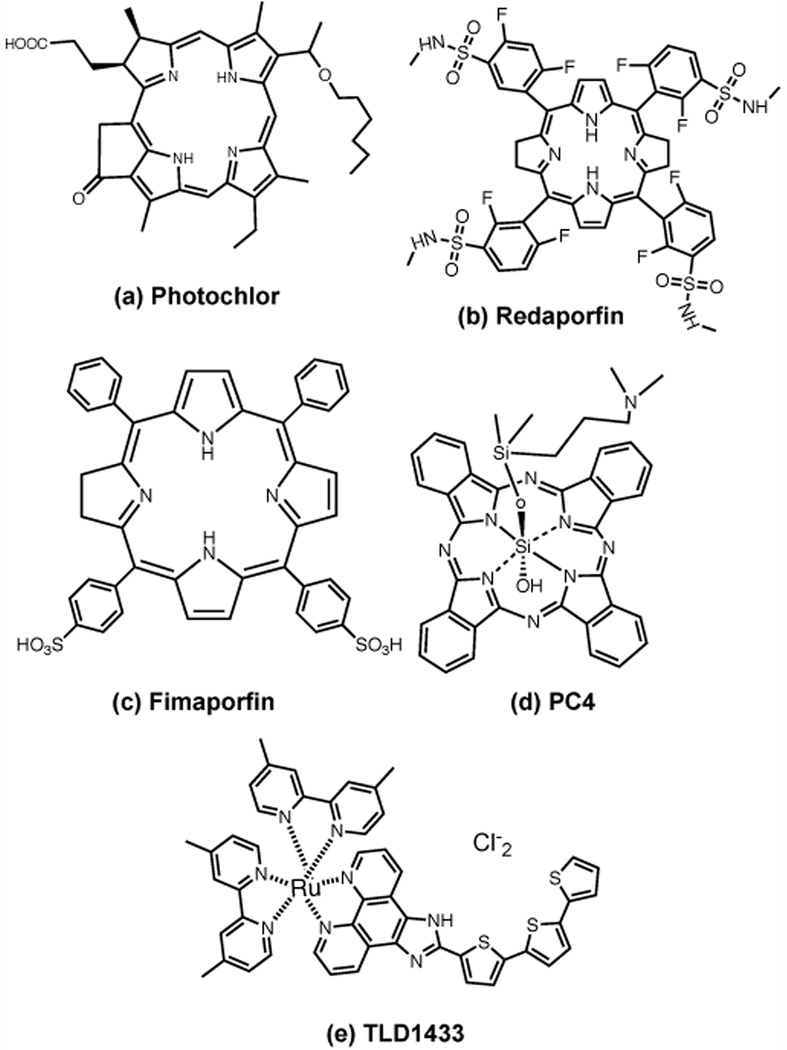
This compound also known as Photochlor was synthesized by Ravindra Pandey at Roswell Park in collaboration with Thomas Dougherty (43). There were a series of structural analogues of pyropheophorbide-a with different ethers, and the hexyl derivative (Figure 3a) was identified as the most active and suitable compound for PDT (84). HPPH is not water soluble but is formulated by trituration of the solid with 2% ethanol and 0.1% polyethylene sorbitanmonooleate (Tween 80) followed by suspension in 5% dextrose (85). HPPH PDT has been studied in several different types of cancer including malignant pleural mesothelioma (86), obstructive esophageal cancer (87), advanced obstructive non-small cell lung cancer (88), oral cancer, and cancer of the larynx (89).
Figure 3.
Chemical structures of (a) Photochlor (HPPH); (b) Redaporfin (LUZ11); (c) Fimaporfin (Amphinex); (d) Silicon phthalocyanine (PC4); (e) TLD1433.
Redaporfin
Redaporfin also known as LUZ11 or F-2BMet is chemically 5,10,15,20-tetrakis(2,6-difluoro-3-N-methylsulfamoylphenyl)-bacteriochlorin (Figure 3b) that was synthesized by Louis Arnaut and colleagues at the University of Coimbra in Portugal, and is manufactured by Luzitin SA Portugal (90). As a bacteriochlorin it has a large absorption peak at 750 nm, but since it is not water-soluble it is formulated in Cremophor-ethanol forming micelles in PBS (91). However, recently it was proposed to change the formulation to Pluronic 123-based micelles (92). Redaporfin was tested in a clinical trial for advanced head and neck cancer using a dose escalation strategy (93). The investigators studying Redaporfin have investigated the induction of anti-tumor immune response in animal models (94), and a case report used administration of a checkpoint inhibitor (anti-PD1 antibody) after Redaporfin PDT in a patient with recurrent cancer in the oral cavity to produce a sustained complete response (95).
Fimaporfin
The concept of photochemical internalization (PCI) was developed by Kristian Berg at the University of Oslo Radium Hospital in Norway (96). Although it uses a PS of the type that has previously been tested for PDT of cancer, PCI is being clinically developed in combination with a traditional anti-cancer chemotherapeutic drug. The idea is that the PS localizes in the membrane of endocytic vesicles that also contain the chemotherapy drug, and when light is delivered the vesicles are ruptured thus delivering the drug into the cytosol of the cancer cells. The first photosensitzers used for PCI were asymmetric porphyrin or phthalocyanine disulfonates but when the procedure was licensed to PCI Biotech, Oslo, Norway, an asymetrical PS was specially designed to mediate PCI called Amphinex, disulfonated tetraphenyl chlorin (TPCS2a) (Figure 3c) (97). The phase 1 clinical trial involved patients with localized recurrent, advanced, or metastatic cutaneous or subcutaneous malignancies in combination with bleomycin (98). Patients were given TPCS2a (0·25 – 1.5 mg/kg) on day 0 by slow intravenous injection, followed by a fixed dose of 15,000 IU/m2 bleomycin by intravenous infusion on day 4. After 3 h, the tumor was illuminated with 60 J/cm2 of 652 nm laser. At day 28, there was 58% complete response, 11% partial response, and 11% stable disease. Side effects were dose-related skin photosensitivity. The ongoing trials are now using gemcitabine instead of bleomycin as the drug whose delivery will be potentiated by PCI. These trials are designed for inoperable extrahepatic cholangiocarcinoma and involve a 0.22 mg/kg dose of Fimaporfin, followed 4 days later by a standard infusion of gemcitabine (1000 mg/m2) and intraluminal laser application (99, 100). Up to 2 PCI treatments can be given and additional cycles of cisplatin/gemcitabine chemotherapy will be given.
Silicon phthalocyanine PC4
PC4 (Figure 3d) is an axially substituted silicon phthalocyanine that was synthesized by Malcolm Kenney at Case Western University OH (101, 102). It has not been licensed to any company and is produced under GMP by the NCI It was investigated as an antimicrobial photosensitizer (103) and for photochemical decontamination of blood products (104). PC4 has been found to be a specific inducer of apoptosis in cancer cells via causing photodamage to Bcl2 and cardiolipin (105). PC4 is not water-soluble and efforts were made to devise a formulated delivery vehicle in PEG-polycaprolactone micelles (106). PC4 has been tested as a topically applied PS for various cutaneous malignancies. A phase 1 trial was conducted for cutaneous T-cell lymphoma of mycosis fungoides type with a dose escalation of topical PC4 (0.01, 0.05, or 0.10 mg/mL) under occlusion for 1 hour followed by increasing light fluence of 675 nm laser (50–100 J/cm2; 100 mW/cm2) (107, 108). 14 of 35 subjects demonstrated a clinical response. Another trial using dose escalation for primary or metastatic cutaneous cancers was terminated before the maximum tolerated dose was reached to poor recruitment (109).
TLD1433
TLD1433 is a water-soluble photosensitizer with a novel chemical structure, consisting of a ruthenium-based dipyridyl coordination complex with an attached terthienyl group (Figure 3e) (110). It was synthesized by the laboratory of Sherri McFarland in Nova Scotia, Canada and was licensed to Theralase, Toronto, Canada. Lothar Lilge helped develop the light device and light protocol to activate TLD1433 for treating non-muscle invasive bladder cancer with PDT (111). It is activated by green light (530 nm) and was originally tested by McFarland for anticancer applications, and then as an antimicrobial PS that was also active in hypoxic conditions (110). A Phase Ib trial studied intravesical PDT in patients with non-muscle invasive bladder cancer at high risk of progression who are refractory to Bacillus Calmette-Guerin therapy (111, 112). A single instillation of TLD-1433 at a dose 0.7 mg/cm2 was infused intravesically into the bladder for approximately 60 minutes. PDT was performed after TLD1433 had been rinsed from the bladder. The Phase 1b trial used a single treatment, but the Phase 2 trial that is underway will use two treatment procedures, one treatment at Day 0 and a second treatment at Day 180.
CLINICAL PHOTOSENSITIZERS IN NON-WESTERN COUNTRIES
Photolon
Photolon (or Fotolon) is a preparation of chlorin(e6) formulated (1:1 w/w) in polyvinylpyrrolidone (av MW 12,000) (113). It is manufactured by RUE “Belmedpreparaty” (Minsk, Republic of Belarus) and was approved in Belarus in 2001 and in Russia in 2004. Photolon has been tested clinically in several different tumor types mostly in Russia (114). These were skin tumors, cervical intraepithelial neoplasia, lung cancer, disseminated forms of melanoma, primary and metastatic brain tumors (intraoperative). Photolon is usually administered IV at a dose of 2-2.5 mg/kg followed after 3 hours by delivery of 662 nm laser at doses of 50-600 J/cm2 depending on the tumor location.
Radachlorin
Radachlorin also known as Bremachlorin (115) is a mixture of the sodium salts of chlorin(e6), chlorin(p6) and purpurin and is manufactured by RadaPharma in Moscow, Russia. It is injected IV at a dose of a dose 0.5-2.4 mg/kg with a 3 hour drug-light interval and exposure of the tumor to 662 nm laser at 200-300 J/cm2. It received Russian regulatory approval in 2009. It was tested in a phase 2 clinical trial for skin cancer (115), in obstructive advanced non-small cell lung cancer (116), and in nonresectable cholangiocarcinoma complicated by obstructive jaundice (117).
Photodithazine
Photodithazine (Fotoditazin) is the N-dimethylglucamine salt of chlorin(e6) invented by Ponomarev Helium Vasilievich, prepared by Veta-Grand LLC, Moscow, Russia and has received regulatory approval in Russia (118). It has been mainly studied as an antimicrobial PS, but has been used in clinical trials of basal cell carcinoma of the skin (119) and in case studies in advanced lung cancer, bladder cancer and brain tumors (120).
Photosens
Photosens is a water-soluble mixture of aluminum sulfonated phthalocyanines with various degrees of sulfonation (n = 2, 3 or 4, mean n = 3.1), prepared by State Scientific Center “NIOPIC”, Moscow, Russia (121). It received a series of regulatory approvals in Russia between 2001-2008. It is used at an IV dose of 0.3-0.8 mg/kg, with multiple 675 nm laser irradiations delivered between 24 and 72 hours post-injection each at 80-100 J/cm2 with a total up to 600J/cm2 (120). It has been tested in Russian clinical trials for basal cell carcinoma, advanced lung cancer, recurrent head and neck cancer, primary or recurrent gastric cancer, cancer of the esophagus, pleural mesothelioma and breast cancer metastases (120).
Hemoporfin
Hemoporfin was invented in China and is a single pure compound with the chemical structure of hematoporphyrin monomethyl ether (Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd., Shanghai, China). It received Chinese regulatory approval for PDT of port-wine stains in 2012, and there are ongoing trials for this indication in children (122). It has been proposed that hemoporfin could also be investigated for endoluminal cancer.
CONCLUSIONS AND FUTURE OUTLOOK
Clinical PDT for cancer is considered as having had a somewhat checkered history (with the exception of topical PDT using ALA and ALA esters). The reasons for this are many and various, and are not directly connected to any failure in the underlying premise of PDT, namely that photochemically generated ROS will efficiently destroy tumors by a variety of biological mechanisms. The fact that Photofrin was developed as the first widely used PS may explain some of the doubtful opinions about the long-term acceptance of anti-cancer PDT. While Photofrin is still widely used all over the world, it is beset with several problems. The most troubling is the long-lasting skin photosensitivity, which can prove troublesome, especially for patients with advanced cancer. These patients (who may not have very long left to live) do not relish being confined to semi-darkness, when they could be out in the sunshine with their loved ones. Moreover the preparation is an undefined mixture of chemical compounds which is not preferred by regulatory authorities, and its absorption peak is small and occurs at relatively short red wavelengths. The drug-light interval of 48 hours is also not convenient for clinicians.
Foscan was approved in Europe but not in USA, which did not encourage its wider adoption throughout the world. Foscan may be even too powerful a PS, as shown by the low clinical dose required and the incidence of skin burns at the injection site. Particularly in the UK, the cost of treatment is an issue to the National Health Service, and Foscan-PDT has been extensively analyzed for its approved indication in advanced head and neck cancer (123). Moreover the formulation of Foscan is not ideal, and efforts to formulate it in liposomes (Foslip) and pegylated liposomes (Fospeg) have not been clinically successful (124).
Verteporfin or Visudyne was very successful as a PS for wet AMD and other ophthalmological indications, and it was only the advent of monoclonal antibodies that led to its relative decline. It is only fairly recently that verteporfin has been investigated as a PS for cancer. If QLT had decided to advance it in clinical cancer indications much earlier then the story might have been different. Its satisfactory formulation, good optical absorption peak, and fast clearance pharmacokinetics are distinct advantages.
The failure of several companies that had raised considerable funding to carry out clinical trials of their proprietary PS for cancer was also decidedly pessimistic for the entire field. Perhaps these companies were victims of their own initial success that encouraged unrealistic expectations.
However, there have been some recent successes in the anti-cancer PDT field that may provide some “light at the end of the tunnel”. Despite the fact that hydrophobic non–water soluble PS are usually more powerful in the laboratory compared to water-soluble compounds, the disadvantages of requiring a formulation or a delivery vehicle, longer clearance times which translate into more prolonged skin photosensitivity, have motivated a switch to using water-soluble PS. This was exemplified by the switch from Tookad to Tookad soluble.
Another consideration for the future is the extent to which PDT can stimulate an anti-tumor immune response. Although this has been widely studied in animal models, it has not been much investigated in humans. The emphasis placed by the investigators studying Redaporfin, is the first real attempt to include this consideration in clinical trials. The move towards shorter drug-light intervals (minutes or hours rather than days) makes it much easier for both patients and clinicians to schedule treatments, and the fact that Tookad VTP for prostate cancer is an out-patient procedure will favor wider adoption. The translation of photochemical internalization into clinical trials is also an interesting new direction.
In conclusion, newer types of PS, better formulations, increased understanding of the tumor types that are best suited to PDT, more convenient patient procedures, fewer adverse side effects, will all tend to work in concert to overcome the pessimistic outlook which is still somewhat prevalent among mainstream medical opinion. The long-term legacy of Thomas Dougherty will then have been firmly established.
Acknowledgments
FUNDING AND CONFLICTS OF INTEREST
MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE.
Footnotes
This article is part of a Special Issue dedicated to Dr. Thomas Dougherty.
References
- 1.Raab O (1900) Uber die Wirkung fluoreszierender Stoffe auf Infusorien. Z Biol 39 524–546. [Google Scholar]
- 2.Von Tappenier H (1900) Uber die Wirkung fluoreszierender Stoffe auf Infusorien nach Versuchen von O. Raab. Muench Med Wochenschr 47, 5. [Google Scholar]
- 3.Jesionek A and von Tappenier H (1903) Therapeutische Versuche mit fluoreszierenden Stoffen. Muench Med Wochneshr 47, 2042–2044. [Google Scholar]
- 4.Von Tappeiner H and Jodlbauer A (1904) Uber Wirkung der photodynamischen (fluorieszierenden) Stoffe auf Protozoan und Enzyme. Dtsch Arch Klin Med 80, 427–487. [Google Scholar]
- 5.Von Tappeiner H and Jodlbauer A (1907) Die Sensibilisierende Wirkung fluorieszierender Substanzer Gesammte Untersuchungen uber die photodynamische Erscheinung. F. C. W. Vogel, Leipzig. [Google Scholar]
- 6.Manganiello LO and Figge FH (1951) Cancer detection and therapy II. Methods of preparation and biological effects of metalloporphyrins. Bull School Med Univ Maryland 36, 3–7. [PubMed] [Google Scholar]
- 7.Peck GC, Mack HP and Holbrook WA (1955) Use of hematoporphyrin fluorescence in biliary and cancer surgery. Ann Surg 21, 181–188. [PubMed] [Google Scholar]
- 8.Rassmussan-Taxdal DS, Ward GE and Figge FH (1955) Fluorescence of human lymphatic and cancer tissues following high doses of intravenous hematoporphyrin. Cancer 8, 78–81. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SK, Absolon K and Vermund H (1955) Some relationships of porphyrins, x-rays and tumours. Univ Minn Med Bull 27, 7–8. [Google Scholar]
- 10.Lipson RL, Baldes EJ and Gray MJ (1967) Hematoporphyrin derivative for detection and management of cancer. Cancer 20, 2255–2257. [DOI] [PubMed] [Google Scholar]
- 11.Lipson RL, Baldes EJ and Olsen AM (1961) Hematoporphyrin derivative: a new aid for endoscopic detection of malignant disease. J Thorac Cardiovasc Surg 42, 623–629. [PubMed] [Google Scholar]
- 12.Diamond I, Granelli SG, McDonagh AF, Nielsen S, Wilson CB and Jaenicke R (1972) Photodynamic therapy of malignant tumours. Lancet 2, 1175–1177. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty TJ (1974) Activated dyes as antitumor agents. J Natl Cancer Inst 52, 1333–1336. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty TJ, Grindey GB, Fiel R, Weishaupt KR and Boyle DG (1975) Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst 55, 115–121. [DOI] [PubMed] [Google Scholar]
- 15.Kelly JF and Snell ME (1976) Hematoporphyrin derivative: a possible aid in the diagnosis and therapy of carcinoma of the bladder. J Urol 115, 150–151. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D and Mittleman A (1978) Photoradiation therapy for the treatment of malignant tumors. Cancer Res 38, 2628–2635. [PubMed] [Google Scholar]
- 17.Dougherty TJ (2007) A Personal History of Photodynamic Therapy In Regional Cancer Therapy. Cancer Drug Discovery and Development. (Edited by Schlag PM, Stein U and Eggermont AMM). Humana Press, Totowa, NJ. [Google Scholar]
- 18.Dougherty TJ (2008) The Roswell Park History of PDT: 1972 to the Present: A Personal Perspective In Photodynamic Therapy of Diseases of the Head and Neck (Edited by Biel MA). Plural Publishing, San Diego, CA. [Google Scholar]
- 19.Hamblin MR and Huang YY (2013) Handbook of Photomedicine. CRC Press, Boca Raton, FL. [Google Scholar]
- 20.Fargnoli MC and Peris K (2015) Photodynamic therapy for basal cell carcinoma. Future Oncol 11, 2991–2996. [DOI] [PubMed] [Google Scholar]
- 21.Szeimies RM (2007) Methyl aminolevulinate-photodynamic therapy for basal cell carcinoma. Dermatol Clin 25, 89–94. [DOI] [PubMed] [Google Scholar]
- 22.Levy J and Levt E (2016) Photofrin-PDT from bench to bedside: some lessons learned In Handbook Of Photodynamic Therapy: Updates On Recent Applications Of Porphyrin-based Compounds. (Edited by Pandey RK, Dougherty TJ and Kessel D). World Scientific Publishing, Singapore. [Google Scholar]
- 23.Bonnett R and Berenbaum MC (1983) HPD - a study of its components and their properties. Adv Exp Med Biol 160, 241–250. [DOI] [PubMed] [Google Scholar]
- 24.Kessel D (1986) Proposed structure of the tumor-localizing fraction of HPD (hematoporphyrin derivative). Photochem Photobiol 44, 193–196. [DOI] [PubMed] [Google Scholar]
- 25.Moan J, Sandberg S, Christensen T and Elander S (1983) Hematoporphyrin derivative: chemical composition, photochemical and photosensitizing properties. Adv Exp Med Biol 160, 165–179. [DOI] [PubMed] [Google Scholar]
- 26. https://clinicaltrials.gov/ct2/show/NCT02082522.
- 27. https://clinicaltrials.gov/ct2/show/NCT01506115.
- 28. https://clinicaltrials.gov/ct2/show/NCT01966809.
- 29. https://clinicaltrials.gov/ct2/show/NCT01770132.
- 30. https://clinicaltrials.gov/ct2/show/NCT03727061.
- 31. https://clinicaltrials.gov/ct2/show/NCT02153229.
- 32. https://clinicaltrials.gov/ct2/show/NCT01872104.
- 33.Moriwaki SI, Misawa J, Yoshinari Y, Yamada I, Takigawa M and Tokura Y (2001) Analysis of photosensitivity in Japanese cancer-bearing patients receiving photodynamic therapy with porfimer sodium (Photofrin). Photodermatol Photoimmunol Photomed 17, 241–243. [DOI] [PubMed] [Google Scholar]
- 34.Yachimski P, Puricelli WP and Nishioka NS (2008) Patient predictors of esophageal stricture development after photodynamic therapy. Clin Gastroenterol Hepatol 6, 302–308. [DOI] [PubMed] [Google Scholar]
- 35. https://clinicaltrials.gov/ct2/show/NCT00243490.
- 36.Stranadko EP, Skobelkin OK and Mironov AE (1994) Photodynamic therapy of cancer by photogem. Proc. SPIE 2078, 10.1117/1112.168695. [DOI] [Google Scholar]
- 37.Sun M, Zhou C, Zeng H, Puebla-Osorio N, Damiani E, J. C, Wang HW, Li G , Yin F, Shan L, Zuo D, Liao Y, Wang Z, Zheng L, Hua Y and Cai Z (2015) Hiporfin-Mediated Photodynamic Therapy in Preclinical Treatment of Osteosarcoma. Photochem Photobiol 91, 533–544. [DOI] [PubMed] [Google Scholar]
- 38. https://clinicaltrials.gov/ct2/show/NCT02955771.
- 39. https://www.ema.europa.eu/en/medicines/human/EPAR/foscan.
- 40. https://clinicaltrials.gov/ct2/show/NCT03003065.
- 41. https://clinicaltrials.gov/ct2/show/NCT01637376.
- 42. https://clinicaltrials.gov/ct2/show/NCT01086488.
- 43.Bryce R (2000) Burns after photodynamic therapy. Drug point gives misleading impression of incidence of burns with temoporfin (Foscan). BMJ 320, 1731–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott LJ and Goa KL (2000) Verteporfin. Drugs Aging 16, 139–146; discussion 147-138. [DOI] [PubMed] [Google Scholar]
- 45.Houle JM and Strong HA (2002) Duration of skin photosensitivity and incidence of photosensitivity reactions after administration of verteporfin. Retina 22, 691–697. [DOI] [PubMed] [Google Scholar]
- 46.Birngruber R, Miller JW, van den Burgh H and T. S. group) (1999) Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials--TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 117, 1329–1345. [PubMed] [Google Scholar]
- 47.Gao Y, Yu T, Zhang Y and Dang G (2018) Anti-VEGF Monotherapy Versus Photodynamic Therapy and Anti-VEGF Combination Treatment for Neovascular Age-Related Macular Degeneration: A Meta-Analysis. Invest Ophthalmol Vis Sci 59, 4307–4317. [DOI] [PubMed] [Google Scholar]
- 48.Newman DK (2016) Photodynamic therapy: current role in the treatment of chorioretinal conditions. Eye (Lond) 30, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. https://clinicaltrials.gov/ct2/show/NCT02872064.
- 50. https://clinicaltrials.gov/ct2/show/NCT02939274.
- 51. https://clinicaltrials.gov/ct2/show/NCT03033225.
- 52. https://clinicaltrials.gov/ct2/show/NCT00002647.
- 53. https://clinicaltrials.gov/ct2/show/NCT00049959.
- 54. https://clinicaltrials.gov/ct2/show/NCT00007969.
- 55.Scherz A and Salomon Y (2014) The story of Tookad: from bench to bedside In Handbook of Photomedicine. (Edited by Hamblin MR and Huang YY). CRC Press, Boca Raton, FL. [Google Scholar]
- 56.Betrouni N, Boukris S and Benzaghou F (2017) Vascular targeted photodynamic therapy with TOOKAD(R) Soluble (WST11) in localized prostate cancer: efficiency of automatic pre-treatment planning. Lasers Med Sci 32, 1301–1307. [DOI] [PubMed] [Google Scholar]
- 57.Azzouzi AR, Lebdai S, Benzaghou F and Stief C (2015) Vascular-targeted photodynamic therapy with TOOKAD(R) Soluble in localized prostate cancer: standardization of the procedure. World J Urol 33, 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azzouzi AR, Vincendeau S, Barret E, Cicco A, Kleinclauss F, van der Poel HG, Stief CG, Rassweiler J, Salomon G, Solsona E, Alcaraz A, Tammela TT, Rosario DJ, Gomez-Veiga F, Ahlgren G, Benzaghou F, Gaillac B, Amzal B, Debruyne FM, Fromont G, Gratzke C, Emberton M and P. C. M. S. Group (2017) Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 18, 181–191. [DOI] [PubMed] [Google Scholar]
- 59. https://clinicaltrials.gov/ct2/show/NCT03849365.
- 60. https://clinicaltrials.gov/ct2/show/NCT03315754?
- 61. https://clinicaltrials.gov/ct2/show/NCT01573156.
- 62.Wang S, Bromley E, Xu L, Chen JC and Keltner L (2010) Talaporfin sodium. Expert Opin Pharmacother 11, 133–140. [DOI] [PubMed] [Google Scholar]
- 63.Hargus JA, Fronczek FR, Vicente MG and Smith KM (2007) Mono-(L)-aspartylchlorin-e6. Photochem Photobiol 83, 1006–1015. [DOI] [PubMed] [Google Scholar]
- 64. https://clinicaltrials.gov/ct2/show/NCT00355355.
- 65. https://clinicaltrials.gov/ct2/show/NCT00068068.
- 66.Sessler JL and Miller RA (2000) Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem Pharmacol 59, 733–739. [DOI] [PubMed] [Google Scholar]
- 67.Yeung PF (2001) Motexafin lutetium (Pharmacyclics). IDrugs 4, 351–359. [PubMed] [Google Scholar]
- 68.Patel H, Mick R, Finlay J, Zhu TC, Rickter E, Cengel KA, Malkowicz SB, Hahn SM and Busch TM (2008) Motexafin lutetium-photodynamic therapy of prostate cancer: short- and long-term effects on prostate-specific antigen. Clin Cancer Res 14, 4869–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young SW, Woodburn KW, Wright M, Mody TD, Fan Q, Sessler JL, Dow WC and Miller RA (1996) Lutetium texaphyrin (PCI-0123): a near-infrared, water-soluble photosensitizer. Photochem Photobiol 63, 892–897. [DOI] [PubMed] [Google Scholar]
- 70.Du KL, Mick R, Busch TM, Zhu TC, Finlay JC, Yu G, Yodh AG, Malkowicz SB, Smith D, Whittington R, Stripp D and Hahn SM (2006) Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg Med 38, 427–434. [DOI] [PubMed] [Google Scholar]
- 71.Finlay JC, Zhu TC, Dimofte A, Stripp D, Malkowicz SB, Busch TM and Hahn SM (2006) Interstitial fluorescence spectroscopy in the human prostate during motexafin lutetium-mediated photodynamic therapy. Photochem Photobiol 82, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dimofte A, Zhu TC, Hahn SM and Lustig RA (2002) In vivo light dosimetry for motexafin lutetium-mediated PDT of recurrent breast cancer. Lasers Surg Med 31, 305–312. [DOI] [PubMed] [Google Scholar]
- 73. https://clinicaltrials.gov/ct2/show/NCT00087191.
- 74.Wang HW, Finlay JC, Lee K, Zhu TC, Putt ME, Glatstein E, Koch CJ, Evans SM, Hahn SM, Busch TM and Yodh AG (2007) Quantitative comparison of tissue oxygen and motexafin lutetium uptake by ex vivo and noninvasive in vivo techniques in patients with intraperitoneal carcinomatosis. J Biomed Opt 12, 034023. [DOI] [PubMed] [Google Scholar]
- 75. https://clinicaltrials.gov/ct2/show/NCT00005808.
- 76.Kereiakes DJ, Szyniszewski AM, Wahr D, Herrmann HC, Simon DI, Rogers C, Kramer P, Shear W, Yeung AC, Shunk KA, Chou TM, Popma J, Fitzgerald P, Carroll TE, Forer D and Adelman DC (2003) Phase I drug and light dose-escalation trial of motexafin lutetium and far red light activation (phototherapy) in subjects with coronary artery disease undergoing percutaneous coronary intervention and stent deployment: procedural and long-term results. Circulation 108, 1310–1315. [DOI] [PubMed] [Google Scholar]
- 77.Criswell MH, Ciulla TA, Lowseth LA, Small W, Danis RP and Carson DL (2005) Anastomotic vessels remain viable after photodynamic therapy in primate models of choroidal neovascularization. Invest Ophthalmol Vis Sci 46, 2168–2174. [DOI] [PubMed] [Google Scholar]
- 78.Selman SH, Qin BS, James HR, Keck RW, Garbo GM and Morgan AR (1990) Hemodynamic effect of the metallopurpurin SnET2 and light on transplantable FANFT induced bladder tumor. J Urol 143, 630–633. [DOI] [PubMed] [Google Scholar]
- 79.Hunt DW (2004) Rostaporfin: PhotoPoint SnET2, Purlytin, Sn(IV) etiopurpurin, SnET2, tin ethyl etiopurpurin. Drugs R D 5, 58–61. [DOI] [PubMed] [Google Scholar]
- 80.Mang TS, Allison R, Hewson G, Snider W and Moskowitz R (1998) A phase II/III clinical study of tin ethyl etiopurpurin (Purlytin)-induced photodynamic therapy for the treatment of recurrent cutaneous metastatic breast cancer. Cancer J Sci Am 4, 378–384. [PubMed] [Google Scholar]
- 81. https://clinicaltrials.gov/ct2/show/NCT00002167.
- 82. https://clinicaltrials.gov/ct2/show/NCT00157976.
- 83.Isele U, van Hoogevest P, Hilfiker R, Capraro HG, Schieweck K and Leuenberger H (1994) Large-scale production of liposomes containing monomeric zinc phthalocyanine by controlled dilution of organic solvents. J Pharm Sci 83, 1608–1616. [DOI] [PubMed] [Google Scholar]
- 84.Henderson BW, Bellnier DA, Greco WR, Sharma A, Pandey RK, Vaughan LA, Weishaupt KR and Dougherty TJ (1997) An in vivo quantitative structure-activity relationship for a congeneric series of pyropheophorbide derivatives as photosensitizers for photodynamic therapy. Cancer Res 57, 4000–4007. [PubMed] [Google Scholar]
- 85.Bellnier DA, Greco WR, Nava H, Loewen GM, Oseroff AR and Dougherty TJ (2006) Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother Pharmacol 57, 40–45. [DOI] [PubMed] [Google Scholar]
- 86.Zhu TC, Ong Y, Kim MM, Liang X, Finlay JC, Dimofte A, Simone Nd CB, Friedberg JS, Busch TM, Glatstein E and Cengel KA (2019) Evaluation of Light Fluence Distribution Using an IR Navigation System for HPPH-mediated Pleural Photodynamic Therapy (pPDT). Photochem Photobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. https://clinicaltrials.gov/ct2/show/NCT00060268.
- 88. https://clinicaltrials.gov/ct2/show/NCT00528775.
- 89.Shafirstein G, Rigual NR, Arshad H, Cooper MT, Bellnier DA, Wilding G, Tan W, Merzianu M and Henderson BW (2016) Photodynamic therapy with 3-(1’-hexyloxyethyl) pyropheophorbide-a for early-stage cancer of the larynx: Phase Ib study. Head Neck 38 Suppl 1, E377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dabrowski JM, Arnaut LG, Pereira MM, Monteiro CJ, Urbanska K, Simoes S and Stochel G (2010) New halogenated water-soluble chlorin and bacteriochlorin as photostable PDT sensitizers: synthesis, spectroscopy, photophysics, and in vitro photosensitizing efficacy. ChemMedChem 5, 1770–1780. [DOI] [PubMed] [Google Scholar]
- 91.Rocha LB, Schaberle F, Dabrowski JM, Simoes S and Arnaut LG (2015) Intravenous Single-Dose Toxicity of Redaporfin-Based Photodynamic Therapy in Rodents. Int J Mol Sci 16, 29236–29249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pucelik B, Arnaut LG, Stochel G and Dabrowski JM (2016) Design of Pluronic-Based Formulation for Enhanced Redaporfin-Photodynamic Therapy against Pigmented Melanoma. ACS Appl Mater Interfaces 8, 22039–22055. [DOI] [PubMed] [Google Scholar]
- 93. https://clinicaltrials.gov/ct2/show/NCT02070432.
- 94.Gomes-da-Silva LC, Zhao L, Arnaut LG, Kroemer G and Kepp O (2018) Redaporfin induces immunogenic cell death by selective destruction of the endoplasmic reticulum and the Golgi apparatus. Oncotarget 9, 31169–31170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos LL, Oliveira J, Monteiro E, Santos J and Sarmento C (2018) Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep Oncol 11, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berg K, Selbo PK, Prasmickaite L and Hogset A (2004) Photochemical drug and gene delivery. Curr Opin Mol Ther 6, 279–287. [PubMed] [Google Scholar]
- 97.Selbo PK, Bostad M, Olsen CE, Edwards VT, Hogset A, Weyergang A and Berg K (2015) Photochemical internalisation, a minimally invasive strategy for light-controlled endosomal escape of cancer stem cell-targeting therapeutics. Photochem Photobiol Sci 14, 1433–1450. [DOI] [PubMed] [Google Scholar]
- 98.Sultan AA, Jerjes W, Berg K, Hogset A, Mosse CA, Hamoudi R, Hamdoon Z, Simeon C, Carnell D, Forster M and Hopper C (2016) Disulfonated tetraphenyl chlorin (TPCS2a)-induced photochemical internalisation of bleomycin in patients with solid malignancies: a phase 1, dose-escalation, first-in-man trial. Lancet Oncol 17, 1217–1229. [DOI] [PubMed] [Google Scholar]
- 99. https://clinicaltrials.gov/ct2/show/NCT04099888.
- 100. https://clinicaltrials.gov/ct2/show/NCT01900158.
- 101.Anderson CY, Freye K, Tubesing KA, Li YS, Kenney ME, Mukhtar H and Elmets CA (1998) A comparative analysis of silicon phthalocyanine photosensitizers for in vivo photodynamic therapy of RIF-1 tumors in C3H mice. Photochem Photobiol 67, 332–336. [PubMed] [Google Scholar]
- 102.He J, Larkin HE, Li YS, Rihter D, Zaidi SI, Rodgers MA, Mukhtar H, Kenney ME and Oleinick NL (1997) The synthesis, photophysical and photobiological properties and in vitro structure-activity relationships of a set of silicon phthalocyanine PDT photosensitizers. Photochem Photobiol 65, 581–586. [DOI] [PubMed] [Google Scholar]
- 103.Dimaano ML, Rozario C, Nerandzic MM, Donskey CJ, Lam M and Baron ED (2015) The photodynamic antibacterial effects of silicon phthalocyanine (Pc) 4. Int J Mol Sci 16, 7851–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ben-Hur E, Chan WS, Yim Z, Zuk MM, Dayal V, Roth N, Heldman E, Lazo A, Valeri CR and Horowitz B (2000) Photochemical decontamination of red blood cell concentrates with the silicon phthalocyanine PC 4 and red light. Dev Biol (Basel) 102, 149–155. [PubMed] [Google Scholar]
- 105.Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McCormick T, Colussi V, Kenney ME, Cooper KD and Oleinick NL (2007) Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharmacol 224, 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Master AM, Rodriguez ME, Kenney ME, Oleinick NL and Gupta AS (2010) Delivery of the photosensitizer Pc 4 in PEG-PCL micelles for in vitro PDT studies. J Pharm Sci 99, 2386–2398. [DOI] [PubMed] [Google Scholar]
- 107.Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hanneman KK, Hsia AH, Oleinick NL, Colussi VC and Cooper KD (2010) Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a phase 1 clinical trial. Lasers Surg Med 42, 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. https://clinicaltrials.gov/ct2/show/NCT01800838.
- 109.Kinsella TJ, Baron ED, Colussi VC, Cooper KD, Hoppel CL, Ingalls ST, Kenney ME, Li X, Oleinick NL, Stevens SR and Remick SC (2011) Preliminary clinical and pharmacologic investigation of photodynamic therapy with the silicon phthalocyanine photosensitizer pc 4 for primary or metastatic cutaneous cancers. Front Oncol 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arenas Y, Monro S, Shi G, Mandel A, McFarland S and Lilge L (2013) Photodynamic inactivation of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus with Ru(II)-based type I/type II photosensitizers. Photodiagnosis Photodyn Ther 10, 615–625. [DOI] [PubMed] [Google Scholar]
- 111. https://clinicaltrials.gov/ct2/show/NCT03053635.
- 112. https://clinicaltrials.gov/ct2/show/NCT03945162.
- 113.Isakau HA, Parkhats MV, Knyukshto VN, Dzhagarov BM, Petrov EP and Petrov PT (2008) Toward understanding the high PDT efficacy of chlorin e6-polyvinylpyrrolidone formulations: photophysical and molecular aspects of photosensitizer-polymer interaction in vitro. J Photochem Photobiol B 92, 165–174. [DOI] [PubMed] [Google Scholar]
- 114.Istomin YP, Kaplan MA, Shliakhtsin SV, Lapzevich TP, Cerkovsky DAA, Marchanka LM, Fedulov AS and Trukhachova TV (2009) Immediate and long-term efficacy and safety of photodynamic therapy with Photolon (Fotolon): a seven-year clinical experience. Proc. SPIE 7380, 73806V [Google Scholar]
- 115.Kochneva EV, Filonenko EV, Vakulovskaya EG, Scherbakova EG, Seliverstov OV, Markichev NA and Reshetnickov AV (2010) Photosensitizer Radachlorin(R): Skin cancer PDT phase II clinical trials. Photodiagnosis Photodyn Ther 7, 258–267. [DOI] [PubMed] [Google Scholar]
- 116.Ji W, Yoo JW, Bae EK, Lee JH and Choi CM (2013) The effect of Radachlorin(R) PDT in advanced NSCLC: a pilot study. Photodiagnosis Photodyn Ther 10, 120–126. [DOI] [PubMed] [Google Scholar]
- 117.Shiryaev AA, Musaev GK, Levkin VV, Reshetov IV, Loshchenov MV, Alekseeva PM, Volkov VV, Linkov KG, Makarov VI, Shchekoturov IO, Borodkin AV and Loschenov VB (2019) Combined treatment of nonresectable cholangiocarcinoma complicated by obstructive jaundice. Photodiagnosis Photodyn Ther 26, 218–223. [DOI] [PubMed] [Google Scholar]
- 118. http://fotoditazin.com/napravleniya/14-fotodinamicheskaya-terapiya-v-onkologii.html.
- 119.Romanko YS, Kaplan MA, Ivanov SA, Galkin VN, Molochkova YV, Kuntsevich ZS, Tretiakova EI, Sukhova TE, Molochkov VA and Molochkov AV (2016) Efficacy of photodynamic therapy for basal cell carcinoma using photosensitizers of different classes. Vopr Onkol 62, 447–450. [PubMed] [Google Scholar]
- 120.Vakulovskaya EG (2014) Photodynamic therapy and fluorescent diagnostics in the Russian Federation In Handbook of Photomedicine. (Edited by Hamblin MR and Huang YY). CRC Press, Boca Raton, FL. [Google Scholar]
- 121.Brilkina AA, Dubasova LV, Sergeeva EA, Pospelov AJ, Shilyagina NY, Shakhova NM and Balalaeva IV (2019) Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J Photochem Photobiol B 191, 128–134. [DOI] [PubMed] [Google Scholar]
- 122. https://clinicaltrials.gov/ct2/show/NCT04106258.
- 123.Hopper C, Niziol C and Sidhu M (2004) The cost-effectiveness of Foscan mediated photodynamic therapy (Foscan-PDT) compared with extensive palliative surgery and palliative chemotherapy for patients with advanced head and neck cancer in the UK. Oral Oncol 40, 372–382. [DOI] [PubMed] [Google Scholar]
- 124.Gaio E, Scheglmann D, Reddi E and Moret F (2016) Uptake and photo-toxicity of Foscan(R), Foslip(R) and Fospeg(R) in multicellular tumor spheroids. J Photochem Photobiol B 161, 244–252. [DOI] [PubMed] [Google Scholar]