Abstract
Background:
Identifying e-cigarette product characteristics that moderate the effects of non-tobacco flavors and nicotine on user appeal can inform regulations issued in tandem with e-cigarette nicotine and flavor policies aimed to protect young adult health. An e-cigarette device’s electrical power affects the amount of solution aerosolized per puff, leading to more concentrated or diluted aerosol, which may alter product appeal. This laboratory experiment tested whether e-cigarette device power moderated the independent and interactive effects of non-tobacco flavors and nicotine on appeal in young adults.
Method:
In a within-subject design single-visit protocol, young adult e-cigarette users (N=100) administered standardized doses of e-cigarette solutions varying in flavor (fruit, menthol, tobacco) and nicotine (nicotine-containing [6 mg/mL], nicotine-free). Solutions were administered via a variable-voltage tank-style device at low (7.3W[3.3V@1.5Ω resistance]) and high (12.3W[4.3V@1.5Ω resistance]) power settings. Participants rated each dose’s appeal (0-100 scale).
Results:
The high (vs. low) power setting attenuated the appeal-enhancing effects of menthol (vs. tobacco) flavors (Menthol×Power, estimate=−5.44, P=.03). Power did not moderate the appeal-enhancing effects of fruit flavors. High (vs. low) power amplified the appeal-reducing effects of nicotine-containing (vs. nicotine-free) solutions (Nicotine×Power, estimate=6.69, P< 001) and augmented the extent to which fruit and menthol flavors suppressed nicotine’s appeal-reducing effects (Flavor×Nicotine×Power, estimates=9.40-14.85, Ps≤.03).
Conclusion:
E-cigarette device power appears to moderate flavor- and nicotine-induced changes in product appeal in nuanced ways, including by augmenting the ability of non-tobacco flavors to mask nicotine’s appeal-reducing effects. Regulatory restrictions on high-powered e-cigarette devices warrant consideration in efforts to protect young adult health.
1. Introduction
The prevalence of current e-cigarette use among U.S. young adults increased substantially this decade (Dai and Leventhal, 2019). Due to concerns regarding exposure to nicotine and toxins that may be present in e-cigarette aerosol (U.S. DHHS, 2016; NASEM, 2018), young adult e-cigarette use is a public health problem. The U.S. Food and Drug Administration (FDA) and other regulatory agencies can restrict or prohibit the manufacturing, marketing, and sales of e-cigarette products with particular characteristics scientifically demonstrated to appeal to young people. Thus, evidence on e-cigarette product characteristics that increase user appeal in young adults is needed.
As young adults typically use e-cigarettes in non-tobacco flavors (e.g., fruit or menthol; Villanti et al., 2017) and are vulnerable to nicotine dependence (U.S. DHHS, 2016), non-tobacco flavors and nicotine are key product characteristics studied in young adult e-cigarette product appeal research. When young adults are administered e-cigarettes in laboratory experiments, puffs taken from products in non-tobacco flavors produce higher appeal ratings (e.g., liking, willingness to use again) versus tobacco-flavored or flavorless products (Audrain-McGovern et al., 2016; Goldenson et al., 2016; Krishnan-Sarin et al., 2017). While nicotine in e-cigarettes generates reinforcing pharmacological effects once absorbed into the blood stream (Benowitz et al., 2009), nicotine also has instantaneous sensory effects that causes e-cigarette aerosol to be perceived as bitter and harsh, which may reduce product appeal (Rosbrook and Green, 2016; Krishnan-Sarin et al., 2017; Pullicin et al., 2019, DeVito et al., 2019). Nicotine-induced aversive sensory effects can be suppressed by non-tobacco flavors that mask nicotine’s bitterness and harshness (Rosbrook and Green, 2016; Krishnan-Sarin et al., 2017; DeVito et al., 2019). Hence, non-tobacco flavors dually influence e-cigarette product appeal by directly increasing their desirability and by suppressing the aversive qualities of nicotine, which could facilitate the acquisition and maintenance of persistent vaping behavior in young adults.
While the direct and interactive appeal-altering effects of flavor and nicotine have been well-studied, evidence identifying other e-cigarette product characteristics that moderate (i.e., augment or suppress) the appeal of flavors or nicotine in young adults is lacking but could have regulatory implications. Such evidence could indicate: (1) whether the impact of regulatory restrictions on high-nicotine or flavored e-cigarette products on young adult vaping would generalize across e-cigarettes with varying product characteristics; (2) whether supplementary regulation of other product characteristics (in tandem with flavor and nicotine regulations) could synergistically impact young adult vaping, and (3) whether, in the absence of direct regulations on flavors or nicotine, regulating other product characteristics could moderate the appeal-enhancing effects of high-nicotine or flavored products.
E-cigarettes operate by delivering power to the device’s heating element—the component that heats e-cigarette solutions into aerosol, with higher temperatures corresponding with greater aerosol production (DeVito and Krishnan-Sarin, 2018). A device’s power level determines the aerosol particulate mass per unit volume of air (i.e., the density or ‘richness’ of the aerosol in each puff; Robinson et al., 2018; Floyd et al., 2018). At high power levels, devices produce puffs that may be more concentrated with flavorants, aerosol, and other constituents in e-cigarette solutions, which could augment the appeal-altering effects of flavors and nicotine relative to less-powerful devices that produce diluted aerosols.
In-vivo human studies which have compared the effects of administering e-cigarette devices with different power outputs are scant (Wagner et al., 2017; Hiler, 2019; Farsalinos et al., 2013). One study found that higher power settings e-cigarettes increased subjective rewarding effects when combined with lower nicotine concentrations (Hiler, 2019). No in-vivo human study has examined the simultaneous effects of e-cigarette flavor and device power on user outcomes.
This article is a secondary analysis of a laboratory study of the effects of non-tobacco flavors and nicotine on e-cigarette product appeal in young adult e-cigarette users (Leventhal et al., 2019). The primary outcomes paper reported that fruit and menthol flavored e-cigarette solutions increased appeal, nicotine reduced appeal, and non-tobacco flavored solutions attenuated the appeal-reducing effects of nicotine (i.e., flavor-by-nicotine interaction). Each solution in the study was administered at low- and high-power device settings, providing the current opportunity to test whether device power moderated the main and interactive effects of flavor and nicotine on user appeal. Given that higher power generates more concentrated aerosol, we hypothesized that the appeal-enhancing effects of non-tobacco flavors, appeal-reducing effects of nicotine, and suppression of nicotine’s appeal-reducing effects by non-tobacco flavors (i.e., flavor-by-nicotine interaction) would be amplified at high vs. low power settings.
2. Method
2.1. Participants
Young adults (N=100) who met inclusion (18-35 years old; current e-cigarette use ≥1 day/week for ≥1 month; and used nicotine-containing e-cigarettes) and exclusion (impending plan to quit vaping; smoking cessation medication use; pregnant/breastfeeding) criteria were recruited. Subjects provided written informed consent. The University of Southern California Institutional Review Board approved the protocol.
2.2. Design and Materials
A flavor (fruit, menthol, tobacco) × nicotine concentration (nicotine-containing [6mg/mL free base nicotine] vs. nicotine-free) within-participant double-blind factorial design was used. The procedure utilized nicotine-containing and nicotine-free versions of 9 flavors—5 fruit (Blackberry, Strawberry, Blueberry, Watermelon, Peach), 2 menthol (Portal Blend, Triple Menthol) and 2 tobacco (Red USA, Desert Ship; Dekang Biotechnology Co., Ltd). There were 18 total solutions with a mean Propylene Glycol/Vegetable Glycerin (PG/VG) of 51/49 (SD=4.3/4.3). Of 9 nicotine-containing solutions, the mean nicotine concentration was 6.1 (SD=0.53) mg/mL.
Each solution was administered at two voltage settings via a variable-voltage Joyetech “Delta 23 Atomizer” tank device and “eVic Supreme” battery: (1) low power (7.3W [3.3 V@1.5Ω resistance]) and (2) high power (12.3W [4.3V@1.5Ω resistance]). These settings were selected based on previous research (Ramôa et al., 2015; Sleiman et al., 2016).
2.3. Procedure
Participants were instructed to abstain from using nicotine/tobacco products for 2-hours prior to the study visit; biochemical verification of abstinence was not obtained. The appeal testing procedure involved 36 experimental trials (18 solutions presented at both low and high-power settings). Each participant received a randomly assigned ordering of the 36 total e-cigarette solution-power setting combinations. Trial sequence number (1-36) and trial sequence × nicotine interactions were not significant, as reported previously (Leventhal et al. 2019), indicating no evidence that participants became habituated, fatigued, or sensitized to the procedure or to nicotine’s appeal-altering effects throughout testing. Each trial consisted of a guided controlled puffing procedure involving 2-puff cycles (10-second preparation, 4-second inhalation, 1-second hold, and 2-second exhale intervals) per product, followed by appeal ratings, with 1-minute inter-trial intervals, during which participants were provided with water. The procedure was separated into four 8-trial blocks. Blocks were separated by 30 minutes, during which participant characteristic questionnaires were completed. The procedure lasted 3-hr total.
2.4. Measures
2.4.1. Appeal.
After each 2-puff trial, participants provided ratings of the product they just vaped on visual analogue scales (VAS; 0-100 range): 1) “How much did you like it?” (“Not at all” - “Extremely” anchors); 2) “How much did you dislike it?” (“Not at all” - “Extremely”); and 3) “Would you use it again?” (“Not at all” - “Definitely”). For each trial, a composite appeal score based on the average of “Liking,” “Disliking” (reverse-scored),” and “Willingness to use again” ratings was calculated (Cronbach’s α=.93). Interitem correlations were large for liking-disliking (r = −.81), liking-willing to use again (r = .89), and disliking-willingness to use (r=−.76).
2.4.2. Participant characteristics.
Participants completed demographic and tobacco product use history questionnaires (see question responses in Table 1) and the Penn State Electronic Cigarette Dependence Index (PSECD; Foulds et al., 2015), an e-cigarette dependence measure (range: 0-20). NicAlert™ test strips (LiveWellTesting.com, San Diego, CA) that provide a semi-quantitative index of salivary cotinine and exhaled carbon monoxide (CO) were collected to provide descriptive data on nicotine and combusted tobacco exposure, respectively.
Table 1.
Descriptive Statistics of Participant Characteristics in Study Samplea
| Participant Characteristics | N(%) or M(SD) |
|---|---|
| Demographics | |
| Female gender, N (%) | 35 (35.0%) |
| Age, years, mean (SD), | 25.4 (4.4) |
| Race/ethnicity, N (%) | |
| Hispanic | 22 (22.0%) |
| White | 29 (29.0%) |
| Black | 25 (25.0%) |
| Asian | 15 (15.0%) |
| Other | 9 (9.0%) |
| Tobacco Product Use Characteristics | |
| Combustible cigarette smoking status,b N (%) | |
| Never smoker | 22 (22.0%) |
| Former smoker | 25 (25.0%) |
| Current smoker | 53 (53.0%) |
| Preference for menthol combustible cigarettes, N (%) | |
| Among former smokers | 10 (66.7%) |
| Among current smokers | 23 (43.4%) |
| Salivary cotinine semi-quantitative level,c mean (SD) | 2.85 (1.22) |
| Carbon monoxide, ppm,d mean (SD) | 5.00 (5.51) |
| PSECDI e-Cigarette Dependence score, mean (SD) | 7.01 (4.51) |
| Puffs per day, mean (SD) | 74.3 (124.3) |
| Nicotine concentration typically used, mean (SD) | 8.77 (13.92) |
| Duration of e-cigarette use, months, mean (SD) | 19.8 (14.4) |
| e-Cigarette device type typically used, N (%) | |
| Cig-a-like | 12 (12%) |
| Tank/pen | 30 (30%) |
| Advanced personal vaporizer/mod | 58 (58%) |
| Preferred e-cigarette flavor,e N (%) | |
| Fruit or Dessert | 80 (80.0%) |
| Menthol or Mint | 13 (13.0%) |
| Tobacco | 7 (7.0%) |
N=100
Never smokers: smoked less than 100 cigarettes lifetime, Former smokers: smoked ≥100 cigarettes but did not smoke in past 30 days; Current smokers: smoked ≥100 cigarettes lifetime and smoked in past 30 days.
NicAlert Strip (Range 1-6; 0=0-10, 1=10-30, 2=30-100, 3=100-200, 4=200-500, 5=500-1000, 6=>1000 ng/mL).
Carbon monoxide was significantly higher in participants who were current smokers (M[SD]=7.04[5.95]) than former (M[SD]=2.40 [3.04]) and never (M[SD]=3.05[4.73]) smokers (ps<.001)
Response to question, “Which e-cigarette liquid flavor do you usually use?” “Tobacco-flavored” responses were categorized in the tobacco category. “Menthol” or “Mint” responses were categorized as menthol/mint. “Candy (e.g., bubble gum, liquorish),” “Fruit (e.g., strawberry, banana),” or “Chocolate or other sweets (e.g., caramel, cake batter)” responses were classified as fruit or desert. Other possible responses (e.g., clove, spice, alcohol, unflavored) were not selected by any participants.
PSECDI = Penn State Electronic Cigarette Dependence Index
2.5. Data Analyses
The primary analysis used multilevel models (MLMs), which utilized appeal rating outcome data from each trial as separate data points (up to 18 total observations for each power level). MLMs generate results that can be interpreted as effects averaged across all trials within each condition. We tested the main effects of flavor (i.e., fruit, menthol, and tobacco [reference category]) and nicotine content (i.e., nicotine and nicotine-free [reference category]) and the flavor×nicotine interaction effect separately for the low-power and high-power trials. To determine whether each effect was significantly moderated by power (high vs. low [reference category]), we tested flavor×power, nicotine×power, and flavor×nicotine×power interactions. In an exploratory analysis of whether power’s moderating effects varied by smoking history, we tested additional higher order interactions including smoking history (current vs. former vs. never smoker) between-subject type-III effects. As an alternative method of depicting power’s interactive effects to highlight how power alters appeal specifically for each flavor, we tested power main effects and nicotine×power effects, stratified by flavor. Results are reported as unstandardized parameter estimates with standard errors (B±SE), which reflect the difference or difference-in-difference in mean appeal ratings between conditions for main effects and interactions, respectively. P-values were 2-tailed. Benjamini-Hochberg multiple test corrections were used to maintain a .05 study-wise false discovery rate (Benjamini and Hochberg, 1995).
3. Results
3.1. Sample Characteristics
The sample (35% female; M±SDage=25.4±4.4 years) was racially/ethnically diverse, and exhibited, on average, moderate e-cigarette dependence levels. The majority of participants used tank/pen or advanced personal vaporizer mod devices and fruit or dessert flavors; 53% were current combustible cigarette smokers, 25% were former smokers, and 22% were never (≤100 cigarettes lifetime) smokers. Descriptive data on other characteristics are reported in Table 1.
3.2. Primary Results
3.2.1. Flavor and nicotine main effects, by power.
Power did not significantly moderate the effects of fruit (vs. tobacco) flavors on appeal (fruit×power, p=.99; Table 2). Appeal ratings were significantly greater for fruit (vs. tobacco) flavored products in both low-power (fruit effect estimate [fruit – tobacco difference]: 13.9) and high-power (fruit estimate: 13.9; Table 2, Figure 1) conditions. Power significantly moderated menthol (vs. tobacco) flavor effects on appeal (menthol×power, p=.03), such that menthol-induced increases in appeal were larger at the low-power (menthol estimate [menthol – tobacco]: 11.9) than high-power (estimate: 6.4) setting (Table 2, Figure 1). Power significantly moderated nicotine-containing (vs. nicotine-free) effects (nicotine×power, p<.001); nicotine-induced reductions in appeal were more robust at the high-power (nicotine estimate [nicotine-containing – nicotine-free]: −14.5) vs. low-power (nicotine estimate: −7.8) setting (Table 2, Figure 2).
Table 2.
Effect Estimates of Non-Tobacco Flavors and Nicotine Content on Appeal,a Stratified by Power Settingb
| Low Powerc |
High Powerd |
Difference in Effect Estimates, by Powere |
||||
|---|---|---|---|---|---|---|
| B (SE) | P | B (SE) | P | B(SE) | P | |
| Flavor and Nicotine Main Effects, by Power | ||||||
| Fruit vs. Tobacco | 13.9 (1.45) | <.001* | 13.9 (1.52) | <.001* | 0.01 (2.15) | .99 |
| Menthol vs. Tobacco | 11.9 (1.74) | <.001* | 6.4 (1.82) | <.001* | −5.4 (2.56) | .03* |
| Nicotine (6mg/mL) vs. Nicotine-free | −7.8 (1.16) | <.001* | −14.5 (1.21) | <.001* | 6.7 (1.71) | <.001* |
| Flavor × Nicotine effects, by Power | ||||||
| Fruit vs. Tobacco × Nicotine vs. Nicotine-free | 1.6 (2.91) | .59 | 11.0 (3.02) | <.001* | 9.4 (4.29) | .03* |
| Menthol vs. Tobacco × Nicotine vs. Nicotine-free | 4.4 (3.48) | .21 | 19.3 (3.61) | <.001* | 14.9 (5.12) | .004* |
Appeal = Average of “liking,” “willingness-to-use-again” and “disliking” (reverse-scored) (range 0-100).
N=100
7.3W (3.3 V @ 1.5 Ω resistance)
12.3 W (4.3 V @ 1.5 Ω resistance)
Estimates in order from top to bottom are from tests of Fruit × Power, Menthol × Power, Nicotine × Power, Fruit × Nicotine × Power, and Menthol × Nicotine × Power interaction effects, respectively.
Significant effect after Benjamini-Hochberg correction to maintain study-wise false discovery rate of .05
Figure 1.
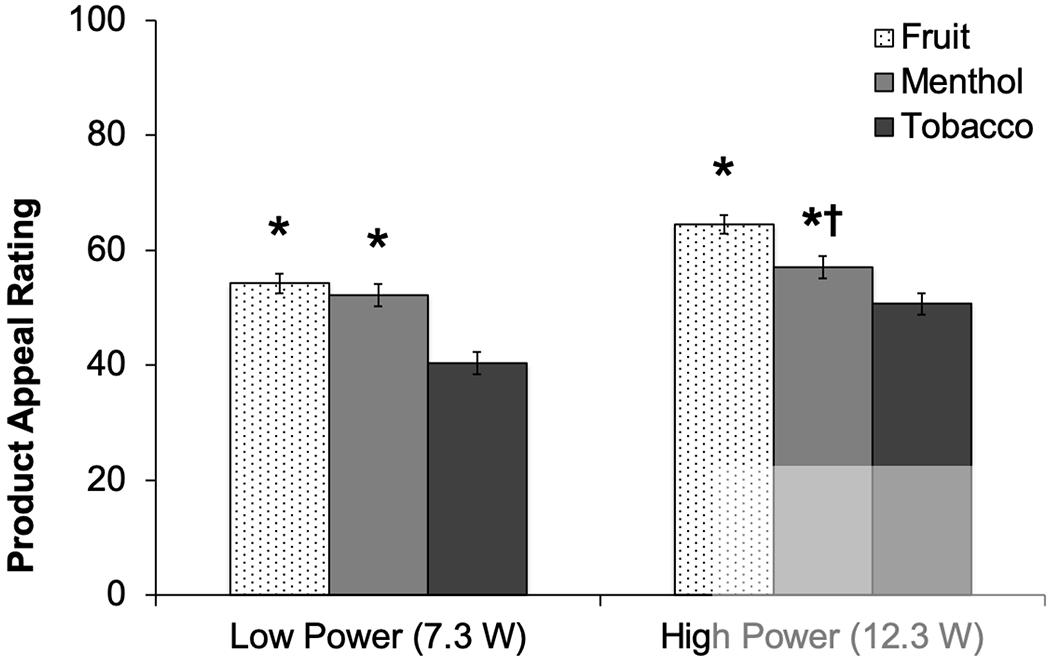
Product Appeal of e-Cigarettes with Fruit, Menthol, and Tobacco Flavored Solutions, by Power Setting (M±SE)
*Appeal rating significantly different between respective flavor condition and tobacco flavor condition at respective power setting (P < .001)
†Extent of difference in appeal between menthol and tobacco flavors significantly differs by power (Menthol × Power, P = .03).
Appeal = Average of “liking,” “willingness-to-use-again” and “disliking” (reverse-scored) (range 0-100).
Figure 2.
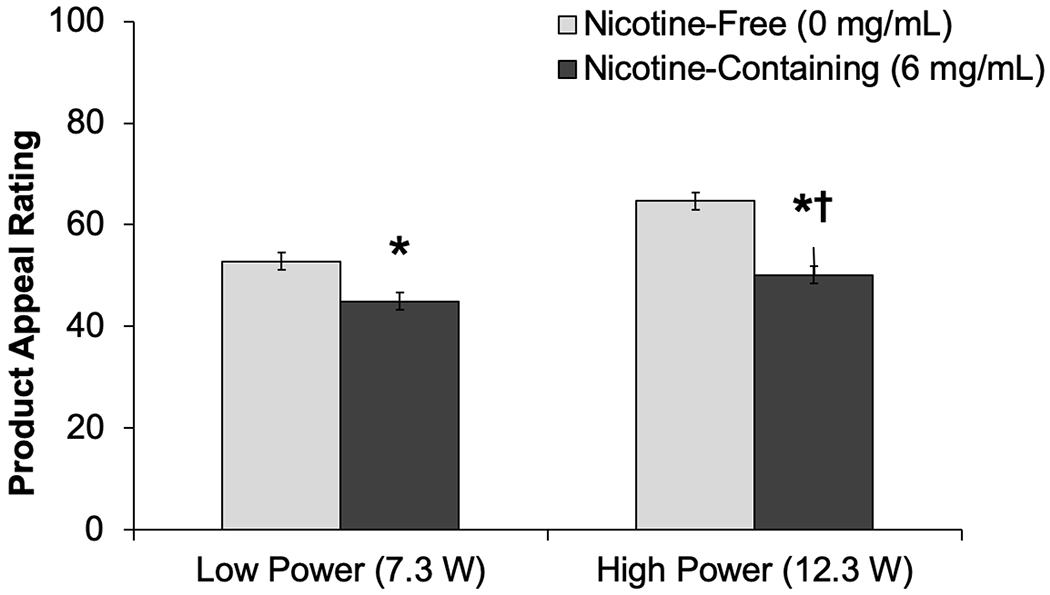
Product Appeal of e-Cigarettes with Nicotine-Containing and Nicotine-Free Solutions, by Power Setting (M±SE)
*Appeal rating significantly different from nicotine-free within respective power setting (P < .001)
†Extent of difference in appeal between nicotine and nicotine-free significantly differs by power setting (Nicotine × Power, < .001).
Appeal = Average of “liking,” “willingness-to-use-again” and “disliking” (reverse-scored) (range 0-100).
3.2.2. Flavor × nicotine interaction effects, by power.
Power significantly moderated flavor×nicotine effects on appeal for both fruit and menthol flavors (flavor×nicotine×power, ps≤.03; Table 2, Figure 3). At the high-power setting, nicotine-related reductions in appeal were significantly attenuated by fruit vs. tobacco flavors (fruit×nicotine, p<.001), with a corresponding fruit×nicotine estimate of 11.0, which is the ‘difference-in-difference’ of the nicotine effect (nicotine-containing – nicotine-free difference score) for fruit flavors subtracted by the nicotine effect for tobacco flavors (−13.9 vs. −24.9). At the low-power setting, fruit did not suppress nicotine-related reductions in appeal (fruit×nicotine, p=.59). Nicotine-related reductions in appeal were also attenuated by menthol vs. tobacco flavors at the high power setting (mentholxnicotine estimate: 19.3, p<.001) but not the low-power setting (menthol×nicotine, p=.21). The M(SE) for each flavor-nicotine-power study condition cell is reported in the supplemental table.
Figure 3.
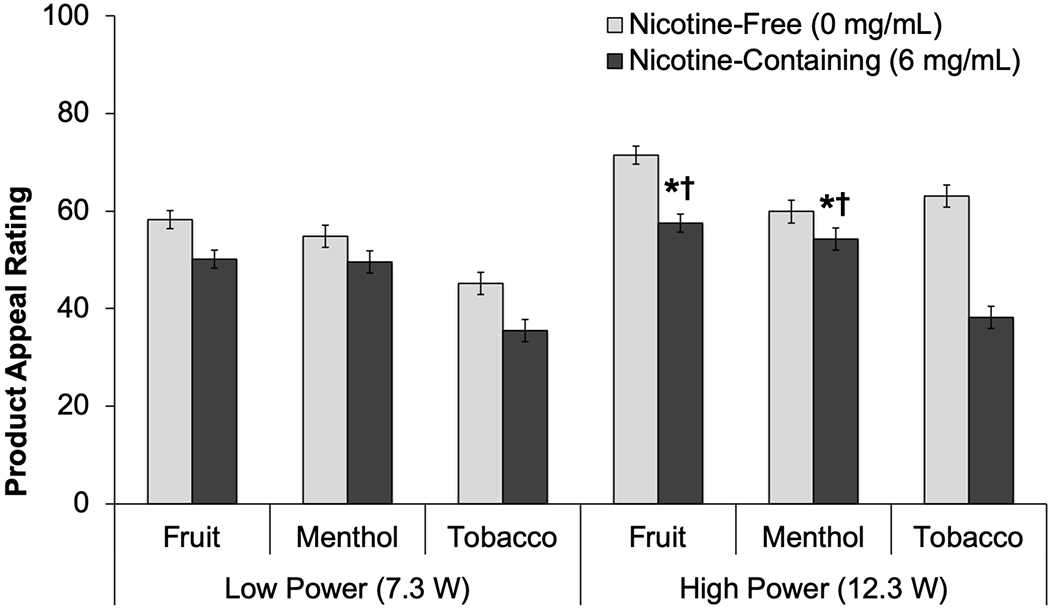
Product Appeal of e-Cigarettes with Nicotine and Nicotine-Free Solutions, by Flavor and Power Setting (M±SE)
*Extent of difference in appeal between nicotine-containing and nicotine-free condition significantly differs between respective flavor and tobacco flavor within high power condition (Flavor × Nicotine, Ps < .001).
†Power level significantly moderates the extent to which the difference in appeal between nicotine-containing and nicotine-free condition varies between respective flavor and tobacco flavor (Flavor × Nicotine × Power, Ps ≤ .03).
Appeal = Average of “liking,” “willingness-to-use-again” and “disliking” (reverse-scored) (range 0-100)
3.3. Exploratory Analyses
3.3.1. Smoking history.
Smoking history did not moderate flavor×power (F=1.47, p=.21), nicotine × power (F=0.17, p=.84), and flavor×nicotine×power (F=1.38, p=.24) effects, indicating no significant differences in power’s moderating effects between current, former, and never smokers.
3.3.2. Power main effects and nicotine × power effects, by flavor.
As an alternative method of disentangling flavor×power and flavor×nicotine×power effects, we tested power main effects and nicotine×power effects stratified by flavor. Power significantly increased appeal in the tobacco (power estimate [high – low]: 17.9, p<.001) and fruit (power estimate: 13.2, p<.001) flavors but not for menthol flavors (power estimate: 5.1, p=.11). The extent to which power amplified nicotine’s appeal-reducing effects was robust for tobacco flavors (nicotine×power estimate [difference-in-difference of nicotine vs. nicotine free at high vs. low power]: −15.2, p<.001), moderate and statistically significant for fruit flavors (nicotine×power estimate: −5.8, p=.02), and non-significant for menthol flavors (nicotine×power estimate: −0.4, p=.90).
4. Discussion
This study demonstrated that e-cigarette device power moderated the main and interactive effects of non-tobacco flavors and nicotine on product appeal in young adults. The main findings were that increasing device power: (1) attenuated menthol’s appeal-enhancing effects but did not alter the appeal-enhancing effects of fruit flavors, (2) amplified nicotine’s appeal-reducing effects, and (3) allowed non-tobacco flavors to mask nicotine’s appeal-reducing effects. These are the first data showing that e-cigarette device power interacts with flavors and nicotine-flavor combinations to alter the user experience of vaping.
We hypothesized that appeal preferences for non-tobacco flavors would be magnified at higher power levels because power increases the amount of e-liquid that is aerosolized into each puff (Robinson et al., 2018; Floyd et al., 2018), but the results ran counter to this prediction. The relative preference for fruit over tobacco flavors was unchanged by power setting; i.e., power increased the appeal of both of these flavors. Preferences for menthol over tobacco flavors were actually larger at the low power setting, mainly because power increased the appeal of tobacco flavors, but did not change the appeal of menthol. We suspect power uniquely altered menthol’s appeal because menthol possesses intense sensory effects unparalleled by other flavors. An aerosol with a low concentration of menthol produced by a modest power setting may nonetheless contain enough menthol to generate coolness and other desirable sensations to the user, whereas an aerosol that is highly-concentrated with menthol may generate exceedingly intense (and potentially unpleasant) sensations that may not be appealing. Previous research shows that menthol exposure at lower concentrations primarily activates TRPM8 receptors, which may produce pleasant cooling sensations, whereas high menthol concentration exposure activates TRPM8 receptors while additionally activating TRPA1 receptors, which may generate irritating sensations (Karashima et al., 2007; Lemon et al., 2019). With flavorants that simulate the taste of fruit or tobacco, however, diluted aerosols produced at low-power settings may be less flavorful and appealing than concentrated aerosols emitted at high-power settings. Previous studies manipulating flavorant concentration in e-cigarette solutions (which, in turn, could increase the flavorant concentration in aerosol) found results consistent with this interpretation. Administering e-cigarettes in 2.5% vs. 0.5% menthol solutions increased user sensations of coolness but did not affect product appeal in one study (Krishnan-Sarin et al., 2017). A study comparing e-cigarette solutions with different concentrations of cherry flavoring found suggestive evidence that appeal was enhanced at higher cherry concentrations (Pulliccin et al., 2019). Thus, the appeal-altering effects of flavorant concentration in e-cigarette aerosol may depend on the flavor.
Consistent with hypotheses, nicotine-containing solutions were less appealing than nicotine-free solutions and power moderated (augmented) nicotine’s appeal-reducing effects. If nicotine, instead of power, were be to conceptualized as the moderator, this same result can be conceptualized as evidence that the extent to which power increases appeal is less robust with nicotine-containing than nicotine-free solutions. A previous study found that lowering device coil resistance (which, in turn, increases electrical power) enhanced the subjective rewarding effects of vaping (Hilter, 2019). That study utilized an abuse liability paradigm in which a single product is administered per study visit with a time course that allows for post-exposure nicotine absorption and can detect effects driven by pharmacologically-mediated reinforcement. The current appeal testing methodology in which multiple products are administered in the same visit can detect perceived appeal based on the sensations elicited while vaping, but not pharmacological effects. Nicotine is a respiratory irritant and has bitter qualities, which, when in e-cigarettes, reduces the immediate appeal of the user experience despite its neuropharmacologically-rewarding effects, as demonstrated in previous studies utilizing appeal testing paradigms similar to the current study (Rosbrook and Green, 2016; Pullicin et al., 2019; Devito et al., 2019; Mead et al., 2019). As in prior studies that increased the amount of nicotine in aerosol by varying nicotine concentration in e-cigarette solutions (Rosbrook and Green, 2016; Pullicin et al., 2019; Devito et al., 2019; Mead et al., 2019), increasing device power in this experiment likely increased aerosol nicotine concentration emitted from the nicotine-containing solutions, which may have reduced user appeal.
This study adds to previous evidence that non-tobacco flavors offset nicotine’s appeal-reducing effects in e-cigarettes (Rosbrook et al., 2016; Krishnan-Sarin et al., 2017; Devito et al., 2019) by showing amplification of flavor-by-nicotine interactions by device power. Menthol may mask the harshness and irritating airway sensations caused by nicotine by producing cooling-sensations (Rosbrook et al., 2016; Krishnan-Sarin et al., 2017; Devito et al., 2019), and fruit flavors could provide sweet and fruity olfactory or orosensory perceptions that offset nicotine’s bitter taste. One interpretation of this result is that by increasing the concentration of both nicotine and flavorants in aerosol, power also increases flavor-by-nicotine sensory interactions, with more nicotine eliciting worse sensations and more flavorant levels eliciting counterpoising sensations that offset nicotine’s appeal-reducing sensations. The capability of non-tobacco flavors to mask nicotine’s appeal-reducing effects may drive young adults to continue vaping nicotine despite its aversive sensory effects, which ultimately may increase exposure to nicotine and risk of nicotine dependence. The current results suggest that this concern could be compounded with more powerful devices.
While we suspect that the moderating effects of e-cigarette device power on product appeal demonstrated in this study are explained by power-related changes in aerosol volume, other explanations may underpin the results. In addition to increasing the total amount of particulate matter in aerosol, higher power settings have also been shown to increase particle size (Floyd et al., 2018). Given that particle size affects respiratory tract deposition (Zhang et al., 2013), aerosol produced at higher power levels could affect the perceived harshness of the aerosol, and, in turn, the appeal of the product. Furthermore, heat generated by more power may also cause thermal decomposition and the emission of new compounds (including aldehydes), which may be unappealing to the user (Farsalinos et al., 2015). Future research conducting aerosol constituent analysis in tandem with user appeal reports could test these explanations.
This study had limitations. First, this sample was heterogenous with regards to smoking history, and overall e-cigarette dependence was relatively low. While a supplementary analysis found that smoking history did not alter the moderating effects of device power in this study, older adult chronic smokers or those with high e-cigarette dependence may respond differently to changes in device power than the young adults in this study. Second, it is unclear whether the current data collected using a tank-style device with free-base nicotine solutions will generalize to recently-popular pod-mod style e-cigarette products that have smaller batteries and utilize protonated (salt-based) nicotine formulations (Barrington-Trimis and Leventhal et al., 2018). Third, this initial study tested one nicotine concentration and only two variable-voltage power settings, both of which may be lower than concentrations and power levels tested previously and preferred by some users (Cameron et al., 2014). For solutions with higher nicotine concentrations, power’s moderating effect could be exaggerated or perhaps blunted due to ceiling effects; high-nicotine concentration solutions could already be considerably aversive at lower power settings. Fourth, the appeal measure used here was not capable of delineating appeal reductions due to absence of reward versus the presence of aversion, which should be addressed using separate measures of each construct with sufficient discriminant validity. Finally, the concentration of menthol and other flavoring constituents in e-cigarette solutions were unavailable.
5. Conclusion
Young adults almost invariably prefer non-tobacco to tobacco flavors and may be vulnerable to the adverse effects of nicotine exposure, including dependence and nicotine-induced neurobehavioral changes (U.S. DHHS, 2016; Villanti et al., 2017). Furthermore, young people who vape typically do not use e-cigarettes as smoking cessation aids and therefore do not garner any health benefits from vaping (Evans-Polce et al., 2018). Consequently, regulatory restrictions on products with non-tobacco flavors or high nicotine concentrations have been proposed as means of preventing vaping among young populations (Drazen et al., 2019; Wasowicz et al., 2015). While the FDA announced an intention to prioritize enforcement of certain unauthorized flavored e-cigarette products on the market that are in violation of FDA’s premarket review requirements (U.S. FDA, 2020), the FDA does not have product standards prohibiting sales of flavored e-cigarettes and exempted menthol flavors and non-cartridge products from their enforcement plan. Thus, e-cigarette makers could eventually legally sell non-tobacco flavored products pending successful FDA premarket review. The current study suggests that, in the absence of certain nicotine and flavor regulations, regulating e-cigarette device power could indirectly impact young adult vaping by altering the effects of flavors and nicotine on user appeal. Given evidence in this study that increasing power allowed non-tobacco flavors to mask nicotine’s appeal-reducing effects in concert with past data demonstrating that power increases nicotine delivery and toxicant emissions in aerosol (Hiler, 2019; Sleidman, 2016), restrictions on high powered devices could have a net positive effect on the health of young adult population. However, given the nuanced effects of how power interacted with flavor and nicotine in this study, it cannot be concluded that restrictions on high powered devices would invariably reduce the appeal of all e-cigarette products among young adults.
Supplementary Material
Highlights.
Users rated appeal of e-cigarettes varying in flavor/nicotine at high & low power
High power reduced the appeal-enhancing effects of menthol flavors
High power amplified the appeal-reducing effects of nicotine containing solutions
High power enhanced fruit/menthol’s ability to suppress nicotine’s appeal-reduction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No conflicts of interest.
References
- Audrain-McGovern J, Strasser AA, & Wileyto EP (2016). The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence, 263–267. 10.1016/j.drugalcdep.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis J & Leventhal AM (2018). Adolescents’ use of “pod mod” e-cigarettes – urgent concerns. The New England Journal of Medicine, 379, 1099–1102. 10.1056/NEJMp1805758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. https://www.jstor.org/stable/2346101. [Google Scholar]
- Benowitz NL (2009). Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annual Review of Pharmacology and Toxicology, 49, 57–71. 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JM, Howell D, White JR, Andrenyak DM, Layton ME, & Roll JM (2014). Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tobacco Control, 23(1), 77–78. 10.1136/tobaccocontrol-2012-050604. [DOI] [PubMed] [Google Scholar]
- Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, & Walker N (2008). Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine & Tobacco Research, 10(4), 607–612. 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- Dai H & Leventhal AM (2019). Prevalence of e-cigarette use among adults in the United States, 2014-2018. Journal of the American Medical Association. https://doi.org/1001/jama.2019.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Jensen KP, O’Malley SS, Gueorguieva R, Krishnan-Sarin S, Valentine G, … Sofuoglu M (2019). Modulation of ‘protective’ nicotine perception and use profile by flavorants: preliminary findings in e-cigarettes. Nicotine & Tobacco Research, ntz057 10.1093/ntr/ntz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, & Krishnan-Sarin S (2018). E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Current Neuropharmacology, 16(4), 438–459. 10.2174/1570159X15666171016164430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen JM, Morrissey S, & Campion EW (2019). The dangerous flavor of e-cigarettes. The New England Journal of Medicine, 380, 679–680. 10.1056/NEJMe1900484. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, & Shihadeh A (2015). Nicotine flux: a potentially important tool for regulating electronic cigarettes. Nicotine & Tobacco Research, 17(2), 165–167. 10.1093/ntr/ntu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission Public Health. (2018). European Tobacco Products Directive. Brussels, Belgium. [Google Scholar]
- Evans-Polce RJ Patrick ME, Lanza ST, Miech RA, O’Malley PM, & Johnston LD (2018). Reasons for vaping among U.S. 12th graders. Journal of Adolescent Health, 62(4), 457–462. 10.1016/j.jadohealth.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V (2013). Impact of flavour variability on electronic cigarette use experience: an internet survey. International Journal of Environmental Research and Public Health, 10(12), 7272–7282. 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Voudris V, Poulas K (2015). E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction, 110(8), 1352–1356. 10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- Floyd EL, Queimado L, Wang J, Regens JL, & Johnson DL (2018). Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoS ONE, 13(12), e0210147 10.1371/journal.pone.0210147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J Veldeer S Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, & Eissenberg T (2015). Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine & Tobacco Research, 17(2), 186–192. 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman IG Kistler KA, Stewart EW, & Paolantonia AR (2016). Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regulatory Toxicology and Pharmacology, 75, 58–65. 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Goldenson N, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Samet, … Leventhal AM (2016). Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug and Alcohol Dependence, 168, 176–180. 10.1016/j.drugalcdep.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler M (2019). The influence of electronic cigarette heating coil resistance on nicotine delivery, heart rate, subjective effects, and puff topography. Virginia Commonwealth University, Richmond, Virginia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera T, Segal A, Voets T, & Nilius B Bimodal action of menthol on the transient receptor potential channel TRPA1. Journal of Neuroscience, 12(31), 9874–9884. 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Morean M, Cavallo D, Camenga DR, & Krishinan-Sarin S (2014). Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine & Tobacco Research, 17(7), 847–854. 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Green BG, Kong G, Cavallo DA, Jatlow P, Gueorguieva R, … O’Malley SS (2017). Studying the interactive effects of menthol and nicotine among youth: An examination using e-cigarettes. Drug and Alcohol Dependence, 180, 193–199. 10.1016/j.drugalcdep.2017.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon C, Norris E, & Heldmann A The TRPA1 ion channel contributes to sensory-guided avoidance of menthol in mice. eNeuro. 10.1523/ENEURO.0304-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Goldenson NI, Barrington-Trimis JL, Pang RD, & Kirkpatrick MG (2019). Effects of non-tobacco flavors and nicotine on e-cigarette product appeal among young adult never, former, and current smokers. Drug & Alcohol Dependence, 203, 99–106. 10.1016/j.drugalcdep.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EL, Duffy V, Oncken C, & Litt MD (2019). E-cigarette palatability in smokers as a function of flavorings, nicotine content and propylthiouracil (PROP) taster phenotype. Addictive Behaviors, 91, 37–44. 10.1016/j.addbeh.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Academies of Sciences, Engineering, Medicine. (2018). Public Health Consequences of E-Cigarettes. Retrieved from http://nationalacademies.org/hmd/Reports/2018/public-health-consequences-of-e-cigarettes.aspx.
- Pullicin AJ, Kim H, Brinkman MC, Buehler SS, Clark PC, & Lim J (2019). Impacts of nicotine and flavoring on the sensory perception of e-cigarette aerosol. Nicotine & Tobacco Research, ntz058 10.1093/ntr/ntz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Râmoa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, … Eissenberg T (2015). Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tobacco Control, 25(E1), e6–69. 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Eddingsaas NC, DiFrancesco AG, Jayasekera S, & Hensel EC Jr. (2018). A Framework to Investigate the Impact of Topography and Product Characteristics on Electronic Cigarette Emissions. PLoS ONE, 13(11), e0206341 10.1371/journal.pone.020634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbrook K, & Green BG (2016). Sensory effects of menthol and nicotine in an e-cigarette. Nicotine & Tobacco Research, 18(7), 1588–1595. 10.1093/ntr/ntw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, & Destaillats H (2016). Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environmental Science & Technology, 50(17), 9644–9651. 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (2019a). FDA issues proposed rule for premarket tobacco product applications as part of commitment to continuing strong oversight of e-cigarettes and other tobacco products. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-issues-proposed-rule-premarket-tobacco-product-applications-part-commitment-continuing-strong.
- U.S. Food and Drug Administration. (2019b). Trump administration combating epidemic of youth e-cigarette use with plan to clear market of unauthorized, non-tobacco-flavored e-cigarette products. Retrieved from https://www.fda.gov/news-events/press-announcements/trump-administration-combating-epidemic-youth-e-cigarette-use-plan-clear-market-unauthorized-non.
- U.S. Food and Drug Administration. (2020). FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children
- U.S. Department of Health and Human Services. (2016). 2016 Surgeon General’s Report: E-cigarette use among youth and young adults: a report of the surgeon general. Retrieved from https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, … Queimado L (2016). Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control, 26(e1), e23–e28. 10.1136/tobaccocontrol-2016-05304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasowicz A, Feleszko W, & Goniewicz ML (2015). E-cigarette use among children and young people: the need for regulation. Expert Review of Respiratory Medicine, 9, 507–509. 10.1586/17476348.2015.1077120. [DOI] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, … Hyland A (2017). Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013-2014). American Journal of Preventative Medicine, 53(2), 139–151. 10.1016/j.amepre.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sumner W, & Chen DR (2013). In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine & Tobacco Research, 15(2), 501–508. 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


