Summary
Cytolethal distending toxins (Cdt) are a family of toxins produced by several human pathogens which infect mucocutaneous tissue and induce inflammatory disease. We have previously demonstrated that the Aggregatibacter actinomycetemcomitans Cdt induces a pro-inflammatory response from human macrophages which involves activation of the NLRP3 inflammasome. We now demonstrate that in addition to activating caspase-1 (canonical inflammasome), Cdt treatment leads to caspase-4 activation and involvement of the noncanonical inflammasome. Cdt-treated cells exhibit pyroptosis characterized by cleavage of gasdermin-D (GSDMD), release of HMGB1 at 24 hr and LDH at 48 hr. Inhibition of either the canonical (caspase-1) or noncanonical (caspase-4) inflammasome blocks both Cdt-induced release of IL-1β and induction of pyroptosis. Analysis of upstream events indicates that Cdt induces Syk phosphorylation (activation); furthermore, blockade of Syk expression and inhibition of pSyk activity inhibit both Cdt-induced cytokine release and pyroptosis. Finally, we demonstrate that increases in pSyk are dependent upon Cdt-induced activation of GSK3β. These studies advance our understanding of Cdt function and provide new insight into the virulence potential of Cdt in mediating the pathogenesis of disease caused by Cdt-producing organisms such as A. actinomycetemcomitans.
Keywords: Microbial-cell interaction, Toxins, Inflammasome, Mechanism of action, Aggregatibacter actinomycetemcomitans
Introduction
Cytolethal distending toxins (Cdt) represent a family of heat-labile protein cytotoxins produced by over 30 γ- and ε-Proteobacteria that are human and/or animal pathogens. These bacteria share the common ability to colonize mucocutaneous tissue such as oral, gastrointestinal, urinary and respiratory tracts (DiRienzo, 2014a, 2014b; Gargi, Reno, & Blanke, 2012; Ge et al., 2017; Ge et al., 2005; Ge et al., 2007; Haghjoo & Galan, 2004; Jinadasa, Bloom, Weiss, & Duhamel, 2011; Lara-Tejero & Galan, 2001; Scuron, Boesze-Battaglia, Dlakic, & Shenker, 2016; Shenker et al., 1999; Thelestam & Frisan, 2004; Whitehouse et al., 1998; Young et al., 2004). Moreover, Cdt-producing bacteria are known to induce disease in these mucocutaneous niches that are characterized by sustained infection and inflammation. Collectively, our studies have demonstrated that Cdt, produced by the periodontal pathogen, Aggregatibacter actinomycetemcomitans, is capable of impairing lymphocyte proliferation and survival while also promoting production of pro-inflammatory mediators from macrophages (Shenker et al., 2005; Shenker, Demuth, & Zekavat, 2006; Shenker, Hoffmaster, McKay, & Demuth, 2000; Shenker et al., 2001; Shenker et al., 1999; Shenker et al., 2015; Shenker, Walker, Zekavat, Dlakic, & Boesze-Battaglia, 2014). Moreover, we have shown that these toxic effects are dependent upon the active Cdt subunit, CdtB, and its ability to function as a phosphatidylinositol (PI)-3,4,5-triphosphate (PIP3) phosphatase which depletes cells of PIP3 and induces blockade of the PI-3K signaling pathway (Shenker, Boesze-Battaglia, et al., 2016; Shenker et al., 2007; Shenker, Walker, Zekavat, & Boesze-Battaglia, 2016; Shenker et al., 2014).
Cdt is a heterotrimeric complex that functions as an AB2 toxin; in addition to CdtB, the toxin consists of two additional subunits, CdtA and CdtC, that are involved in toxin-host cell interactions (reviewed in (Gargi et al., 2012; Scuron et al., 2016). These interactions are dependent upon CdtC (and CdtB) binding to cholesterol within the plasma membrane (Boesze-Battaglia, Alexander, Dlakic, & Shenker, 2016; Boesze-Battaglia et al., 2009; Boesze-Battaglia et al., 2017; Boesze-Battaglia et al., 2015; Guerra et al., 2005; Lai et al., 2013; Zhou, Zhang, Zhao, & Jin, 2012). As noted above, internalization of CdtB leads to PI-3K signaling blockade within two hr in both lymphocytes and macrophages (Shenker, Boesze-Battaglia, et al., 2016; Shenker et al., 2007; Shenker et al., 2014). Toxin treated lymphocytes undergo cell cycle arrest (G2/M) and apoptosis. In contrast, Cdt-mediated PI-3K blockade in non-proliferating cells such as macrophages does not lead to apoptosis; instead the toxin induces a pro-inflammatory cytokine response. This response includes increased synthesis and release of TNFα and IL-6 as well as synthesis, maturation and release of IL-1β and IL-18 (Shenker et al., 2014). Recently, we also demonstrated that Cdt-mediated maturation and release of IL-1β and IL-18 were dependent upon activation of the NLRP3 inflammasome which in turn requires Cdt-dependent increases in extracellular ATP, activation of the P2X7 purinergic receptor and K+ efflux (Shenker et al., 2015).
Recent studies have demonstrated that release of mature IL-1β is not only regulated by canonical inflammasomes which involves caspase-1 activation, but can also involve stimulation of noncanonical inflammasomes leading to activation of other caspases such as caspase-4 and caspase- 5 (caspase-11 in mice) (Platnich & Muruve, 2019; Vigano et al., 2015). In light of these observations, we extended our studies on the pro-inflammatory effects of Cdt on macrophages and now report that Cdt-induced IL-1β release is also dependent upon caspase-4 activation. Furthermore, we demonstrate that Cdt-mediated activation of both the canonical and noncanonical inflammasome is regulated upstream by activation of spleen tyrosine kinase (Syk) and GSK3β, Activation of both inflammasomes leads downstream to pyroptosis exemplified by the release of both the high mobility group box 1 protein (HMGB1) and lactic dehydrogenase (LDH) along with cleavage of gasdermin D (GSDMD).
Results
Cdt activates the noncanonical inflammasome leading to pyroptosis
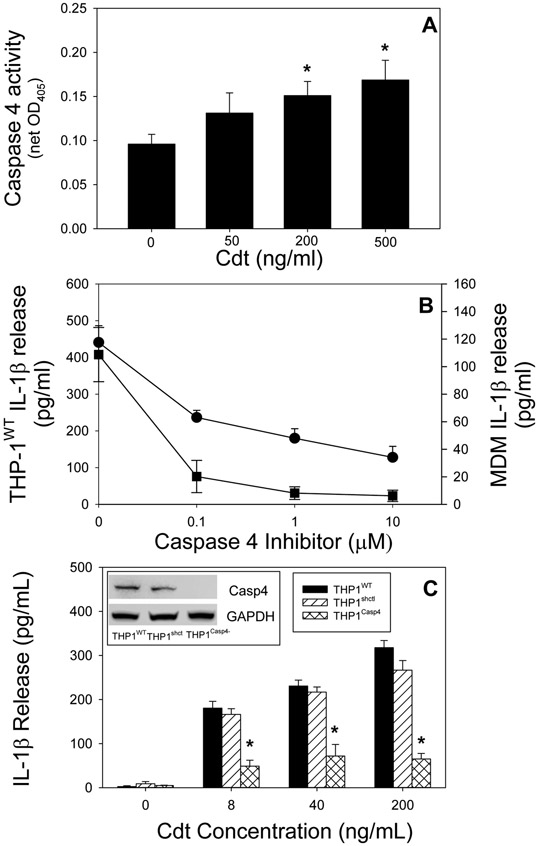
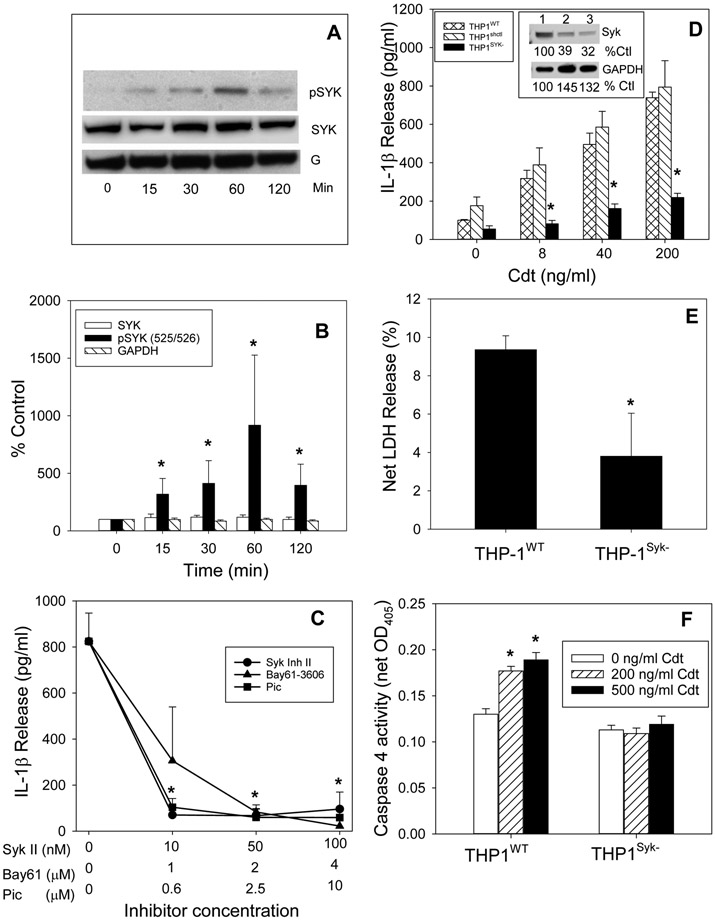
The goal of this study was to explore the contribution of noncanonical inflammasome pathways in mediating the Cdt-induced pro-inflammatory response in human macrophages derived from both THP-1 cells and monocyte-derived macrophgages (MDM). In this regard, the most studied mediator of the noncanonical inflammasome is caspase-11 in murine macrophages and caspase-4 (and -5), the human analog of caspase-11. Therefore, we focused our study on caspase-4 in macrophages derived from the human leukemia monocytic cell line (wild-type), THP-1WT. THP-1 WT-derived macrophages were treated with 0-500 ng/ml Cdt for 2 hrs and the cells analyzed for caspase-4 activation (Fig. 1A). Caspase 4 activity was monitored by measuring the net change in OD405 resulting from the release of the chromophore p-NA from LEVD–pNA as described in Materials and Methods. Caspase-4 activity exhibited a statistically significant dose-dependent increase over baseline levels representing increases of 44%, 67% and 89% in the presence of 50, 200 and 500 ng/ml Cdt, respectively. The magnitude of the increase in caspase-4 activity was comparable to the increment observed with LPS transfection (Fig. 1S).
Fig. 1.
Cdt-induced release of IL-1β is dependent upon caspase-4 activation. Panel A: THP-1WT-derived macrophages were treated with Cdt and analyzed for caspase-4 activation. Results are plotted as Cdt concentration vs caspase-4 activity (net OD405) and are the mean±SEM of four experiments. Panel B: THP-1WT- (circle) and monocyte (square)-derived macrophages were pre-treated with varying amounts of the caspase-4 inhibitor LEVD-CHO followed by the addition of 200 ng/ml Cdt. Supernatants were harvested 5 hrs later and analyzed for IL-1β by ELISA. Results are plotted as mean±SEM pg/ml IL-1β vs caspase-4 inhibitor concentration. Results are the mean of 3 experiments; note: samples receiving caspase-4 inhibitor were significantly lower at all concentrations than samples receiving Cdt alone (p<0.05). Panel C: Macrophages derived from THP-1WT (solid bar), THP-1shctrl (hatched bars) and THP-1Casp4− (cross-hatched bars) were treated with varying concentrations of Cdt for 5 hrs. Supernatants were harvested and analyzed by ELISA for IL-1β. Results are plotted as the mean±SEM pg/ml IL-1β for four experiments versus Cdt concentration. Inset shows caspase-4 protein levels (Western blot) in cell extracts obtained from THP-1WT , THP-1shctrl and THP-1CasP4− derived macrophages, *indicates statistical significance (p<0.05 relative to THP-1WT-derived macrophages treated with the same concentration of Cdt.
In order to determine if the increase in caspase-4 activity was critical to Cdt-induced release of mature IL-1β, THP-1WT macrophages were pre-treated with 0-10 μM of the cell permeable caspase-4 inhibitor (LEVD-CHO) (Fig. 1B). In the presence of 50 ng/ml Cdt, IL-1β levels increased to 441±46 pg/ml versus 41.7±2.9 pg/ml in untreated cells. Pre-treatment of macrophages with 0.1, 1.0 and 10 μM inhibitor reduced IL-1β release to 54%, 41% and 29% of levels observed with Cdt alone. The requirement for caspase-4 in mediating Cdt-induced release of IL-1β was also assessed in MDM; as shown in Fig. 1B, 200 ng/ml Cdt-induced an increase in IL-1β release to 108.8±19.7 pg/ml over baseline levels of 0.2±0.2 pg/ml. In the presence of 0.1, 1.0 and 10 μM caspase-4 inhibitor the amount of cytokine release was significantly reduced to <20% of levels observed in cells treated with toxin alone.
To rule out possible off-target effects of the caspase-4 inhibitor and more directly implicate caspase-4 in the pro-inflammatory response to Cdt, THP-1 cells deficient in caspase-4 expression (THP-1Casp4−) were assessed for their susceptibility to Cdt-induced release of IL-1β. As shown in Fig. 1C (inset), Western blot analysis of the protein levels of caspase-4 were not detectable in the THP-1Casp4− cells; in contrast, caspase-4 was present in THP-1WT cells. Cells were then treated with Cdt for 5 hr and the supernatants analyzed for IL-1β levels by ELISA. THP-1WT macrophages exhibited a dose-dependent increase in IL-1β release when challenged with 8, 40 and 200 ng/ml Cdt. In contrast, THPCasp4− cells were severely impaired in their ability to produce mature IL-1β as they released 48.9±13.5, 72.0±26.1 and 65.2±12.4 pg/ml in the presence of 8, 40 and 200 ng/ml Cdt, respectively. It should be noted that caspase-4 deficient cells retained the capacity to exhibit canonical inflammasome activation in response to Cdt as previously reported (Shenker et al., 2015); Cdt treated THP-1Casp4− derived macrophages demonstrated both an increase in pro-IL-1β and active caspase-1 (p20) in response to Cdt treatment (Fig. 2S).
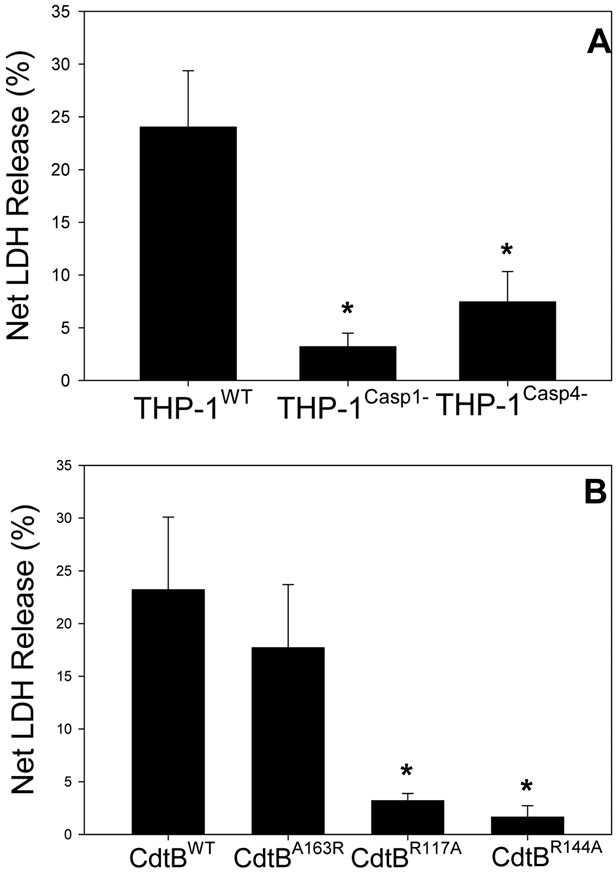
Our observations demonstrate that Cdt-induced release of IL-1β is dependent upon caspase-4 activation; this finding is significant as this caspase is involved in not only, the noncanonical inflammasome, but also pyroptosis, a form of inflammatory caspase-mediated lytic cell death (Martel, Lai, Ko, Young, & Ojcius, 2016; Taabazuing, Okondo, & Bachovchin, 2017; Xia, Wang, Zheng, Jiang, & Hu, 2019). In the next series of experiments we focused on the relationship between Cdt, caspase-4 activation, pyroptosis and ultimately the release of cytokines. First, we assessed Cdt’s ability to induce the release of LDH and HMGB1. THP-1WT derived macrophages were treated with Cdt for 48 hr and then cell supernatants were assessed for LDH activity. As shown in Fig. 2A, THP-1WT cells exhibited a 24% net increase in the release of LDH in the presence of Cdt (50 ng/ml). It should be noted that in preliminary experiments, LDH release was not observed at earlier time points. In contrast, THP-1Casp4− cells exhibited a 7% net increase in LDH; this was a statistically significantly less than the amount of LDH release observed with Cdt-treated THP-1WT cells. In addition to the noncanonical inflammasome, it has been reported that pyroptosis may also be induced by the canonical inflammasome (Shi, Gao, & Shao, 2017). Therefore, LDH release was also assessed in caspase-1 deficient cells. THP-1Casp−1− -derived macrophages were treated with Cdt for 48 hr and the supernatants assessed for enzymatic activity; these cells released 3% net LDH which was also a statistically significantly less than the amount observed with Cdt-treated THP-1WT cells.
Fig.2.
Cdt-induces LDH release from macrophages. Panel A: macrophages derived from THP-1Wl, THP-1CasP4− and THP-1Casp1− cells were incubated with 50 ng/ml Cdt; supernatants were harvested 48 hr later and analyzed for LDH activity. Results are plotted as the net release of LDH (%) mean±SEM obtained from four experiments vs Cdt concentration. Panel B: THP-1WT-derived macrophages were treated with 50 ng/ml Cdt containing CdtBWT, CdtBA163R, CdtBR117A or CdtBR144A Supernatants were obtained at 48 hrs and assayed for LDH activity. Results are plotted as in panel A and are the mean±SEM of four. * indicates statistical significance p<0.05 when compared to respective control (0 Cdt).
In another series of experiments we determined whether CdtB associated lipid phosphatase activity was required to induce LDH release from THP-1WT-derived macrophages. Cells were treated with 50 ng/ml Cdt containing either CdtBWT or CdtB mutant proteins that retain (CdtBA163R) or lack (CdtBR117A and CdtBR144A) lipid phosphatase activity; these mutants have previously been characterized and it should be noted that the latter two mutants have also been shown to be deficient in toxic activity (Shenker, Boesze-Battaglia, et al., 2016). Supernatants were harvested 48 hr later and assessed for LDH activity; as shown in Fig. 2B, THP-1WT cells treated with Cdt containing CdtBWT exhibited 23% LDH release. Cells treated with Cdt containing the CdtBA163R mutant protein, which retains both phosphatase and toxic activities, exhibited 18% LDH release; this was not statistically different from the release observed with THP-1WT cells. In contrast, cells treated with the enzymatically deficient CdtB mutants, CdtBR117A or CdtBR144A, exhibited low levels of LDH release which were statistically significantly lower than the amount released by cells treated with toxin containing CdtBWT subunit.
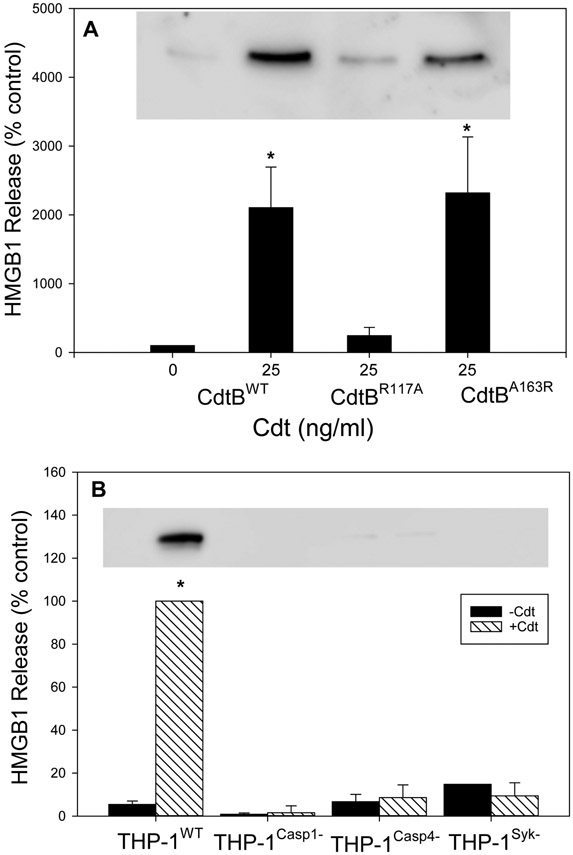
In addition to LDH, supernatants were assessed for the release of HMGB1, a pro-inflammatory mediator whose release is associated with pyroptosis and often employed as a marker for the activation of this process (Bui et al., 2016; Johnson et al., 2015). As shown in Fig. 3A, THP-1WT cells treated with 25 ng/ml Cdt were analyzed by Western blot for HMGB1. Cells exhibited a significant increase in release of HMGB1 at 24 hr relative to untreated (control) cells. Furthermore, as we observed with LDH release, the release of HMGB1 was dependent upon CdtB’s ability to function as a lipid phosphatase. Cells treated with Cdt containing the active CdtBA163R subunit exhibited significant HMGB1 release; these levels were comparable to the release observed with cells treated with Cdt containing CdtBWT In contrast, cells treated with Cdt containing the phosphatase deficient subunit, CdtBR117A , exhibited much lower levels of HMGB1 release. It should also be noted that cells treated with 25 ng/ml Cdt containing CdtBWT also exhibited a 40% reduction in total cellular HMGB1 (Fig. S3).
Fig. 3.
Cdt-induces HMGB1 release from macrophages. Panel A: THP-1WT-derived macrophages were incubated with Cdt containing CdtBWT, CdtBR117A or CdtBA163R Following incubation for 24 hrs, supernatants were harvested and analyzed by Western blot for the presence of HMGB1. Results are plotted as HMGB1 levels (% of levels released by control cells; 0 Cdt) versus Cdt concentration and CdtB subunit type; results represent the mean±SEM of four experiments. A representative Western blot is shown for HGMB1. Panel B: Macrophages derived from THP-1WT , THP-1Casp1−, THP-1Casp4− and THP-1Syk− cells incubated with medium (solid bars) or 50 ng/ml Cdt (hatched bars); 24 hrs later, supernatants were harvested and analyzed for the presence of HMGB1. Results are plotted as HMGB1 release (% of release observed in THP-1WT- derived macrophages incubated with Cdt) vs cell type. Results are the mean±SEM of three experiments. * indicates statistical significance (p<0.05) when compared to control cells incubated in medium alone (panel A) or to THP-1WT- derived macrophages incubated with Cdt. A representative full blot is shown in Fig. 5S.
The requirement for caspase-1 and caspase-4 activation leading to the release of HMGB1 was also assessed (Fig. 3B). THP-1WT-derived macrophages were treated with 50 ng/ml Cdt and the supernatants analyzed 24 hr later for HMGB1. Results are plotted as a percentage of HMGB1 observed in the supernatants of Cdt-treated THP-1WT cells (100%); as observed above, Cdt induced a significant increase in HMGB1 release in THP-1WT cells. In contrast both THP-1Casp1− and THP-lCasp4− cells exhibited low levels of HMGB1 in their superanatants: 1.6±3.2 and 8.6±5.9 percent of toxin treated THP-1WT cells, respectively; these levels were not significantly higher than that observed with the untreated control cells.
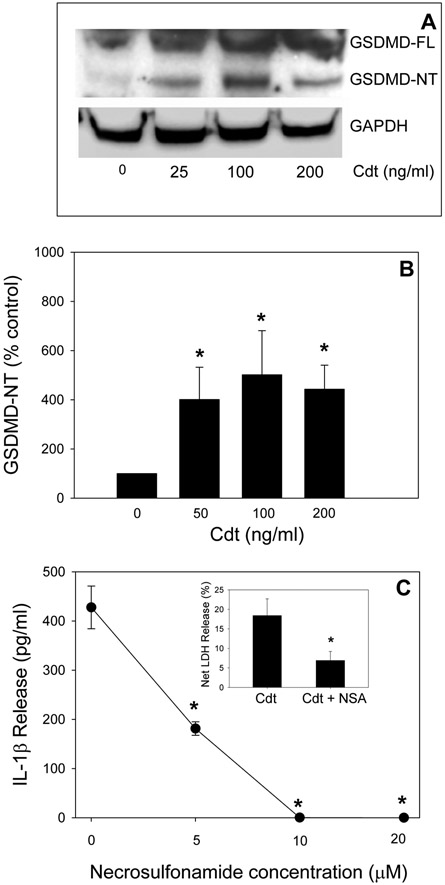
One of the key elements in caspase-1 and −4-mediated activation of pyroptosis and the resulting release of pro-inflammatory cytokines is the cleavage of GSDMD. Caspase-1 and caspase-4 cleavage removes the C-terminal fragment; this releases and activates the N-terminal fragment, GSDMD-NT. This fragment is directly responsible for pore formation in the plasma membrane which in turn enhances cytokine release and ultimately leads to cell death as indicated by LDH release (Liu et al., 2016). As shown in Fig. 4A and B, THP-1WT-derived macrophages treated with Cdt for 24 hr exhibited an increase in the kDa GSDMD-NT fragment. The levels of GSDMD-NT increased in cells treated with Cdt to 401 (50 ng/ml Cdt), 501 (100 ng/ml Cdt) and 443 (200 ng/ml Cdt) percent over that observed with cells incubated in medium alone; these increases were statistically significant. Further evidence of GSDMD-NT involvement in Cdt-induced cytokine release was demonstrated by its pharmacologic inhibition. NSA has recently been shown to bind to GSDMD and inhibit both pore formation by GDMD-NT and the subsequent onset of pyroptosis (Rathkey et al., 2018). In Fig 4C, we demonstrate that THP-1WT-derived macrophages pre-treated with 5, 10 and 20 μM NSA blocked Cdt-induced release of IL-1β; in the presence of toxin alone, cells released 427 pg/ml cytokine. In contrast pre-treatment with NSA reduced IL-1β release to 181, 0.2 and 0 pg/ml IL-1β. Furthermore, pre-treatment with 10 μM NSA reduced LDH release from 18% with Cdt alone to 6.9% (insert, Fig. 4C).
Fig. 4.
Cdt-treated macrophages exhibit GSDMD cleavage. Panel A: Macrophages derived from THP-1WT cells were incubated with varying amounts of Cdt for 24 hr. Cell extracts were analyzed by Western blot for the presence of GSDMD-NT. Panel A shows a representative blot. Panel B: shows the results of three experiments: data are plotted as percent release (relative to control cells) mean±SEM. Panel C: THP-1WT-derived macrophages were pre-incubated with varying amounts of NSA for 60 min followed by the addition of 200 ng/ml Cdt. Supernatants were analyzed 5 hrs later for the presence of IL-1β by ELISA. Results are plotted as the mean±SEM of three experiments vs NSA concentration. * indicates statistical signfiance <0.05. A representative full blot is shown in Fig. 5S.
Preparations of recombinant proteins expressed in E. coli typically contain some level of LPS contamination. While our preparations typically contain low levels of LPS, we routinely prepare samples of Cdt in which we have significantly reduced the levels of LPS (Cdt-LD). This preparation of toxin is employed in select experiments to rule out contribution of LPS contamination as a significant contributor to our experimental observations. As shown in Fig. S4, we compared our standard Cdt preparation to Cdt-LD for their ability to activate caspase-4, induce the release of both HMGB1 and LDH as well as the cleavage of GSDMD. In each instance, we failed to detect a significant difference in the potency of the two Cdt preparations.
Upstream events critical to Cdt-induced activation of noncanonical pyroptosis
In the next series of experiments we explored the role of the nonreceptor protein tyrosine kinase, Syk, as an upstream mediator of Cdt-induced activation of both canonical and noncanonical inflammasomes. Cdt-treated macrophages were first assessed for changes in both total Syk and its activated form, phosphorylated Syk (pSyk; 525/526). THP-1WT-derived macrophages were treated with Cdt (200 ng/ml) and analyzed by Western blot over time [(0-120 min); Fig. 5A,B], The levels of Syk and pSyk, respectively, are presented as a percentage of the amount of each protein observed in untreated control cells. Total Syk levels remained relatively constant over the 2 hr time period. In contrast, pSyk levels exhibited a statistically significant increase (318% of control values) at 15 min; these levels continued to rise to 412% (30 min) and 917% (60 min) above control levels. The levels remained elevated at 120 min but lower than the peak levels observed at 60 min (395%).
Fig. 5.
Cdt-induced pro-inflammatory response and noncanonical inflammasome activation is dependent upon Syk. Panels A and B: THP-1WT-derived macrophages were incubated with 200 ng/ml Cdt for 0-120 min; cell extracts were analyzed for the Syk and pSyk levels by Western blot. A representative blot including GAPDH (G) is shown in panel A and pooled results from three experiments in panel B; data are plotted as a percentage of untreated (time 0) levels and represent the mean±SEM of four experiments. * indicates statistical significance (p<0.05) when compared to cells exposed to medium only. Panel C: THP-1WT-derived macrophages were pre-incubated with varying amounts of one of three Syk inhibitors: Syk II (circle), Bay61 (triangles) or piceatannol (Pic) for 60 min followed by the addition of 200 ng/ml Cdt. Supernatants were harvested 5 hr later and analyzed by ELISA for IL-1β. Results are plotted as mean±SEM of IL-1β (pg/ml) based on three experiments vs Syk inhibitor concentration. Note: all samples treated with inhibitor and Cdt were statistically significantly lower when compared to cells treated with Cdt alone.. Panel D: macrophages derived from THP-1WT (cross hatched bars), THP-1shctrl (hatched bars) and THP-1Syk− (solid bars) cells were incubated with varying amounts of Cdt. Supernatants were harvested 5 hrs later and analyzed by ELISA for IL-1β. Results are plotted as mean±SEM of IL-1β (pg/ml) of three experiments vs Cdt concentration. * indicates statistical significance (p<0.05) when compared to control cells (0 Cdt). Panel E: THP-1WT- and THP-1Syk− -derived macrophages were incubated with medium or 200 ng/ml Cdt (cross-hatched bars) for 48 hr; supernatants were analyzed for LDH activity and plotted as net LDH release (%) (mean±SEM for four experiments). * indicates statistical significance (p<0.05) when compared to Cdt-treated THP-1WT control cells (0 Cdt). Panel F: THP-1WT- and THP-1Syk− -derived macrophages were incubated with medium (open bars), 200 ng/ml Cdt (hatched bars) and 500 ng/ml Cdt (solid bars) for 2 hr. Cell extracts were analyzed for caspase 4 activity (net OD405). Results are the mean±SEM for four experiments; * indicates statistical significance when compared to control cells (0 Cdt).
To determine if Syk is critical to Cdt-mediated inflammasome activation and the release of IL-1β, we utilized both a pharmacologic and molecular approach to block its activation. First, three Syk inhibitors were employed: Syk Inhibitor II [(Syk II); 0-100 nM], Bay61-3606 [(Bay61); 0-4 μM] and piceatanol [(pic);0-1 μM] (Fig. 5C). THP-WT-derived macrophages were pre-incubated with each inhibitor for one hr followed by the addition of 200 ng/ml Cdt; after an additional 5 hr incubation, supernatants were collected and analyzed for the presence of IL-1β by ELISA. Cells treated with Cdt in the absence of inhibitor released 824±122 pg/ml IL-1β as compared to 108±10 pg/ml for cells exposed to medium only. Pre-treatment of cells with the lowest dose of each inhibitor resulted in significant reductions in IL-1β release: 70±19, 306±233 and 104±38 pg/ml in the presence of Syk II inhibitor (10 nM), Bay61-3606 (1 μM) and piceatanol (0.6 μM), respectively. IL-1β release continued to decline in the presence of 50 and 100 nM Syk II inhibitor to 67±28 and 95±74 pg/ml. No further decreases were observed with Bay61-3606 and piceatanol.
Lentivirus particles containing Syk shRNA sequences were used to transfect THP-1 cells (THP-1Syk−) and reduce Syk expression (Fig. 5D inset). These cells were employed to further analyze the requirement for Syk in Cdt-induced IL-1β maturation and release. As shown in Fig. 5D, treatment of THP-1WT cells with increasing concentrations of Cdt (0-200 ng/ml) resulted in a dose-dependent increase in the release of IL-1β into the medium: 100±4.3 (0 Cdt), 318±43 (8 ng/ml Cdt), 496±58 (40 ng/ml Cdt), and 738±29 pg/ml (200 ng/ml Cdt). In contrast, THP-1Syk− cells were severely compromised in their ability to respond to Cdt as these cells released 55 (0 Cdt), 82 (8 ng/ml Cdt), 161 (40 ng/ml Cdt) and 219 pg/ml IL-1β (200 ng/ml Cdt).
In addition to Cdt-induced release of IL-1β in THP-1Syk− cells, the release of both LDH and HMGB1 into cell supernatants was assessed. As shown in Fig. 5E, the supernatants of THP-1WT cells contained LDH (9%) in the presence of 200 ng/ml Cdt. In contrast, macrophages derived from THP-1Syk− cells exhibited significantly less LDH (3.8%) in the cell supernatants. Similarly, THP-1Syk− -derived macrophages failed to release significant amounts of HMGB1 (Fig. 3B).
In the next series of experiments the requirement for Syk in mediating Cdt-induced caspase-4 activation was assessed. As shown in Fig 5F, THP-1WT-derived macrophages cells an increase in caspase-4 activity in the presence of Cdt (net OD405): 0.13±0.006 (0 Cdt), 0.18±0.005 (200 ng/ml Cdt) and 0.190±0.009 (500 ng/ml Cdt). In comparison THP-1Syk− -derived macrophages did not exhibit an increase in caspase-4 activity over levels observed with control cells.
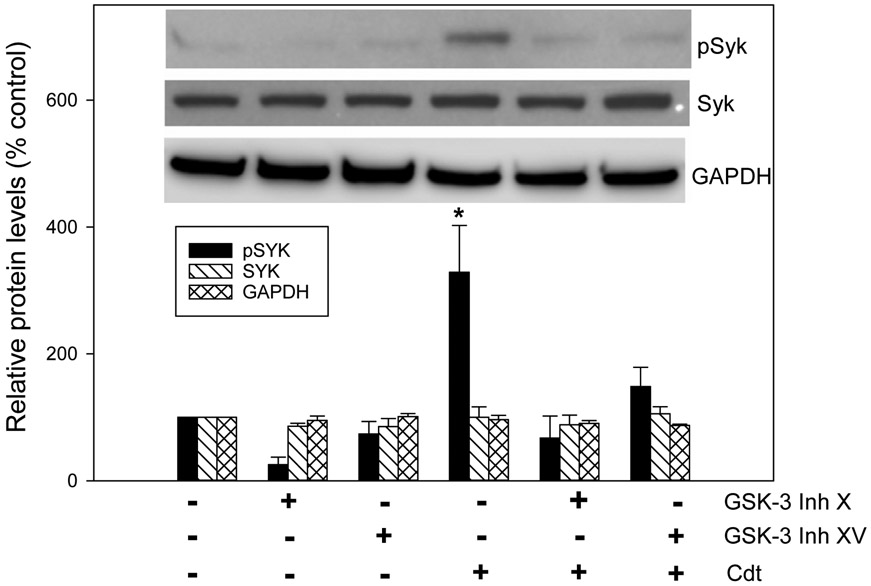
In previous studies we established that the action of A. actinomycetemcomitans Cdt on lymphocytes and macrophages was dependent upon CdtB’s ability to function as a PIP3 phosphatase (Shenker, Boesze-Battaglia, et al., 2016; Shenker et al., 2007; Shenker et al., 2014). Depletion of PIP3 results in PI-3K signaling blockade: reduced PIP3, reduced pAkt (loss of kinase activity) and reduced pGSKβ (activation of kinase activity). The significance of these observations was demonstrated by the ability of GSK3β inhibitors to block Cdt toxicity including activation of the canonical NLRP3 inflammasome (Shenker, Boesze-Battaglia, et al., 2016; Shenker et al., 2014). In order to build upon our previous observations and link them with our current studies, we next explored the possibility that the observed increases in Cdt-mediated Syk phosphorylation might also be linked to PI-3K signaling. Therefore, we assessed the ability of two of these inhibitors, GSK-3 inhibitor X and XV, to block Cdt-induced increases in pSyk. As shown in Fig. 6, both inhibitors reduced background levels of pSyk to: 25.4% (GSK-3 X) and 73.8% (GSK-3 XV) of levels observed in untreated control cells. In the presence of Cdt, pSyk levels increased to 328.8% above control cell levels. The inhibitors blocked the effects of Cdt on pSyk levels as they remained below levels observed with Cdt alone. Total Syk levels were not significantly altered by either of the inhibitors.
Fig. 6.
GSK3β inhibitors block Cdt-induced Syk phosphorylation. THP-1 WT-derived macrophages were pre-incubated with medium, 10μM GSK-3 Inh X or 1.0 μM GSK-3 Inh XV for 60. Medium or 200 ng/ml Cdt was then added to cell cultures and cells harvested 2 hr later. Cell extracts were analyzed for Syk and pSyk by Western blot. GAPDH was used as a loading control. Results are plotted as the relative protein levels (% control) and represent the mean±SEM of three experiments. A representative blot is shown in the inset.* indicates statistical significance relative to cells treated with medium.
Discussion
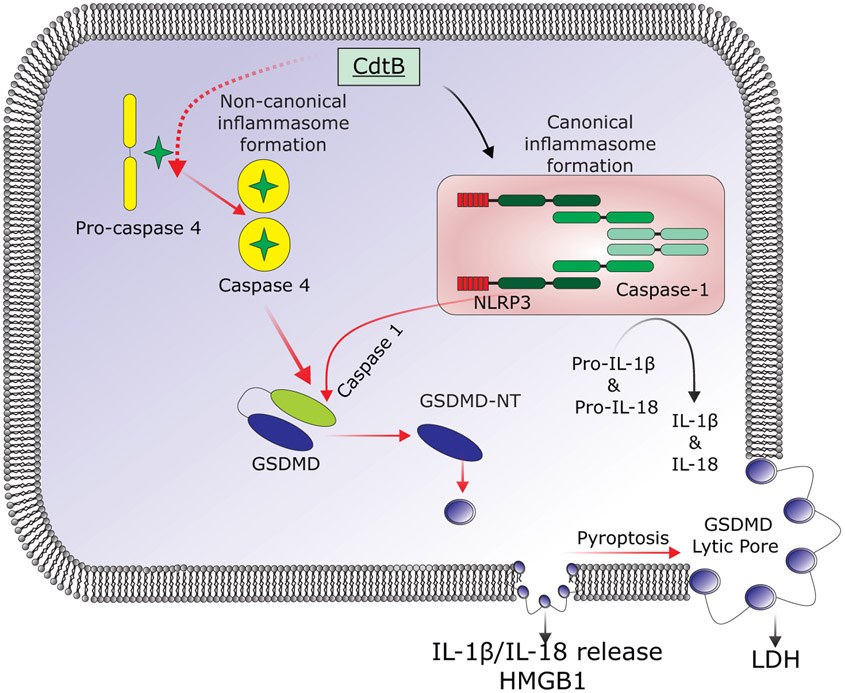
Cdt toxicity is typically associated with induction of irreversible cell cycle arrest and eventually apoptotic cell death in proliferating cells such as lymphocytes (reviewed in (Gargi et al., 2013; Scuron et al., 2016) ). In previous studies, however, we have demonstrated that non-proliferating cells, such as human monocytes and macrophages, are resistant to A. actinomycetemcomitans Cdt-induced apoptosis (Shenker et al., 2015; Shenker et al., 2014). Instead, the toxin induces macrophages derived from either human monocytes or from the acute monocytic leukemia cell line, THP-1, to synthesize and release pro-inflammatory cytokines (IL-1β, IL-18, TNFα and IL-6). Additionally, we have shown that the Cdt-induced pro-inflammatory response involves activation of the canonical NLRP3 inflammasome (Fig. 7). In those studies we employed both specific inhibitors and stable transfection (shRNA) of cell lines to demonstrate requirements for NLRP3, ASC as well as caspase-1. Furthermore, Cdt-mediated inflammasome activation was found to be dependent upon upstream danger signals including: reactive oxygen species (ROS) and Cdt-induced increases in extracellular ATP. The latter contributed to the activation of the P2X7 purinergic receptor and K+ efflux. Additionally, we have demonstrated that induction of this pro-inflammatory response was dependent upon CdtB associated PIP3 phosphatase activity. Specifically, enzymatic depletion of PIP3 results in blockade of the PI-3K signaling pathway leading to both Akt inactivation and GSK3β activation (REFS). In the current study we demonstrate that, in addition to the canonical inflammasome, the Cdt-induced pro-inflammatory response is also dependent upon activation of the noncanonical inflammasome and further that activation of both inflammasomes contribute to cytokine release which is dependent upon GSDMD cleavage which in turn leads to pyroptosis (summarized in Fig 7).
Fig. 7.
Schematic showing an overview of the proposed activation of the noncanonical inflammasome and pyroptosis by Cdt. Internalization of CdtB leads to activation of caspase-4 and caspase-1; the later involves activation of the canonical inflammasome that we have previously described in detail (Shenker et al., 2015). Both caspase-4 and caspase-1 cleave GSDMD to release the pore-forming fragment, GSDMD-NT. Initially, this leads to the formation of small pore that facilitate the release of IL-1β, IL-18 and HMGB1. Eventually, these pores enlarge and become lytic allowing LDH to escape from the dying cells.
Pyroptosis was first recognized as a form of cell death that was distinct from apoptosis as it was independent of caspase-3 (Tang, Kang, Berghe, Vandenabeele, & Kroemer, 2019; Xia et al., 2019). The two forms of cell death exhibit other distinguishing characteristics. Morphologically, pyroptotic cells display loss of plasma membrane integrity which results from pore formation within the plasma membrane and ultimately leads to cell swelling and release of cytoplasmic content. Biochemically, pyroptosis is mediated by inflammatory caspases-1, −4, −5 and −11 (murine cells) (Casson et al., 2015; Sollberger, Strittmatter, Kistowska, French, & Beer, 2012; Taabazuing et al., 2017; Tang et al., 2019; Vigano et al., 2015). As noted above, caspase-1 is typically activated by a multi-protein complex, the inflammasome, consisting of a NOD-like receptor (NLR), a linker protein such as ASC and pro-caspase-1 and is often referred to as the canonical inflammasome (He, Hara, & Nunez, 2016). Activation of the canonical inflammasome leads to cleavage and activation of caspase-1 and, in turn, maturation of IL-1β. Cell death mediated by this complex is referred to as canonical pyroptosis and typically occurs in monocytes, macrophages and dendritic cells (Russo, Behl, Banerjee, & Rathinam, 2018; Shi et al., 2017). In contrast, noncanonical pyroptosis is mediated by activated caspases-4, −5 −11 and occurs in a variety of cell types (Shi et al., 2017; Xia et al., 2019). Activation of this pathway was initially identified as occurring as a result of direct interaction between intracellular bacterial lipopolysaccharide (LPS) and caspase-4/5; cell surface interactions involving TLR4-LPS do not activate these caspases (Shi et al., 2014). Whereas activated caspase-1 directly cleaves pro-IL-1β and pro-IL-18 leading to the formation of the mature cytokine, caspases −4,−5 and −11 do not promote cleavage. However, each of these caspases, along with caspase-1, cleave GSDMD releasing the active, pore forming, N-terminal fragment GSDMD-NT (Evavold & Kagan, 2019; Feng, Fox, & Man, 2018; Liu et al., 2016; Shi et al., 2017).
We now demonstrate that in addition to caspase-1, caspase-4 is activated in Cdt-treated macrophages and, further, the release of IL-1β is also dependent upon caspase-4. Cells pre-treated with the caspase-4 inhibitor LEVD-CHO or deficient in caspase-4 expression were resistant to Cdt-induced cytokine release. Cdt treated macrophages become pyroptotic within 48 hr and exhibit both GSDMD cleavage and an increase in membrane permeability(Kovacs & Miao, 2017; Sborgi et al., 2016; Shi et al., 2015). The latter was demonstrated by the release of both LDH and HMGB1 into culture supernatants. The release of HMGB1 is particularly significant as it not only represents a marker for the onset of pyroptosis but is also a potent inducer of pro-inflammatory responses(Lotze & Tracey, 2005). In contrast, macrophages derived from either THP-1Casp1− or THP-1Casp4−2 cells did not exhibit GSDMD cleavage or increased membrane permeability as evinced by the failure to release either LDH or HMGB1. These results suggest that not only are these cells resistant to Cdt-induced pyroptosis but also that both caspase-1 and caspase-4 were required for this lytic form of cell death. These caspases are thought to act in a synergistic role in mediating GSDMD cleavage and in turn, pyroptosis. Therefore, it was surprising that depletion of either caspase-1 or caspase-4 was sufficient to block pyroptosis and IL-1β release. These results suggest a cooperative role such that the activation of either caspase alone is insufficient to induce pyroptosis and facilitate cytokine release or alternatively, that caspase-4 is upstream of caspase-1.
GSDMDs are a family of cytosolic proteins with diverse function and expression profiles; one member of this family, GSDMD, as noted above is cleaved by caspases-1 and −4. The liberated N-terminus, GDSMD-NT, is the pore forming fragment (Evavold & Kagan, 2019; Feng, Fox, et al., 2018; Liu et al., 2016; Shi et al., 2017). Indeed, we demonstrate that not only do Cdt-treated macrophages contain significant increases in GSDMD-NT when exposed to Cdt, but further, that NSA, an inhibitor of GSDMD cleavage, blocks the release of both IL-1β and LDH (Rathkey et al., 2018). Following cleavage, GSDMD-NT has been shown to bind to phosphtidylinositol (PI) phosphates on the inner leaf of plasma membranes as well as on mitochondrial, lysosomal, phagocytic and endosomal membranes (reviewed in (Feng, Fox, et al., 2018)). This interaction is critical to subsequent pore formation as it promotes oligomerization of GSDMD-NT. One of the phosphoinositides that GSDMD-NT binds to is PI-3,4-P2; this PI-disphosphate is of particular relevance to our study as it is the enzymatic breakdown product of CdtB-mediated PIP3 degradation (Shenker et al., 2007). Thus, it is interesting to speculate that a secondary effect of CdtB on macrophages is to not only induce PI-3K signaling blockade and activate the canonical and noncanonical inflammasomes, but to further potentiate pore formation by providing additional docking sites for the GDSMD-NT. These potential interactions are the subject of ongoing investigation.
One critical feature of pores formed by GSDMD-NT is that they are selective and further that selectivity is determined by pore size (Liu et al., 2016). Initially, the pores are small (10-15 nm inner diameter), large enough for the release of cytokines and the danger signal HMGB1. This is critical for the release of mature IL-1β and IL18 (~4.5 nm diameter) which lack N-terminal signal sequences necessary for secretion via biosynthetic vesicle-mediated pathways. Furthermore, it has been proposed that the formation of these small GSDMD-NT-dependent pores is consistent with macrophages being in a hyperactive/pro-inflammatory state (Evavold & Kagan, 2019). Under these conditions the pores are not lytic and cells are able to survive. Our findings are consistent with this scheme as we observe the release of IL-β and HMGB1 during the first 24 hrs of exposure to Cdt; we do not observe the release of LDH at this time. LDH requires larger lytic pores to escape cells as it exists as a tetramer in the cytosol. Eventually, the pores enlarge and reach a threshold that leads to the lytic phase of pyroptosis characterized by the release of LDH and other intracellular components (Evavold & Kagan, 2019; Shi et al., 2017; Taabazuing et al., 2017). Indeed, we do not observe LDH release in Cdt-treated cells until 48 post treatment with the toxin. The mechanism leading to transition to larger lytic pores is not clear, but it has been proposed that initially the number and/or size of the GDSMD-NT pores is maintained by an endosomal sorting complex required for transport (ESCRT)-III repair mechanism (Ruhl et al., 2018). It has been suggested that failure of this repair mechanism ultimately contributes to pyroptotic cell death.
Previously, we have detailed the relationship between CdtB associated lipid phosphatase activity, PI-3K signaling and Cdt toxicity and, as noted above, one of the key outcomes of this blockade is the activation of GSK3β (i.e., reduced phosphorylation). Thus, it is consistent that the ability of Cdt to activate canonical and noncanonical inflammasomes and thereby induce pyroptosis requires that the toxin complex contain a CdtB subunit that retains PIP3 phosphatase activity (e.g., CdtBWT or CdtBA163R). Likewise, it was not surprising that CdtB mutants that lack lipid phosphatase and toxic activity (CdtBR117A and CdtBR144A) were unable to induce LDH and HMGB1 release as well as activate caspase-4. These observations point to a potential linkage between the induction of pyroptosis and CdtB-mediated blockade of the PI-3K signaling pathway. The activation of this kinase and the potential cross-talk between PI-3K signaling and inflammasome activation is further discussed below as it may also relate to Syk activation.
Syk is a nonreceptor tyrosine kinase; its activation is typically considered to be the result of interaction with immunoreceptor tyrosine-based motif (ITAM)-containing proteins. It is becoming increasingly clear that Syk is activated in macrophages following stimulation via pathogen recognition receptors (PRRs) and further that activation of this tyrosine kinase has been shown to be critical for pro-inflammatory cytokine gene expression (reviewed in (Mocsai, Ruland, & Tybulewicz, 2010)). Additionally, canonical inflammasome activation has been shown to be regulated by pSyk via phosphorylation of ASC, a requirement for its oligomerization. Indeed, we demonstrate that within 15 min of exposure to Cdt, there is a significant increase in pSyk. Moreover, pre-incubation of cells with Syk inhibitors blocks Cdt-induced release of mature IL-1β. Similarly, macrophages derived from Syk deficient cells (THP-1Syk−) were resistant to Cdt-induced IL-1β release. These results are consistent with the reported role of Syk in mediating activation of the NLRP3 inflammasome (Feng, Huang, et al., 2018; Fitzpatrick, 2015; Said-Sadier, Padilla, Langsley, & Ojcius, 2010). We also observed that THP-1Svk− -derived macrophages failed to exhibit an increase in caspase-4 activity in the presence of Cdt and further that the toxin did not induce the release of either HMGB1 or LDH in these cells. Thus, in addition to canonical inflammasome activation, Syk appears to also regulate Cdt-induced activation of the noncanonical inflammasome and ultimately pyroptosis.
In order to account for CdtB-dependent Syk activation, we explored the possibility that GSK3β might be involved. As noted earlier, we have previously demonstrated that Cdt-induced PI-3K signaling blockade in both lymphocytes and macrophages results in GSK3β activation (Scuron et al., 2016; Shenker, Boesze-Battaglia, et al., 2016; Shenker et al., 2007; Shenker et al., 2015; Shenker et al., 2014). Moreover, we have shown that GSK3β inhibitors blocked toxin-induced IL-1β release, clearly indicating a role for this kinase in canonical and/or noncanonical inflammasome activation. We now report that these same inhibitors block Cdt-induced Syk phosphorylation. These studies demonstrate a potential link between GSK3β activation and/or a downstream PI-3K signaling component, Syk activation and IL-1β release via canonical and/or noncanonical pathways
In conclusion, we propose that pyroptosis, in general, and Cdt-induced pyroptosis, in particular, is critical to the toxins virulence potential as this form of cell death has been implicated to play a critical role in microbial infection and host defense. One key aspect of this lytic cell death pathway is that it provides a mechanism for the release of IL-1β and IL-18, cytokines that lack signal sequences for secretion, as well as other pro-inflammatory mediators such as HMGB1 and eicosonaids (Fig. 7). Collectively, these pro-inflammatory mediators potentially benefit the host by promoting microbial clearance through both bactericidal and phagocytic mechanisms. In this context, it is also important to note that in addition to creating pores in the plasma membranes of host cells, GSDMD-NT is lethal to bacteria. Pyroptosis also serves to eliminate intracellular pathogens as host cell death releases these organisms where they face elimination by both innate and acquired host defense (Xia et al., 2019). Alternatively, pyroptotic amplification of the release of inflammatory mediators contributes to development of chronic inflammation and tissue injury. It is in this context that we propose that Cdt-hijacks this critical host defense mechanism, amplifies the pro-inflammatory response and eventually contributes to the destruction of tooth supporting tissue, a clinical feature of A. actinomycetemcomitans infection. Morever, A. actinomycetemcomitans Cdt is capable of functioning as a tri-perditious toxin (virulence factor) by altering host defense mechanisms where it impairs acquired immunity, activates innate immunity and damages epithelial barriers (reviewed in (Scuron et al., 2016)). These properties are consistent with the current view of the role for A. actinomycetemcomitans in the pathogenesis of localized aggressive periodontitis. Rather than serve as the causative agent, it has been proposed that A. actinomycetemcomitans is an early colonizer that modifies local host defense; this perturbation facilitates both its own survival and in turn contributes to dysbiosis by advancing the survival of other microbes (Fine, Patil, & Velusamy, 2019). The ability to function in this manner and differentially affect a spectrum of cell types is due to two properties of this AB2 toxin. The binding unit, CdtC, binds to cholesterol, a ubiquitous component of all cell membranes and the active subunit, CdB, is a lipid phosphatase capable of depleting cells of PIP3 resulting in blockade of the PI-3K signaling pathway, a ubiquitous signaling cascade, functional in all cells.
Experimental procedures
Cells and reagents
The human acute monocytic leukemia cell line, THP-1, was obtained from ATCC; cells were maintained in RPMI 1640 containing 10% FBS, 1mM sodium pyruvate, 20 μM 2-mercaptoethanol and 2% penicillin-streptomycin at 37°C with 5% CO2 in a humidified incubator. THP-1 cells stably expressing shRNA against caspase-1 (THP-1Casp1−) and Syk (THP-1Syk−) were provided by D. M. Ojcius and previously described (Abdul-Sater, Said-Sadier, Padilla, & Ojcius, 2010; Chen et al., 2012); caspase-4 deficient cells (THP-1Casp4−) were provided by P.R. Huang and similarly prepared with lentivral oparticles containing the following sequence:5-’-GCAACGTATGGCAGGACAAAT-3". The cells were prepared by transducing THP-1 cells with lentiviral particles, along with nontarget controls, as previously described (Abdul-Sater et al., 2010; Chen et al., 2012). Expression levels were recently reported to be reduced > 95% for THP-1Casp1−, >70% THP-1Syk− and caspase-4 was not detectable by Western blot in THP-1Casp4 cells with no effect in the nontarget controls (Zhu et al., 2015). THP-1 cells were differentiated into macrophages by incubating cells in the presence of 50 ng/ml PMA for 48 hr at which time the cells were washed and incubated an additional 24 hr in medium prior to use.
Human peripheral blood mononuclear cells (HPBMC) were obtained from venous blood of healthy donors as previously described (Shenker et al., 2004). Monocytes were further purified from HPBMC by positive selection using anti-CD14 mAb according to the manufacturers directions (Dynabeads® FlowComp™ Human CD14; ThermoFisher Invitrogen, Waltham, MA). Anti-CD14 mAb-bead complexes were removed from the positively selected monocytes using a release buffer (Invitrogen). Monocytes were routinely found to be >96% pure as determined by immunofluorescent staining and analyzed by flow cytometry. Monocyte-derived macrophages (MDM) were generated by incubating monocytes in DMEM containing 10% fetal bovine serum and 2% penicillin/streptomycin for 6-8 days.
THP-1 derived macrophages and MDM were incubated as described below (or in Figure legends) and treated with recombinant Cdt holotoxin containing CdtB wildtype (CdtBWT) or mutant (CdtBA163R, CdtBR117A or CdtBR144A) which were expressed and purified as described below. In some experiments cells were also treated with: necrosulfonamide (NSA), Syk inihbitor II, Bay61-3606, picceatanol, GSK inhibitor XV (MilliporeSigma, Burlington, MA) and GSK inhibitor X (Santa Cruz Biotechnology, Dallas, TX)
Expression and purification of Cdt holotoxin
Construction and expression of the plasmid containing the cdt genes for the holotoxin (pUCAacdtABChis) have previously been reported (Shenker et al., 2004). The plasmids were constructed so that the cdt genes were under control of the lac promotor and transformed into E. coli DH5α. Cultures of transformed E. coli were grown in 1L LB broth and induced with 0.1 mM IPTG for 2 hr; bacterial cells were harvested, washed and resuspended in 50 mM Tris (pH 8.0). The cells were frozen overnight, thawed and sonicated. The histidine-tagged peptide holotoxin was isolated by nickel affinity chromatograpky as previously described (Shenker et al., 2004).
Analysis of IL-1β release
IL-1β release was measured in THP-1 derived macrophages (2 x 105 cells) or MDM incubated for 5 hr in the presence or absence of varying amounts of Cdt as previously described (Shenker et al., 2015). Briefly, culture supernatants were collected and analyzed by ELISA for IL-1β (Quantikine Elisa Kit; R and D Systems, Minneapolis, MN). In each instance, the amount of cytokine present in the supernatant was determined using a standard curve.
Western blot analysis
Replicate wells of THP-1 derived macrophages (4 x 106 cells/well) were incubated as described with Cdt. The cells were washed and treated with 20 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholate and protease and phosphatase inhibitors (ThermoFisher Pierce Protein Biology, Pittsburgh, PA); replicate wells were pooled and protein concentration determined. HMGB1 release into culture supernatants was determined following precipitation of supernatants in 20% TCA. Samples were separated on 12% SDS-PAGE and then transferred to nitrocellulose. The membrane was blocked with BLOTTO and then incubated with anti-pro-IL-1β, anti-caspase-4, anti-Syk, anti-pSyk, anti-GSDMD or anti-GAPDH antibody (Santa Cruz Biotechnology) for 18 hr at 4°C (Shenker et al., 1999). Membranes were washed, incubated with either goat anti-rabbit IgG or goat-anti-mouse IgG conjugated to horseradish peroxidase. The Western blots were developed using chemiluminescence and analyzed (Licor).
Analysis of LDH release
THP-1-derived macrophages (5 x 104) were incubated as described in Figure Legends. The supernatants were harvested and LDH activity was detected using a commercially available kit (LDH Cytotoxicity Assay Kit; ThermoFisher Pierce Protein Biology). Extracellular LDH in the supernatant was quantified by a coupled enzymatic reaction in which LDH catalyzes the conversion of lactate to pyruvate via NAD+ reduction to NADH. NADH is then utilized by diaphorase to reduce tetrazolium salt to red formazan which is measured at OD490 nm. LDH release is presented as % net LDH release: 100 x (experimental OD490 release-spontaneous OD490 release/maximum OD490 release-spontaneous OD490 release).
Caspase-4 activity
THP-1-derived macrophages (4 x 106) were incubated as described in the figure legends. Caspase-4 activity was measured using a commercially available kit (Caspase-4 Colorimetric Assay Kit; BioVision, Milpitas, CA). Cells were harvested, lysed and following centrifugation the supernatants containing the cytosolic extract were assessed for caspase-4 activity. Briefly, caspase-4 activity was determined by monitoring the release of the chromophore p-nitranilide (pNA) from the labeled substrate, LEVD-pNA at OD405 nm. Background OD405 from cell lysates and buffers were subtracted from measurements obtained from both control and treated samples (net OD405).
Statistical analysis
Mean ± standard error of the mean were calculated for replicate experiments. Significance was determined using a Student’s t-test; differences between multiple treatments were compared by ANOVA paired with Tukeys HSD posttest; a P-value of less than 0.05 was considered to be statistically significant.
Supplementary Material
Figure S1 Comparison of the effects of Cdt and transfected LPS on caspase-4 activation. THP-1WT derived macrophages were treated with Cdt (200 ng/ml), 1:1000 dilution of Lipofectamine 2000 (Invivogen) or 0.01 μg/ml LPS (E. coli 0111:B4) plus Lipofectamine. Caspase-4 activity was measured as described in Materials and Methods. Results are plotted as the mean±SEM of caspase activity (net OD405). *indicates statistical signficance (p<0.05) compared to untreated control cells.
Figure S2 THP-1Casp4− cells are susceptible to Cdt-induced activation of the canonical inflammasome. Panel A: Macrophages derived from THPWT, THP-1shctl or THP-1Casp4− were treated with 200 ng/ml Cdt and cell extracts were assessed by Western blot analysis for the presence of pro-IL-1β; GAPDH is shown as a loading control. Results are representative of three experiments. Panel B: THPWT and THP-1Casp4− derived macrophages were treated with Cdt and analysed for the presence of active caspase-1 (p20) by Western blot. A representative Western blot is shown along with compiled data from three experiments; data is expressed as a percentage of p20 levels in control cells. Results represent the mean±SEM of three experiments: * indicates statistical significance when compared to untreated control cells.
Figure S3 Cdt-treated cells exhibit a reduction in total cellular HMGB1. Macrophages derived from THP-1WT cells were treated with 25 and 50 ng/ml Cdt. Cells extracts were analyzed by Western blot for the presence of HGMB1. A representative blot is shown along with compiled results from three experiments. Data is expressed as a percentage (mean±SEM) of HMGB1 levels observed in control cells (0 Cdt); * indicates statistical significance (p<0.05) when compared to control cells.
Figure S4: Comparison of the effects of Cdt and LPS depleted Cdt (Cdt-LD) on activation of the noncanonical inflammasome and pyroptosis. Cdt preparations were depleted of LPS using the Pierce High Capacity Endotoxin Removal Resin Spin Column and the levels assessed using the LAL Chromogenic Endotoxin Quantification Kit. LPS contamination was reduced to < 6 pg/μg Cdt. Panel A: THP-1WT macrophages were treated with 200 ng/ml Cdt or Cdt-LD. Cell extracts were assessed for caspase-4 activity; results are expressed as a percent of caspase-4 activity observed in control cells and are the mean±SEM of three experiments. Panel B: THP-1WT macrophages were treated with medium alone, Cdt or Cdt-LD and analyzed for both HMGB1 release and GSDMD cleavage (lower band represents GSDMD-NT) . Results are plotted as protein intensity (mean±SEM) of three experiments. Panel C: THP-1WT macrophages were treated with Cdt or Cdt-LD and analyzed for LDH release at 48 hr. Results are plotted as net LDH release (%) and are the mean±SEM of three experiments. No statistical differences were observed between the results obtained with Cdt versus Cdt-LD
Figure S5: Representative Western blots of Cdt-induced HMGB1 release and GSDMD cleavage. The full blot of HMGB1 (top panel) and GSDMD cleavage (bottom panel) is shown; cells treated with medium alone (−) and with Cdt (+) are shown.
Acknowledgments
The authors wish to acknowledge the expertise and assistance of the Flow Cytometry Core Facility and the Live Cell Imaging Core Facility at the University of Pennsylvania School of Dental Medicine. Authors acknowledge that they have no conflicts of interest to declare.
Funding:
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award numbers: R01DE006014 and R01DE023071.
References
- Abdul-Sater AA, Said-Sadier N, Padilla EV, & Ojcius DM (2010). Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect, 12(8-9), 652–661. doi: 10.1016/j.micinf.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Alexander D, Dlakic M, & Shenker BJ (2016). A Journey of Cytolethal Distending Toxins through Cell Membranes. Front Cell Infect Microbiol, 6, 81. doi: 10.3389/fcimb.2016.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Brown A, Walker L, Besack D, Zekavat A, Wrenn S, … Shenker BJ (2009). Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J Biol Chem, 284(16), 10650–10658. doi: 10.1074/jbc.M809094200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Walker LP, Dhingra A, Kandror K, Tang HY, & Shenker BJ (2017). Internalization of the Active Subunit of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Is Dependent upon Cellugyrin (Synaptogyrin 2), a Host Cell Non-Neuronal Paralog of the Synaptic Vesicle Protein, Synaptogyrin 1. Front Cell Infect Microbiol, 7, 469. doi: 10.3389/fcimb.2017.00469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Walker LP, Zekavat A, Dlakic M, Scuron MD, Nygren P, & Shenker BJ (2015). The Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Active Subunit CdtB Contains a Cholesterol Recognition Sequence Required for Toxin Binding and Subunit Internalization. Infect Immun, 83(10), 4042–4055. doi: 10.1128/IAI.00788-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui FQ, Johnson L, Roberts J, Hung SC, Lee J, Atanasova KR, … Ojcius DM (2016). Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome-dependent secretion of IL-1beta and the danger signals ASC and HMGB1. Cell Microbiol, 18(7), 970–981. doi: 10.1111/cmi.12560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, … Shin S (2015). Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci U S A, 112(21), 6688–6693. doi: 10.1073/pnas.1421699112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Tsai SH, Lu CC, Hu ST, Wu TS, Huang TT, … Lai HC (2012). Activation of an NLRP3 inflammasome restricts Mycobacterium kansasii infection. PLoS One, 7(4), e36292. doi: 10.1371/journal.pone.0036292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo JM (2014a). Breaking the Gingival Epithelial Barrier: Role of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin in Oral Infectious Disease. Cells, 3(2), 476–499. doi: 10.3390/cells3020476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo JM (2014b). Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins (Basel), 6(11), 3098–3116. doi: 10.3390/toxins6113098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, & Kagan JC (2019). Defying Death: The (W)hole Truth about the Fate of GSDMD Pores. Immunity, 50(1), 15–17. doi: 10.1016/j.immuni.2018.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Fox D, & Man SM (2018). Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol, 430(18 Pt B), 3068–3080. doi: 10.1016/j.jmb.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Feng S, Huang Q, Ye C, Wu R, Lei G, Jiang J, … Fang R (2018). Syk and JNK signaling pathways are involved in inflammasome activation in macrophages infected with Streptococcus pneumoniae. Biochem Biophys Res Commun, 507(1-4), 217–222. doi: 10.1016/j.bbrc.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Fine DH, Patil AG, & Velusamy SK (2019). Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front Immunol, 10, 728. doi: 10.3389/fimmu.2019.00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick E (2015). Editorial: Flexible Syk: turning on and off the inflammasome as needed. J Leukoc Biol, 97(5), 821–824. doi: 10.1189/jlb.2CE1214-627R [DOI] [PubMed] [Google Scholar]
- Gargi A, Reno M, & Blanke SR (2012). Bacterial toxin modulation of the eukaryotic cell cycle: are all cytolethal distending toxins created equally? Front Cell Infect Microbiol, 2, 124. doi: 10.3389/fcimb.2012.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargi A, Tamilselvam B, Powers B, Prouty MG, Lincecum T, Eshraghi A, … Blanke SR (2013). Cellular interactions of the cytolethal distending toxins from Escherichia coli and Haemophilus ducreyi. J Biol Chem, 288(11), 7492–7505. doi: 10.1074/jbc.M112.448118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Feng Y, Ge L, Parry N, Muthupalani S, & Fox JG (2017). Helicobacter hepaticus cytolethal distending toxin promotes intestinal carcinogenesis in 129Rag2-deficient mice. Cell Microbiol, 19(7). doi: 10.1111/cmi.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Feng Y, Whary MT, Nambiar PR, Xu S, Ng V, … Fox JG (2005). Cytolethal distending toxin is essential for Helicobacter hepaticus colonization in outbred Swiss Webster mice. Infect Immun, 73(6), 3559–3567. doi: 10.1128/IAI.73.6.3559-3567.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Rogers AB, Feng Y, Lee A, Xu S, Taylor NS, & Fox JG (2007). Bacterial cytolethal distending toxin promotes the development of dysplasia in a model of microbially induced hepatocarcinogenesis. Cell Microbiol, 9(8), 2070–2080. doi: 10.1111/j.1462-5822.2007.00939.x [DOI] [PubMed] [Google Scholar]
- Guerra L, Teter K, Lilley BN, Stenerlow B, Holmes RK, Ploegh HL, … Frisan T (2005). Cellular internalization of cytolethal distending toxin: a new end to a known pathway. Cell Microbiol, 7(7), 921–934. doi: 10.1111/j.1462-5822.2005.00520.x [DOI] [PubMed] [Google Scholar]
- Haghjoo E, & Galan JE (2004). Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A, 101(13), 4614–4619. doi: 10.1073/pnas.0400932101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hara H, & Nunez G (2016). Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci, 41(12), 1012–1021. doi: 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinadasa RN, Bloom SE, Weiss RS, & Duhamel GE (2011). Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiology, 157(Pt 7), 1851–1875. doi: 10.1099/mic.0.049536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Atanasova KR, Bui PQ, Lee J, Hung SC, Yilmaz O, & Ojcius DM (2015). Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes Infect, 17(5), 369–377. doi: 10.1016/j.micinf.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs SB, & Miao EA (2017). Gasdermins: Effectors of Pyroptosis. Trends Cell Biol, 27(9), 673–684. doi: 10.1016/j.tcb.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Lai CK, Lin YJ, Hung CL, Chu CH, Feng CL, … Su HL (2013). Characterization of putative cholesterol recognition/interaction amino acid consensus-like motif of Campylobacter jejuni cytolethal distending toxin C. PLoS One, 8(6), e66202. doi: 10.1371/journal.pone.0066202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero M, & Galan JE (2001). CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun, 69(7), 4358–4365. doi: 10.1128/IAI.69.7.4358-4365.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, & Lieberman J (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature, 535(7610), 153–158. doi: 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, & Tracey KJ (2005). High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol, 5(4), 331–342. doi: 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- Martel J, Lai HC, Ko YF, Young JD, & Ojcius DM (2016). Alternative functions for the multifarious inflammasome. Biomed J, 39(3), 183–187. doi: 10.1016/j.bj.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Ruland J, & Tybulewicz VL (2010). The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol, 10(6), 387–402. doi: 10.1038/nri2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platnich JM, & Muruve DA (2019). NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. doi: 10.1016/j.abb.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, … Abbott DW (2018). Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol, 3(26). doi: 10.1126/sciimmunol.aat2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, & Broz P (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science, 362(6417), 956–960. doi: 10.1126/science.aar7607 [DOI] [PubMed] [Google Scholar]
- Russo AJ, Behl B, Banerjee I, & Rathinam VAK (2018). Emerging Insights into Noncanonical Inflammasome Recognition of Microbes. J Mol Biol, 430(2), 207–216. doi: 10.1016/j.jmb.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said-Sadier N, Padilla E, Langsley G, & Ojcius DM (2010). Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One, 5(4), e10008. doi: 10.1371/journal.pone.0010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, … Hiller S (2016). GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J, 35(16), 1766–1778. doi: 10.15252/embj.201694696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuron MD, Boesze-Battaglia K, Dlakic M, & Shenker BJ (2016). The Cytolethal Distending Toxin Contributes to Microbial Virulence and Disease Pathogenesis by Acting As a Tri-Perditious Toxin. Front Cell Infect Microbiol, 6, 168. doi: 10.3389/fcimb.2016.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Besack D, McKay T, Pankoski L, Zekavat A, & Demuth DR (2004). Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt): evidence that the holotoxin is composed of three subunits: CdtA, CdtB, and CdtC. J Immunol, 172(1), 410–417. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Besack D, McKay T, Pankoski L, Zekavat A, & Demuth DR (2005). Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin requires three subunits for maximum activity. J Immunol, 174(4), 2228–2234. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Boesze-Battaglia K, Scuron MD, Walker LP, Zekavat A, & Dlakic M (2016). The toxicity of the Aggregatibacter actinomycetemcomitans cytolethal distending toxin correlates with its phosphatidylinositol-3,4,5-triphosphate phosphatase activity. Cell Microbiol, 18(2), 223–243. doi: 10.1111/cmi.12497 [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Demuth DR, & Zekavat A (2006). Exposure of lymphocytes to high doses of Actinobacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infect Immun, 74(4), 2080–2092. doi: 10.1128/IAI.74.4.2080-2092.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Dlakic M, Walker LP, Besack D, Jaffe E, LaBelle E, & Boesze-Battaglia K (2007). A novel mode of action for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol 3,4,5-triphosphate phosphatase activity. J Immunol, 178(8), 5099–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Hoffmaster RH, McKay TL, & Demuth DR (2000). Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol, 165(5), 2612–2618. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Hoffmaster RH, Zekavat A, Yamaguchi N, Lally ET, & Demuth DR (2001). Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J Immunol, 167(1), 435–441. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, McKay T, Datar S, Miller M, Chowhan R, & Demuth D (1999). Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol, 162(8), 4773–4780. [PubMed] [Google Scholar]
- Shenker BJ, Ojcius DM, Walker LP, Zekavat A, Scuron MD, & Boesze-Battaglia K (2015). Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines. Infect Immun, 83(4), 1487–1496. doi: 10.1128/IAI.03132-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Walker LP, Zekavat A, & Boesze-Battaglia K (2016). Lymphoid susceptibility to the Aggregatibacter actinomycetemcomitans cytolethal distending toxin is dependent upon baseline levels of the signaling lipid, phosphatidylinositol-3,4,5-triphosphate. Mol Oral Microbiol, 31(1),33–42. doi: 10.1111/omi.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Walker LP, Zekavat A, Dlakic M, & Boesze-Battaglia K (2014). Blockade of the PI-3K signalling pathway by the Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces macrophages to synthesize and secrete pro-inflammatory cytokines. Cell Microbiol, 16(9), 1391–1404. doi: 10.1111/cmi.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gao W, & Shao F (2017). Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci, 42(4), 245–254. doi: 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, … Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526(7575), 660–665. doi: 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, … Shao F (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 514(7521), 187–192. doi: 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- Sollberger G, Strittmatter GE, Kistowska M, French LE, & Beer HD (2012). Caspase-4 is required for activation of inflammasomes. J Immunol, 188(4), 1992–2000. doi: 10.4049/jimmunol.1101620 [DOI] [PubMed] [Google Scholar]
- Taabazuing CY, Okondo MC, & Bachovchin DA (2017). Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol, 24(4), 507–514 e504. doi: 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Berghe TV, Vandenabeele P, & Kroemer G (2019). The molecular machinery of regulated cell death. Cell Res, 29(5), 347–364. doi: 10.1038/s41422-019-0164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M, & Frisan T (2004). Cytolethal distending toxins. Rev Physiol Biochem Pharmacol, 152, 111–133. doi: 10.1007/s10254-004-0030-8 [DOI] [PubMed] [Google Scholar]
- Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, & Mortellaro A (2015). Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun, 6, 8761. doi: 10.1038/ncomms9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, & Pickett CL (1998). Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun, 66(5), 1934–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Wang X, Zheng Y, Jiang J, & Hu J (2019). What role does pyroptosis play in microbial infection? J Cell Physiol, 234(6), 7885–7892. doi: 10.1002/jcp.27909 [DOI] [PubMed] [Google Scholar]
- Young VB, Knox KA, Pratt JS, Cortez JS, Mansfield LS, Rogers AB, … Schauer DB (2004). In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect Immun, 72(5), 2521–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Zhang Q, Zhao J, & Jin M (2012). Haemophilus parasuis encodes two functional cytolethal distending toxins: CdtC contains an atypical cholesterol recognition/interaction region. PLoS One, 7(3), e32580. doi: 10.1371/journal.pone.0032580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Jiang J, Said-Sadier N, Boxx G, Champion C, Tetlow A, … Kelly KA (2015). Activation of the NLRP3 inflammasome by vault nanoparticles expressing a chlamydial epitope. Vaccine, 33(2), 298–306. doi: 10.1016/j.vaccine.2014.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of the effects of Cdt and transfected LPS on caspase-4 activation. THP-1WT derived macrophages were treated with Cdt (200 ng/ml), 1:1000 dilution of Lipofectamine 2000 (Invivogen) or 0.01 μg/ml LPS (E. coli 0111:B4) plus Lipofectamine. Caspase-4 activity was measured as described in Materials and Methods. Results are plotted as the mean±SEM of caspase activity (net OD405). *indicates statistical signficance (p<0.05) compared to untreated control cells.
Figure S2 THP-1Casp4− cells are susceptible to Cdt-induced activation of the canonical inflammasome. Panel A: Macrophages derived from THPWT, THP-1shctl or THP-1Casp4− were treated with 200 ng/ml Cdt and cell extracts were assessed by Western blot analysis for the presence of pro-IL-1β; GAPDH is shown as a loading control. Results are representative of three experiments. Panel B: THPWT and THP-1Casp4− derived macrophages were treated with Cdt and analysed for the presence of active caspase-1 (p20) by Western blot. A representative Western blot is shown along with compiled data from three experiments; data is expressed as a percentage of p20 levels in control cells. Results represent the mean±SEM of three experiments: * indicates statistical significance when compared to untreated control cells.
Figure S3 Cdt-treated cells exhibit a reduction in total cellular HMGB1. Macrophages derived from THP-1WT cells were treated with 25 and 50 ng/ml Cdt. Cells extracts were analyzed by Western blot for the presence of HGMB1. A representative blot is shown along with compiled results from three experiments. Data is expressed as a percentage (mean±SEM) of HMGB1 levels observed in control cells (0 Cdt); * indicates statistical significance (p<0.05) when compared to control cells.
Figure S4: Comparison of the effects of Cdt and LPS depleted Cdt (Cdt-LD) on activation of the noncanonical inflammasome and pyroptosis. Cdt preparations were depleted of LPS using the Pierce High Capacity Endotoxin Removal Resin Spin Column and the levels assessed using the LAL Chromogenic Endotoxin Quantification Kit. LPS contamination was reduced to < 6 pg/μg Cdt. Panel A: THP-1WT macrophages were treated with 200 ng/ml Cdt or Cdt-LD. Cell extracts were assessed for caspase-4 activity; results are expressed as a percent of caspase-4 activity observed in control cells and are the mean±SEM of three experiments. Panel B: THP-1WT macrophages were treated with medium alone, Cdt or Cdt-LD and analyzed for both HMGB1 release and GSDMD cleavage (lower band represents GSDMD-NT) . Results are plotted as protein intensity (mean±SEM) of three experiments. Panel C: THP-1WT macrophages were treated with Cdt or Cdt-LD and analyzed for LDH release at 48 hr. Results are plotted as net LDH release (%) and are the mean±SEM of three experiments. No statistical differences were observed between the results obtained with Cdt versus Cdt-LD
Figure S5: Representative Western blots of Cdt-induced HMGB1 release and GSDMD cleavage. The full blot of HMGB1 (top panel) and GSDMD cleavage (bottom panel) is shown; cells treated with medium alone (−) and with Cdt (+) are shown.