Abstract
PURPOSE:
To characterize features of glaucoma associated with a TANK binding kinase 1 (TBK1) gene duplication, which is among the most common molecularly defined causes of normal tension glaucoma (NTG).
DESIGN:
Retrospective observational case series
METHODS:
We conducted a retrospective case series, by reviewing medical records of 7 members of a pedigree with normal tension glaucoma (NTG) caused by TBK1 gene duplications. Clinical features of these patients at diagnosis, throughout management, and at latest follow up were identified including age, intraocular pressure (IOP), central corneal thickness (CCT), optic nerve head appearance, and mean deviation (MD) assessed with Humphrey visual field (HVF) testing protocols.
RESULTS:
At initial diagnosis, the mean age was 35 ± 7 years, IOP was 16 ± 2.1 mm Hg, cup-to-disc (C/D) ratio was 0.9 ± 0.08, and MD assessed via HVF 30-2 and/or 24-2 testing protocols was −9.0 ± 8.9 (range: −1.8 to −27) dB in the 14 study eyes. At initial diagnosis, 4 (28%) of 14 eyes had no visual field defect, 4 (28%) had early visual field defects, and 6 (43%) had severe visual field defects. Patients had a mean follow-up of 21.5 ± 9.0 years and experienced an average reduction of IOP by 28%. Four of 12 eyes (33%) had stable visual fields throughout follow-up, while 8 eyes (67%) had slow to moderate progression. The 30-2 and/or 24-2 HVF tests had an average change in MD of −0.53 ± 0.26 dB/year. No eyes had rapid progression with a MD > 1.0 dB/year. At final follow up, the mean IOP was 11.5 ± 2.9, and C/D ratio was 0.94 ± 0.4. At final follow up, 3 (21%) of 14 eyes had early visual field defects, 4 (29%) of 14 eyes had moderate visual field defects, and 7 (50%) had severe visual field defects. Six (43%) of 14 eyes met criteria for legal blindness.
CONCLUSIONS:
We provide the first report of the clinical features and long-term clinical course in a family of NTG patients with TBK1 gene duplications. TBK1-associated glaucoma exhibits classic features of NTG. Patients present with severe disease at a relatively early age and most (67%) have slow to moderate progression of their visual field fields. The rate of visual field change appears correlated with the magnitude of IOP, suggesting that it may be advantageous to set extremely low IOP targets for some patients with TBK1-associated glaucoma.
Glaucoma is a chronic disease that lasts a lifetime. It is the leading cause of irreversible blindness in the world.1 Primary open-angle glaucoma (POAG) is the most common subtype of glaucoma and is characterized by optic nerve head (ONH) cupping with corresponding visual field loss. Known risk factors of POAG include advanced age, a positive family history, African or Hispanic ancestry, thin central corneal thickness (CCT), and elevated intraocular pressure (IOP).2 Although POAG may occur at any IOP, glaucoma that occurs at lower IOP (i.e. ≤ 21 mm Hg) may be termed normal tension glaucoma (NTG).3
Most cases of open angle glaucoma are caused by a complex interaction of many environmental and genetic risk factors.4, 5 However, to date, we have found that approximately 5% of OAG cases appear to be caused primarily by mutations in single genes.6-8 Myocilin (MYOC) mutations have been shown to cause a large proportion of juvenile-onset primary open angle glaucoma (JOAG) and 3-4% of POAG cases worldwide. Mutations in three glaucoma-causing genes, optineurin (OPTN), TANK binding kinase 1 (TBK1), and MYOC have each been associated with approximately 1% of NTG cases.7-12
TBK1 encodes a serine-kinase that phosphorylates and activates many substrates that are involved in NF-kB signaling and autophagy. One target of TBK1 kinase activity is the autophagy receptor protein OPTN.13 Autophagy is a catabolic process that cells employ to survive nutritional stress and/or to degrade defective proteins, organelles, and intracellular pathogens.14 The recognition that two NTG-causing genes, TBK1 and OPTN, directly interact within the same biological pathway suggests that dysregulation of autophagy may have a role in retinal ganglion cell death and glaucoma pathogenesis in patients with TBK1 or OPTN mutations.
In four independent reports,8-11 TBK1 gene duplications or triplications were detected in 8 (0.72%) of 1172 unrelated NTG patients and in 0 of 1320 controls (p = 0.0018).15 These reports include descriptions of mean maximum IOP, age at diagnosis, and optic disc photos. However, the long-term clinical course of patients with TBK1-related NTG (or any other molecularly defined type of glaucoma) has not yet been described. In the current report, we delineate the clinical features of seven TBK1 glaucoma patients (14 eyes) who are all part of a large previously described pedigree.8 We describe features of their glaucoma at the time of diagnosis as well as their long-term follow-up (up to 31 years of examinations).
MATERIALS AND METHODS
Study Design.
In this retrospective observational case series, we examined the presenting features and clinical course of glaucoma in members of pedigree with a TBK1 gene duplication.
Clinical Analysis of patients.
All study participants provided written informed consent and this research was conducted with the approval of the University of Iowa’s Institutional Review Board (IRB). The study followed the tenets of the Treaty of Helsinki.
Complete ophthalmological examinations including Goldmann applanation tonometry, slit lamp examination, gonioscopy, ophthalmoscopy, and all testing were performed by one of the authors (ALR). Visual acuity (VA) was measured using the Snellen visual acuity eye chart and converted to LogMAR. IOP was measured with Goldmann applanation. Visual fields were assessed with a Humphrey Field Analyzer (Zeiss Meditec, Dublin, CA). A total of 299 fields were available for analysis including 26 (8.7%) full threshold, 255 (85%) SITA-Standard, 10 (3.3%) SITA-FAST, and 8 (2.7%) FAST-PAC. We identified 243 visual fields that were obtained using either Full-Threshold or SITA-Standard protocols that met reliability standard based on the Normal Tension Glaucoma Study criteria.16 SITA-Standard and full threshold tests were included in our analysis given data suggesting acceptable comparability.17, 18 Of the 243 visual field tests included in this study, 114 (46)% were performed in a 10-2 test pattern, 105 (42%) were performed in a 24-2 test pattern, and 24 (12%) were performed in a 30-2 test pattern. Of the 243 visual field tests, 216 (89%) were performed in SITA-Standard and 27 (11%) were performed in full threshold prior to the commercial availability of the SITA protocols. Visual field tests were graded as mild, moderate, or severe using the Hodapp-Parrish-Anderson classification system.19 Retinal nerve fiber layer was measured using spectral domain OCT (Cirrus HD-OCT, Zeiss). Patients with glaucomatous cupping of the optic disc and corresponding visual field defects were diagnosed with NTG as described in a previous report of this pedigree8 and using standard criteria.20 Legal blindness is defined as the best corrected visual acuity being ≤ 20/200 or a visual field defect such that the widest diameter of the visual field is no greater than 20 degrees in the better seeing eye with a Goldmann III4e target or equivalent Humphrey Visual field analyzer stimulus size III, threshold equal or worse than 10db defect.
Genetic analysis.
DNA from each family member was tested for glaucoma-causing mutations. The proband of the pedigree, III-1, tested negative for the Glu50Lys mutation in OPTN and negative for a MYOC mutation using Sanger sequencing assays as previously described,8 but tested positive for a TBK1 gene duplication using a quantitative PCR assay and comparative genome hybridization as part of a previous report.8 The other six family members with NTG in this study also tested positive for the same TBK1 gene duplication.
RESULTS
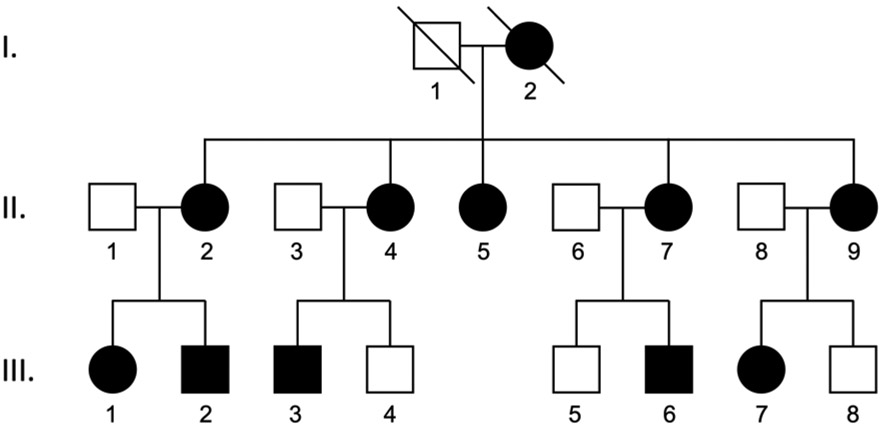
We previously reported a large African American pedigree with 10 family members that were both diagnosed with NTG and had a TANK binding kinase 1 (TBK1) gene duplication as shown in Figure 1.8 Long-term follow up data from March 1985 through May 2018 were available from 7 of these family members (Figure 1: II-2, II-5, II-9, III-1, III-2, III-6, and III-7). Five (71%) of the 7 patients were female. The mean length of follow up was 21.1 ± 10 years with a range of 5 to 31 years. The mean number of clinic visits was 59 ± 44 with a range of 3 to 137 visits per patient. We evaluated ophthalmic features of glaucoma (Table 1) and corresponding visual fields (Table 2) at the first and last available examination. For 3 of 7 patients, we were not able to obtain visual field tests at the time of glaucoma diagnosis as the patients were referred for the question of the etiology of the nerve and visual field changes. Visual fields were assessed with the 24-2 or 30-2 testing protocols initially, however, 3 of 7 family members were later tested using the 10-2 test protocol. The earliest and latest optic disc photos and Humphrey visual field tests available are shown for each eye in Figure 2.
Figure 1. A three generation African American pedigree with NTG caused by a TBK1 gene duplication.
Family members diagnosed with NTG are indicated by shaded symbols. All ten living family members with NTG carry a TBK1 gene duplication. This family has been described in a previous report.8
Table 1. Clinical Features of patients with a TBK1 gene duplication at initial examination and at last available examination.
Age, visual acuity, IOP, CCT, cup-todisc ratio, and average retinal nerve fiber layer thickness from OCT was measured in 14 eyes of 7 patients from a single African American pedigree with NTG caused by a TBK1 gene duplication. Patients are indicated by their pedigree symbol identification number from Figure 1. Abbreviations: OD, right eye; OS, left eye; NA, not available.
| II-2 | II-5 | II-9 | III-1 | III-2 | III-6 | III-7 | Mean (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family member (Pedigree symbol) |
Initial Exam |
Final Exam |
Initial Exam |
Final Exam |
Initial Exam |
Final Exam |
Initial Exam |
Final Exam |
Initial Exam |
Final Exam |
Initial Exam |
Final Exam |
Initial Exam |
Final Exam |
Mean Initial Exam (SD) |
Mean Final Exam (SD) |
| Age (years) | 46 | 77 | 43 | 73 | 35 | 60 | 31 | 57 | 34 | 48 | 36 | 41 | 22 | 39 | 35 (8) | 56 (15) |
| VA (LogMAR) | 0.0 OD 0.0 OS |
0.3 OD 0.6 OS |
0.0 OD 0.0 OS |
0.3 OD 0.4 OS |
0.0 OD 0.0 OS |
0.1 OD 0.1 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 OD 0.0 OS |
0.0 (0.0) | 0.13 (0.2) |
| IOP (mm Hg) | 14.0 OD 14.0 OS |
6.0 OD 7.0 OS |
18.0 OD 17.0 OS |
16.0 OD 9.0 OS |
17.0 OD 16.0 OS |
12.0 OD 13.0 OS |
15.0 OS 14.5 OA |
10 OD 10 OS |
20.0 OD 20.0 OS |
14.0 OD 14.0 OS |
15.0 OD 15.0 OS |
14.0 OD 14.0 OS |
14.0 OD 14.0 OS |
11.0 OS 11.0 OD |
16.0 (2.1) | 11.5 (2.9) |
| CCT (microns)* | 532 OD 541 OS |
NA | 533 OD 526 OS |
NA | 487 OD 504 OS |
NA | 496 OD 497 OS |
NA | 490 OD 486 OS |
NA | 524 OD 533 OS |
NA | 474 OD 463 OS |
NA | 506 (25) | NA |
| C/D ratio | 0.99 OD 0.99 OS |
0.99 OD 0.99 OS |
0.99 OD 0.99 OS |
0.99 OD 0.99 OS |
0.80 OD 0.80 OS |
0.90 OD 0.90 OS |
0.90 OD 0.90 OS |
0.99 OD 0.99 OS |
0.80 OD 0.90 OS |
0.90 OD 0.95 OS |
0.90 OD 0.90 OS |
0.90 OD 0.90 OS |
0.80 OD 0.80 OS |
0.90 OD 0.99 OS |
0.90 (0.1) | 0.94 (0.04) |
| OCT nerve fiber layer (microns) | NA | 58.0 OD 49.0 OS |
NA | 61.0 OD 61.0 OS |
NA | 62.0 OD 90.0 OS |
NA | 54.0 OD 62.0 OS |
NA | 86.0 OD 71.0 OS |
NA | 88.0 OD 80.0 OS |
NA | 58.0 OD 56.0 OS |
NA | 66.6 (13.6) |
A single measurement of corneal thickness was made at some point during follow-up and is listed under initial exam column.
Table 2. Humphrey visual field (HVF) test parameters of patients with a TBK1 gene duplication at initial and last available examination.
The first available Humphrey visual field (HVF), last available HVF, change in HVF, and rate of change in HVF was recorded in 14 eyes of 7 patients from a single African American pedigree with NTG caused by a TBK1 gene duplication. Rate of change was calculated by compared first and last available HVF tests and by plotting the best linear fit to all of the available HVF data. The rate of change in MD was categorized as “slow” if the rate was less than −0.5 dB/year and “moderate” if the rate was between −0.5 dB/year and −1.0 dB/year. The rate of change in MD was categorized as no change or “NC” if the rate was not statistically different from 0 dB/year. Abbreviations: OD, right eye; OS, left eye; dB, decibels; Mod, moderate; NA, not available due to limited data; NC, no significant change.
| Family Member (Pedigree Symbol) |
II-2 | II-5 | II-9 | III-1 | III-2 | III-6 | III-7 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HVF Protocol | 24-2 (dB) | 10-2 (dB) | 30-2 or 24-2 (dB) |
10-2 (dB) | 24-2 (dB) | 30-2 or 24-2 (dB) |
10-2 (dB) | 24-2 (dB) | 10-2 (dB) |
24-2 (dB) | 24-2 (dB) | ||||||||||
| Eye | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OS | OD | OS | OD | OS |
| First Available HVF | −24.3 | −27.0 | −20.5 | −21.9 | −18.7 | −17.3 | −13.1 | −23.6 | −2.7 | −2.96 | −9.6 | −8.4 | −11.7 | −5.2 | −1.8 | −3.0 | −3.9 | −1.8 | −3.5 | −2.9 | −2.9 |
| Last Available HVF | −25.1 | −28.7 | −28.2 | −27.1 | −23.1 | −28.3 | −23.8 | −32.0 | −3.6 | −3.0 | −29.0 | −27.5 | −25.6 | −15.1 | −2.1 | −8.53 | −4.6 | −1.2 | −2.6 | −2.1 | −2.7 |
| Change in HVF | −0.8 | −1.7 | −7.7 | −5.2 | −4.4 | 11.0 | −10.7 | −8.4 | −0.9 | 0.04 | −19.4 | −19.1 | −13.9 | −9.9 | −0.3 | −5.53 | −0.7 | 0.6 | 0.9 | 0.8 | 0.2 |
| Years of Follow-up | 2 | 2 | 19 | 19 | 15 | 15 | 20 | 20 | 20 | 20 | 24 | 24 | 16 | 16 | 14 | 14 | 2 | 5 | 5 | 15 | 15 |
| Rate of change in HVF (dB/year) | |||||||||||||||||||||
| First and last test only | −0.40 | −0.85 | −0.41 | −0.27 | −0.29 | −0.73 | −0.54 | −0.42 | −0.01 | 0.00 | −0.81 | 0.80 | −0.87 | −0.68 | −0.02 | −0.40 | −0.35 | 0.12 | 0.18 | 0.1 | 0.01 |
| Best linear fit (all data) | NA | NA | −0.30 | −0.22 | −0.44 | −0.77 | −0.57 | −0.48 | 0.00 | −0.03 | −0.80 | 0.84 | −0.74 | −0.52 | −0.23 | −0.51 | NA | −0.04 | 0.06 | 0.02 | −0.04 |
| Rate of Change Category | NA | NA | Slow | Slow | Slow | Mod | Mod | Slow | NC | NC | Mod | Mod | Mod | Mod | Slow | Slow | NA | NA | NA | NC | NC |
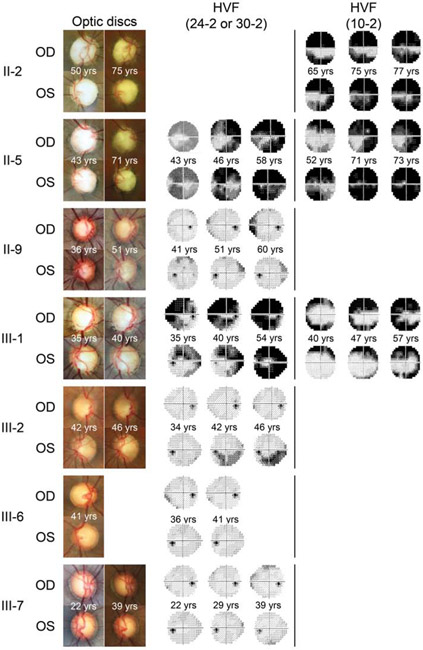
Figure 2. First and last available disc photos and Humphrey visual field tests.
Long term clinical follow-up data were available from seven family members with NTG caused by a TBK1 gene duplication. First and last available disc photos demonstrate progressive optic disc cupping. First and last available Humphrey visual field tests (30-2, 24-2, and/or 10-2 protocols) demonstrate progressive glaucomatous visual field loss. Visual field tests that were obtained at the same time as optic disc photos are also presented. Patients are indicated by their pedigree symbol identification number from Figure 1. Only one set of disc photos was available from patient III-6.
Ophthalmic features at diagnosis / initial exam.
Ocular examination.
The mean age at diagnosis for these 7 patients was 35 ± 8 years with a range of 22 to 46 years. All eyes presented with a Snellen visual acuity of 20/20 (LogMAR = 0). All eyes had an IOP less than or equal to 21 mm Hg at presentation and throughout follow-up with a mean initial IOP of 16.0 ± 2.1 mm Hg with a range of 14 to 20 mm Hg. Central corneal thickness (CCT) was thin in some patients and average in others with a mean thickness of 506.1 ± 25.1 microns and range of 463 to 541 microns, which is thinner than the reported mean CCT for African Americans of ~530 microns.21,22 Gonioscopy revealed wide-open angles without visible pathology in all cases. At initial examination, glaucomatous optic nerve damage was detected in all cases with ophthalmoscopy and optic disc photos. All 14 eyes had extensive cupping with a mean cup-to-disc (C/D) ratio of 0.90 ± 0.08 and range of 0.80 to 0.99 as determined by physician assessment. At initial examination, 1 eye (7%) met criteria for legal blindness.
Visual field and optic nerve image analyses.
Humphrey Visual Field Analyzer perimetry data (24-2 and 30-2 protocol) were available from 10 (71%) of the 14 eyes at or within one year of initial examination and from 4 (29%) of the 14 eyes within 5 to 8 years of initial examination. Reduced global sensitivity was detected with a mean MD of −9.0 ± 8.9 (range: −1.8 to −27) dB. Using the Hodapp-Parrish-Anderson classification system,19 4 (29%) of 14 eyes had no glaucomatous visual field defects, 4 (29%) of 14 eyes had early visual field defects, and 6 (43%) of 14 eyes had severe defects. Visual field defects typical for glaucoma, i.e. paracentral scotomas, arcuate defects and nasal steps,23 were detected in patients with TBK1-associated glaucoma (Figure 2). Analysis of the 4 eyes with early defects on presentation indicates that 2 eyes had arcuate defects and 2 eyes had nasal step defects. OCT analyses were not available at initial examination.
Clinical course during long-term follow up examinations.
The mean IOP of the seven members of this pedigree with glaucoma caused by a TBK1 gene duplication was lowered by topical medications, laser trabeculoplasty, and/or trabeculectomy from 16.0 ± 2.1 mm Hg to 11.5 ± 2.9 mm Hg, for a mean reduction of 4.5 ± 2.4 mm Hg, a decrease in IOP of 28% ± 15%. Eleven (79%) of 14 eyes were managed only with topical anti-hypertensives and/or argon laser trabeculoplasty, while 3 (21%) of 14 eyes underwent glaucoma surgery (trabeculectomy) at some point during follow up. Several patients also underwent cataract extraction when indicated to restore vision. No optic disc hemorrhages were detected during any of the optic disc examinations or identified retrospectively in disc photos from any patients.
Visual Field analysis.
We analyzed visual fields and determined the rate of change in MD for each glaucoma patient as a measure of overall change in severity of visual field damage (Table 2).
30-2 and 24-2 Humphrey visual field tests.
Of the 14 eyes in this study, all had 30-2 and or 24-2 HVF tests. These eyes had a total of 129 Humphrey 30-2 or 24-2 visual field tests with a mean number of 9.2 ± 5.1 visual field tests per eye. Ten (71%) of 14 eyes had more than five 30-2 and or 24-2 visual field tests that were suitable for assessing progression rate. The rate of change in MD for these ten eyes ranged from −0.84 to 0.017 dB/year and the average rate of change was −0.38 ± 0.36 dB/year. Four eyes had no statistically significant change in MD during follow up. The average rate of change in MD increased to −0.62 ± 0.23 dB/year if these four stable eyes were excluded. For each of these ten eyes, we also compared the mean of IOP measurements during follow-up with the rate of change in MD and found a correlation of r = 0.71 and r2 = 0.51 (p = 0.019). IOP and rate of change of MD in Humphrey 24-2 visual fields in this family were moderately correlated.
10-2 Humphrey visual field tests.
Of the 14 eyes in this study, 7 had 10-2 HVF tests. These eyes had a total of 114 Humphrey 10-2 visual field tests with a mean number of 14.3 ± 9.5 visual field tests per eye. Six (42%) of the 14 eyes had more than five 10-2 visual field tests that were suitable for assessing progression rate. The rate of change in MD for these six eyes ranged from −0.21 dB/year to −0.57 dB/year with a mean rate of change of −0.47 ± 0.19 dB/year. The rate of change in MD of 10-2 visual field tests was highly correlated to mean IOP with r = 0.81 and r2 = 0.66 (p = 0.05).
None of the family members eyes had progression > 1 dB/year with Humphrey visual field tests (24-2 or 10-2), which is a frequently used threshold for rapid progression.24
We placed the 12 eyes with more than 5 visual field tests into three groups: 1) stable MD, 2) slowly progressive MD (< 0.5 dB/year), and 3) moderately progressive MD (0.5 – 1.0 dB/year).
Group 1. Stable MD.
Four (33%) of 12 eyes (II-9 OU and III-7 OU) had visual fields with stable MD throughout follow-up. For each of these eyes, the rate of change in MD was not statistically different from zero dB/year (no change) and ranged from −0.04 to 0.017 dB/year (mean = −0.02 ± 0.03 dB/year). Two (50%) of the 4 eyes (II-9 OD and III-7 OS) had no glaucomatous visual field loss at initial examination but both developed moderate visual field defects at final examination. Two (50%) of the 4 eyes (II-9 OS and III-7 OD) had an early defect on visual field testing at initial examination and one developed a moderate visual field defect at final examination. Thus, all four eyes with stable MD, did in fact have progression of glaucomatous changes in their visual fields when measured with other criteria (i.e. Hodapp-Parrish-Anderson classification system). The Collaborative Normal Tension Glaucoma Study set a target for a 30% reduction in IOP for their normal tension glaucoma study patients.25 The mean baseline IOP for these four patients was 15.5 ± 1.5 mm Hg (range 14 to 17 mm Hg) and 30% reduction from baseline IOP was reached at 38% of follow up visits. The mean IOP of these eyes during follow up was 11.4 mm Hg (range 5-16) with a mean decrease in IOP by 22.7% ± 4.6%.
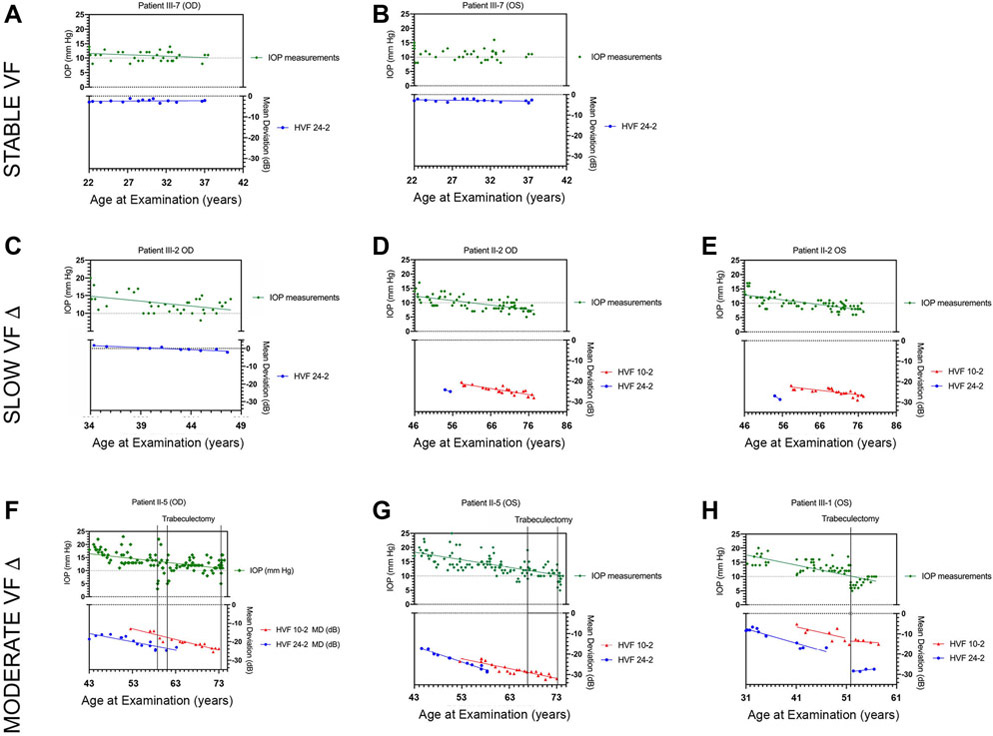
The MD of visual field tests and IOP measurements of Patient III-7 right and left eye are depicted in Figure 3A and 3B as an example of the stable visual fields in this patient group. Additional diagrams for the rest of study patients are shown in Supplementary Figure 1.
Figure 3. Longitudinal measures of IOP and Humphrey visual field tests.
Panels A-H show longitudinal plots of IOP and mean deviation (MD) from HVF tests for 8 eyes of patients with NTG caused by a TBK1 gene duplication. Linear fits Panel A and B show plots for Patient III-7 OD and OS that have stable HVF tests throughout follow-up. Panel C – E show plots for Patient III-2 OD and Patient II-2 OD and OS that have HVF tests with slow progression with a change in MD < 0.5 dB / year. Panel F – H show plots for Patient III-5 OD and OS and Patient III-1 OS that have HVF tests with moderate progression with a change in MD of 0.5 – 1.0 dB / year. The time at which a trabeculectomy surgery was performed is indicated by a vertical line in these patients.
Group 2. Slow progression of MD.
Three (25%) of 12 eyes (II-2 OU and III-2 OD) had worsening Humphrey visual fields (30-2, 24-2 and/or 10-2) with relatively slow rates of change in MD (< 0.5 dB / year). On initial exam, one of these eyes (III-2 OD) had an essentially normal 24-2 visual field that developed damage at a rate of −0.23 dB / year with IOPs that were mostly between 10 – 15 mm Hg (Figure 3C). Two other eyes (II-2 OD and II-2 OS) had severe 10-2 visual field defects on first available visual field with MDs of −20.53 dB and 21.90 dB that worsened at a rate of −0.29 dB/year and −0.21 dB/year respectively. Notably, these two eyes consistently worsened despite successful lowering of IOP to levels frequently < 10 mm Hg (Figure 3D and 3E). The three eyes in this group had a mean baseline IOP of 16.0 ± 3.5 mm Hg (range 14 to 20 mm Hg) and a 30% reduction of IOP from baseline was reached at 62% of follow up visits, while the mean IOP of these eyes during follow-up was 10.8 mm Hg with a mean decrease in IOP by 45% ± 14.1%.
Group 3. Moderate progression of MD.
Five (42%) of 12 eyes (III-1 OU, III-2 OS, and II-5 OU) had more rapidly worsening visual fields (0.5 to 1.0 dB/year) with either 10-2, 24-2, and/or 30-2 protocols. These five eyes had 30-2 or 24-2 visual field defects on initial examination that ranged from mild (MD = − 3.0 dB) to severe defects (MD = −18.65 dB). Humphrey visual field 30-2 and 24-2 tests in these five eyes worsened at an average rate of −0.70 ± 0.15 dB/year. Additionally, 10-2 visual field tests were obtained from 4 of these eyes and these visual field tests worsened at a rate of −0.75 ± 0.11 dB/year. The mean baseline IOP for these five eyes was 17.0 ± 2.6 mm Hg (range 14.5 to 20 mm Hg) and a 30% reduction from baseline IOP was reached at 39% of follow-up visits, while the mean IOP of these eyes during follow-up was 12.8 mm Hg (range 9 to 20 mm Hg) with a mean decrease in IOP by 30.5% ± 12.8%. Three (60%) of these 5 more rapidly progressing eyes (III-1 OS, II-5 OU) were treated surgically with trabeculectomy with a mean of two trabeculectomies per eye (range 1 to 3).
We compared the mean IOPs of eyes with stable MD (n = 4), slowly progressive MD (n = 3), and moderately progressive MD (n = 5). There was no significant difference in mean IOP between these groups (p = .0386). There was only a slight difference in mean age between these groups (p = 0.609) with the group that did not have progression of visual field loss being slightly younger.
The eyes that were treated with trabeculectomy (III-1 OS, II-5 OU) had a mean baseline IOP of 16.5 ± 1.8 mm Hg and severe visual field defects at the time of diagnosis. These eyes had a statistically significant change in HVF 24-2 MD throughout follow up with a mean change in MD of −0.73 ± 0.14 dB/year and a statistically significant change in HVF 10-2 MD throughout follow up with a mean change in MD of −0.53 ± 0.26 dB/year. One family member (patient II-5) had 3 trabeculectomies in the right eye and 2 trabeculectomies in the left eye. During the course of follow-up this patient had a mean IOP of 13±1.8 mm Hg in the right eye and a mean IOP of 13.5 ± 3.6 mm Hg in the left eye with a peak IOP of up to 23 mm Hg in both eyes. Despite achieving IOP lowering, her visual fields continued to worsen (Figure 3F and 3G). Another family member (patient III-1) underwent one trabeculectomy in the left eye. In this patient, IOP was ultimately maintained below 10 mm Hg and there was stabilization her visual field (Figure 3H).
Ophthalmic features at last examination.
Ocular examination.
The mean age at final follow up was 56 ± 15 years with a range of 39 to 77 years and the mean visual acuity at final exam was LogMar of 0.13 ± 0.2, equivalent to 20/25 Snellen. A decline in visual acuity was detected in 6 (42%) of 14 eyes. Reduced visual acuity was likely attributable to glaucomatous visual field damage within 5 degrees of fixation in 4 (67%) of 6 eyes and likely attributable to cataract in 2 (33%) of 6 eyes. The mean IOP at final examination was 11.5 ± 2.9 mm Hg (range of 6 to 16 mm Hg), which was a 28 ± 15.5% mean decrease in IOP compared with first examination or a 4.5 ± 2.4 mm Hg mean decrease in IOP. Progressive glaucomatous optic nerve damage was detected in 8 (57%) of 14 eyes with ophthalmoscopy and evaluation of optic disc photos. Of the 6 eyes without progressive glaucomatous optic nerve damage, 4 (67%) had initial C/D ratios of 0.99. At final examination, the mean cup-to-disc ratio was 0.94 ± 0.4 with a range of 0.90 to 0.99.
Visual field and optic nerve image analyses.
At final examination 3 (21%) of 14 eyes had early defects, 4 (29%) of 14 eyes had moderate defects, and 7 (50%) of 14 eyes had severe defects using the Hodapp-Parrish-Anderson classification system. Half of the eyes with early visual field defects at last examination had only 5 years or less follow-up, which is much shorter than the average follow-up in this study. At final examination, 6 (43%) of 14 eyes meet criteria for legal blindness based on visual field testing.
All eyes had thin retinal nerve fiber layers (RNFL) when assessed using spectral domain optical coherence tomography (Cirrus HD-OCT) at last examination. Unfortunately, longitudinal OCT measures were not available for analysis due to the date of introduction of this testing modality. The average RNFL thickness was available for 14 (100%) of 14 eyes at last examination and ranged from 49 to 90 microns with a mean of 66.0 ± 13.6 microns. In most cases, inferior thinning of the RNFL was greater than superior thinning of the RNFL.
Other systemic features of patients.
Systemic hypertension treated with antihypertensive medications was present in two (29%) of seven patients; however, no patient had a known history of associated nocturnal hypotension. No patients had a known history of vasospastic disease such as migraine headache or Raynaud’s phenomenon, autoimmune disease, or obstructive sleep apnea (OSA). A history of iron deficiency anemia associated with heavy menstruation and/or the need for one or more blood transfusions during childbirth was also present in three (60%) of five female patients.
DISCUSSION
Approximately 1% of NTG cases may be due to TBK1 mutations.15 Duplications and triplications of TBK1 have been associated with glaucoma that occurs at low IOP in several reports.8-11, 26 The current report, however, is the first to describe the clinical features of TBK1-associated NTG at the time of diagnosis as well as during long-term (up to 31 years) follow-up. Patients were young (ranging 22 to 46 years old) and had low, untreated IOPs (ranging from 14 to 20 mm Hg) at the time of first examination. All of these patients had extensive optic nerve damage with cup-to-disc ratios ranging from 0.8 to 0.9 and almost half (43%) had severe visual field damage at first exam using the Hodapp-Parrish-Anderson classification.
TBK1 duplications and triplications have been reported in 10 pedigrees that include 27 patients with glaucoma.8-11, 26 These patients were diagnosed with NTG by glaucoma specialists prior to the identification of their TBK1 mutations. However, the longitudinal features of TBK1-associated glaucoma have not yet been delineated. Our research finds that NTG patients with a TBK1 mutation have classic features of NTG: progressive cupping and progressive visual field damage that occur at low IOP. We chose to primarily analyze progression of visual field defects by following change in MD. Although MD does not take the pattern of visual field loss into account, its global assessment facilitates longitudinal comparisons and simple calculation of overall rates of change.24, 27, 28
Analysis of hundreds of visual field tests from our NTG pedigree showed that 33% of patients had stable visual fields and that 67% had progressive worsening during their follow-up examinations (mean of 21.5 years). The rate at which 24-2 visual fields worsened in these patients (average change in MD = −0.57 ± 0.31 dB/year) was similar to the rate of worsening exhibited by patients in the Collaborative Normal Tension Glaucoma Study (average MD = −0.40 to −0.50 dB/year)3 and no family members had rapidly progressing visual fields (change in MD > 1.0 dB/year). The rate of visual field loss was strongly correlated with the magnitude of IOP (r = 0.66 for 24-2 and r = 0.90 for 10-2), which provides evidence that IOP influences the rate of worsening of visual fields in TBK1-related glaucoma. Moreover, the rate of worsening was slowed by IOP reduction in at least one patient. This patient, III-1, had progressive visual field loss in the left eye until her IOP became < 10 mm Hg following a trabeculectomy. With lower IOP, the visual field stabilized (Figure 3G). These data indicate that patients with TBK1-related glaucoma may have similar responses to lowering IOP as other open angle glaucoma patients.24, 29, 30
Although disc hemorrhages have been more frequently detected in patients with NTG than in other forms of glaucoma,31, 32 none were reported nor did we observe any disc hemorrhages in these TBK1-associated NTG patients despite extensive review of all clinical notes and optic disc photographs. A disc hemorrhage was documented, however, in a recent report of another NTG pedigree with glaucoma due to a TBK1 gene duplication.33 Although the follow-up period for this pedigree was long, the sample size may have been too small to detect optic disc hemorrhages. However, these data and observations suggest that disc hemorrhages in this specific pedigree may occur with TBK1-associated NTG, at reduced frequency. Also, a reduced rate of disc hemorrhages has been reported in African American glaucoma patients when compared with Caucasian patients.34, 35 Thus, the African ancestry of our TBK1 glaucoma pedigree may have contributed to the absence of disc hemorrhages in our study. TBK1 duplications and triplications have been also been detected in Caucasian and Asian NTG patients8-11, 26 and rate of optic disc hemorrhages in these patients has not yet been investigated.
Several types of data demonstrate that TBK1 mutations are the primary cause of disease in a subset of NTG cases. The pathogenicity of TBK1 gene duplications has been confirmed by statistical analyses15 and by recapitulation of disease in transgenic animals with analogous TBK1 mutations.36 TBK1-associated glaucoma also has autosomal dominant inheritance, which indicates a single-gene cause of disease.8 Nonetheless, additional coexisting genetic and environmental risk factors may have contributed to disease in members of the pedigree described in this report. First, members of this pedigree have African ancestry, which may increase overall risk for glaucoma and severity of disease. Second, the majority of patients in this study had a thin or very thin central corneal thickness (CCT), with only one patient having an average CCT when compared with African American normative data. Third, three patients had significant iron deficiency anemia related to either heavy menses or postpartum hemorrhage that required blood transfusions. Unfortunately, the amount of blood loss, hemoglobin level, or these patients’ resulting blood pressure was not documented. Also, several patients in this NTG pedigree were taking oral medication for systemic hypertension. None of these patients were known to have nocturnal hypotension as a result of these medications. It is possible that reduced ocular perfusion due to these hemodynamic influences may have contributed to glaucoma in these patients with TBK1 mutations.
Our study had additional limitations. Although our NTG pedigree is one of the largest known families with glaucoma caused by a TBK1 gene duplication, longitudinal clinical data was available from only seven family members. This relatively small sample size from a single pedigree may have biased our results. However, TBK1 mutations were only recently discovered and individuals with this abnormality are just beginning to be recognized. Additionally, this family’s clinical course may not be representative for patients with TBK1-associated glaucoma who are of different racial/ethnic ancestry. The vast majority of the visual field tests in our study were obtained using the SITA-Standard 24-2 protocol. However, we also included 24-2 full threshold, 30-2 SITA-standard, and 30-2 full threshold visual field tests in our analysis. Data from each of these visual field test protocols are not completely interchangeable due to differences in algorithms used to find threshold sensitivities as well as differences in patient fatigue and intertest variability related to differences in test times.37 However, prior analyses have demonstrated that the test results from 24-2 and 30-2 protocols have minimal differences.18 Moreover, global indices, like MD, are determined by comparison with normative databases that are specific to each testing strategy (i.e. SITA-Standard and full threshold). Consequently MD calculated from different testing strategies are very similar with little to no statistical difference38 and we focused our analyses on this robust metric.
This study demonstrates that one must be more cautious and observant in younger patients with glaucoma. Although the mean MD loss per year was not that great, when following a relatively young patient for decades, this small loss/year becomes clinically significant.
In conclusion, this is the first paper to report the natural history of NTG caused by TBK1 gene duplication. These data demonstrated that TBK1 mutations are associated with progressive optic nerve and visual field damage that are typical for NTG. Standard therapies achieved an average IOP reduction of 28% during the course of follow-up and overall family members experienced slow to moderate progressive worsening of Humphrey 24-2 visual fields (rate of MD change was −0.35 dB / year). Although 25% of family members had stable MD throughout follow-up, even these individuals experienced some objective worsening of visual fields with Hodapp-Parrish-Anderson classification. None of the family members had rapidly worsening visual fields (rate of MD change > dB / year), however, substantial visual disability commonly occurred in members of this family, perhaps due in part to the early-onset of disease. Finally, the rate of visual field worsening in these patients was correlated with IOP and in at least one patient reduction of IOP to < 10 mm Hg served to stabilize disease, which indicates that lowering IOP may slow progression of TBK1-associated glaucoma. Such mutation-specific data may provide physicians with better information to counsel patients with TBK1-associated glaucoma about the likely course of their disease and may also assist with setting treatment goals (i.e. target IOP). Finally, documenting the natural history of NTG caused by a TBK1 mutation is important to clinicians to help them counsel and treat their patients and will also be necessary for designing future gene-targeted glaucoma therapies and evaluating their effectiveness. Similar studies in other forms of NTG and POAG will better help clinicians manage their patients.
Supplementary Material
Features of disease were assessed in 7 members of a pedigree with glaucoma caused by a TBK1 gene duplication, a mutation that is responsible for ~1% of normal tension glaucoma cases. Patients had early onset of disease and slow to moderate progression of visual field loss. The rate of visual field change was correlated with IOP and especially low target pressures were beneficial for some of these patients.
ACKNOWLEGEMENTS
A. FUNDING / SUPPORT
This research was funded in part by the National Eye Institute (NIH R01EY023512), Research to Prevent Blindness, and the Hadley-Carver Chair in Glaucoma.
B. FINANCIAL DISCLOSURES
Dr. Tyler S. Quist has no financial Disclosures. Dr. Chris A. Johnson is a consultant for Haag-Streit, Centervue, and M & S Technologies. Dr. Alan L. Robin is a consultant for Versant Health, has received financial support from the National Institutes of Health and Glaucoma Research Foundation, has received honoraria from Aerie Pharmaceuticals, Inc., is an advisory board member for Aravind Eye Foundation, and is the Executive Vice President of the American Glaucoma Society. Dr. John H. Fingert received research support from the National Institutes of Health, The Glaucoma Foundation, and Regeneron Genetics Center and is a consultant for Perfuse Therapeutics.
C. OTHER ACKNOWLEDGEMENTS
The authors gratefully acknowledge the patients that participated in this research project and for expert technical assistance by Teresa Kopel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014;121(1):2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med 2009;360(11):1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126(4):487–497. [DOI] [PubMed] [Google Scholar]
- 4.Fingert JH. Primary open-angle glaucoma genes. Eye 2011;25(5):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet 2017;26(R1):R21–R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science 1997;275(5300):668–70. [DOI] [PubMed] [Google Scholar]
- 7.Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002;295(5557):1077–1079. [DOI] [PubMed] [Google Scholar]
- 8.Fingert JH, Robin AL, Ben RR, et al. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet 2011;20(12):2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawase K, Allingham RR, Meguro A, et al. Confirmation of TBK1 duplication in normal tension glaucoma. Exp Eye Res 2012;96(1):178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritch R, Darbro B, menon G, et al. TBK1 Gene Duplication and Normal-Tension Glaucoma. JAMA Ophthalmol 2014;132(5):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awadalla MS, Fingert JH, Roos BE, et al. Copy Number Variations of TBK1 in Australian Patients With Primary Open-Angle Glaucoma. Am J Ophthalmol 2015;159(1):124–130.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alward WLM, van der Heide CJ, Khanna CL, et al. Myocilin Mutations in Patients With Normal-Tension Glaucoma. JAMA Ophthalmol 2019;137(5):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wild P, Farhan H, McEwan DG, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011;333(6039):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 2013;368(19):1845–1846. [DOI] [PubMed] [Google Scholar]
- 15.Fingert JH, Robin AL, Scheetz TE, et al. Tank-Binding Kinase 1 (TBK1) Gene and Open-Angle Glaucomas. Trans Am Ophthalmol Soc 2016;114:t6[1–11]. [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DR, Study NTG. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol 2003;14(2):86–90. [DOI] [PubMed] [Google Scholar]
- 17.Artes PH, Iwase A, Ohno Y, Kitazawa Y, Chauhan BC. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Invest Ophthalmol Vis Sci 2002;43(8):2654–2659. [PubMed] [Google Scholar]
- 18.Heijl A, Bengtsson B, Chauhan BC, et al. A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology 2008;115(9):1557–65. [DOI] [PubMed] [Google Scholar]
- 19.Chang TC, Ramulu PY, Hodapp E. Diagnosing Glaucoma Clinical Decisions in Glaucoma. Chicago: Mosby - Year Book, Inc., 2016:59–71. [Google Scholar]
- 20.Alward WL, Fingert JH, Coote MA, et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med 1998;338(15):1022–1027. [DOI] [PubMed] [Google Scholar]
- 21.Girkin CA, Nievergelt CM, Kuo JZ, et al. Biogeographic Ancestry in the African Descent and Glaucoma Evaluation Study (ADAGES): Association With Corneal and Optic Nerve Structure. Invest Ophthalmol Vis Sci 2015;56(3):2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sng C, Barton K, Kim H, Yuan S, Budenz DL. Central Corneal Thickness and its Associations With Ocular and Systemic Factors in an Urban West African Population. Am J Ophthalmol 2016;169:268–275. [DOI] [PubMed] [Google Scholar]
- 23.Keltner JL, Johnson CA, Cello KE, et al. Classification of visual field abnormalities in the ocular hypertension treatment study. Arch Ophthalmol 2003;121(5):643–650. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan BC, Malik R, Shuba LM, Rafuse PE, Nicolela MT, Artes PH. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci 2014;55(7):4135–4143. [DOI] [PubMed] [Google Scholar]
- 25.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 1998;126(4):498–505. [DOI] [PubMed] [Google Scholar]
- 26.Kaurani L, Vishal M, Ray J, Sen A, Ray K, Mukhopadhyay A. TBK1 duplication is found in normal tension and not in high tension glaucoma patients of Indian origin. J Genet 2016;95(2):459–461. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol 2008;92(4):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardiner SK, Demirel S. Detecting Change Using Standard Global Perimetric Indices in Glaucoma. Am J Ophthalmol 2017;176(4):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120(10):1268–1279. [DOI] [PubMed] [Google Scholar]
- 30.Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol 2013;91(5):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitazawa Y, Shirato S, Yamamoto T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology 1986;93(6):853–857. [DOI] [PubMed] [Google Scholar]
- 32.Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol 2000;129(6):707–714. [DOI] [PubMed] [Google Scholar]
- 33.Sears NC, Darbro BW, Alward WLM, Fingert JH. Progressive optic disc cupping over 20 years in a patient with TBK1-associated glaucoma. Ophthalmol Glaucoma 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skaat A, De Moraes CG, Bowd C, et al. African Descent and Glaucoma Evaluation Study (ADAGES): Racial Differences in Optic Disc Hemorrhage and Beta-Zone Parapapillary Atrophy. Ophthalmology 2016;123(7):1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budenz DL, Huecker JB, Gedde SJ, Gordon M, Kass M, Group OHTS. Thirteen-Year Follow-up of Optic Disc Hemorrhages in the Ocular Hypertension Treatment Study. Am J Ophthalmol 2017;174(2):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fingert JH, Miller K, Hedberg-Buenz A, et al. Transgenic TBK1 mice have features of normal tension glaucoma. Hum Mol Genet 2017;26(1):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heijl A, Bengtsson B, Patella VM. Glaucoma follow-up when converting from long to short perimetric threshold tests. Arch Ophthalmol 2000;118(4):489–493. [DOI] [PubMed] [Google Scholar]
- 38.Bengtsson B, Heijl A. Comparing significance and magnitude of glaucomatous visual field defects using the SITA and Full Threshold strategies. Acta Ophthalmol Scand 1999;77(2):143–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.