Capsule summary:
After EoE treatment, association of platelets with blood eosinophils, as reported by CD41, predicted esophageal eosinophil count. Percentage CD41+ circulating eosinophils is a potential non-invasive biomarker for EoE disease activity.
Keywords: Eosinophilic esophagitis, αIIb-integrin, CD41, blood eosinophils, platelets, platelet-eosinophil association, peak eosinophil count, disease activity, biomarker, non-invasive
To the Editor:
Eosinophilic esophagitis (EoE), a chronic inflammatory disease characterized by symptoms of esophageal dysfunction and eosinophilic infiltration of the esophageal mucosa, has increased in incidence and prevalence the past two decades.1, 2 The standard for assessing disease activity is performing endoscopy and pathological examination of esophageal biopsies. There is a critical need for a biomarker to replace such invasive monitoring. A number of potential tests have been evaluated but none have been incorporated into guideline recommendations or routine clinical practice.3, 4
We previously demonstrated by flow cytometry that the activation epitope for monoclonal antibody (mAb) N29 on the β1 integrin subunit on circulating eosinophils correlates with and predicts decreased pulmonary function in patients with non-severe asthma.5 Further, we linked N29 epitope appearance to eosinophil engagement by P-selectin (CD62P) on activated platelets and showed that P-selectin-stimulated eosinophil α4β1 integrin activation causes enhanced adhesion to vascular cell adhesion molecule (VCAM)-1, which is induced on activated endothelium.6 We hypothesized that a similar pathway occurs in EoE and undertook the present study to determine if eosinophil surface biomarkers correlate with and predict eosinophil count in esophageal biopsies.
Patients were recruited from the University of Wisconsin (UW) Health Gastroenterology Clinic after failing to respond for two months to proton-pump inhibitor (PPI) therapy and following endoscopy diagnostic of EoE. Informed written consent was obtained at Visit 1 (V1) within two weeks of release of the endoscopy results, according to a protocol approved by the UW-Madison Health Sciences Institutional Review Board (see Online Repository text and Table EI). Patients received standard of care EoE treatment for eight weeks followed by V2 and repeat endoscopy. N29, CD62P, CD41 (αIIb integrin subunit, a reporter of platelet association), and 13 other surface markers (Table EII) were assayed by flow cytometry of eosinophils gated in whole blood. Peak eosinophil count (PEC) per high power field (HPF) was assessed on esophageal biopsy. Flow cytometry, clinical assessments, and statistical analysis are described in the Online Repository.
Twenty-five patients (Table I) completed V1 and V2. At V1, all patients had active disease, as specified by the enrollment criteria. Changes in PEC and disease scores from V1 to V2 are visualized in Fig E1. PEC (Table I) and scores decreased significantly, from means of 11.0 to 4.6 for EoEHSS (histology, P = 0.001), 31.6 to 19.0 for EEsAI (symptoms, P < 0.001), and 4.1 to 2.8 for EREFS (endoscopy, P < 0.001). At V2, eleven patients (44%) had PEC < 6/HPF and three others, for a total of 14 (56%) patients, had PEC < 15/HPF (Fig. E1, A). Correlations among the three scores and PEC at V1 or V2 are shown in Fig E2. The strongest correlation was between EoEHSS and PEC, whereas the other correlations were weaker.
TABLE I.
Patient characteristics
| Variable | Value |
|---|---|
| n | 25 |
| Age (years) (mean ± SD) | 38 ±14 |
| Sex (n [percentage] male) | 19 (76%) |
| Race/ethnicity (white non-Hispanic) (n [percentage]) | 24 (96%)* |
| Time since symptom onset (years) (median [25th, 75th percentiles]) | 5 (2, 10) |
| V1 blood eosinophils (per μl) (median [25th, 75th percentiles]) | 194 (70,299) |
| V2 blood eosinophils (per μl) (median [25th, 75th percentiles]) | 158 (114,273) |
| Allergic rhinitis diagnosis or symptoms (n [percentage]) | 20 (80%) |
| VI RCAT score (mean ± SD) (n = 21) | 24.1 ± 4.3 |
| V2 RCAT score (mean ± SD) (n = 22) | 23.3 ± 4.4 |
| Asthma diagnosis (n [percentage]) | 2 (8%) |
| Treatment from V1 to V2 (n) | 12 topical steroid |
| 7 food elimination | |
| 5 both | |
| 1 other | |
| Reported use of NSAID(s) (including aspirin) (n) | 13 |
| EoE severity: | |
| Required dilations (n [percentage]) | 12 (48%) |
| Required ED visits for EoE (n [percentage]) | 13 (52%) |
| Allergic sensitivities: | |
| Trees (n [percentage]) | 17 (68%) |
| Grass (n [percentage]) | 12 (48%) |
| Ragweed/late fall pollen (n [percentage]) | 10 (40%) |
| Mold (n [percentage]) | 5 (20%) |
| Dust mite (n [percentage]) | 10 (40%) |
| Cat or dog (n [percentage]) | 9 (36%) |
| Food sensitivities: | |
| Egg (n [percentage]) | 9 (36%) |
| 2 (8%) | |
| Peanut (n [percentage]) | 6 (24%) |
| Shrimp (n [percentage]) | 5 (20%) |
| Soy (n [percentage]) | 1 (4%) |
| Wheat (n [percentage]) | 3 (12%) |
| Any above food (n [percentage]) | 13 (52%) |
| V1 PEC (per HPF) (median [25th, 75th percentiles]) | 42 (28, 68) |
| (percentage ≥ 6/HPF or ≥ 15/HPF) | 100% ≥ 6, 96% ≥ 15 |
| V2 PEC (per HPF) (median [25th, 75th percentiles]) | 12 (0, 44)† |
| (percentage ≥ 6/HPF or ≥ 15/HPF) | 56% ≥ 6, 44% ≥ 15 |
ED, emergency department; EoE, eosinophilic esophagitis; HPF, high power field; n, number of subjects; NSAID, non-steroidal anti-inflammatory drug; PEC, peak eosinophil count; RCAT, rhinitis control assessment test; V, visit.
Data are presented as mean ± SD (if data are normally distributed) or median (25th, 75th percentiles) (if data are not normally distributed).
One patient was white and American Indian/Alaska Native.
P = 0.02 versus V1.
For blood eosinophils, P = 0.96 between V2 and V1.
Allergic and food sensitivities are based on skin test.
Note: Information on NSAID use was captured as part of the information on medication but not captured in relation to study visits.
Flow cytometry histogram examples for patients with low or high level activated β1 integrin (N29 signal) or low or high degree αIIb (CD41) positivity are displayed in Fig E3. N29 intensity and levels of and percentage eosinophils positive for P-selectin (CD62P) and CD41 correlated among each other (Fig E4). CD62P and CD41 positivity correlated with PEC at V2, whereas N29 intensity did not (Fig E5). Adjusting for the RCAT (Rhinitis Control Assessment Test) score, allergy and asthma, or treatment including steroid, did not affect these correlations (Table EIII). In addition, patients on steroids did not have significantly different expression of the markers compared to patients not on steroids (Table EIV). None of the other 13 markers correlated significantly with PEC at V2 (Table III). N29 intensity or CD62P or CD41 positivity did not correlate significantly with the other measures of EoE disease activity (Table EV). However, CD41 positivity correlated with eosinophil inflammation and lamina propria fibrosis EoEHSS subscores (Table EVI).
We performed receiver operating characteristic (ROC) analysis to investigate the ability of these potential biomarkers to predict PEC below pre-specified cutoffs. CD62P or CD41 significantly predicted PEC < 6/HPF; CD41 was best with an area under curve (AUC) of 0.84, 93% specificity and 82% sensitivity for the statistically optimal criterion < 22.9% CD41-positive cells (Fig 1, A; Table EVII). CD41 also significantly predicted PEC < 15/HPF (Fig E6 legend). Dividing patients according to median CD41 positivity (26.7%), CD41-low patients had median PEC = 0/HPF and CD41-high patients significantly higher median PEC = 31/HPF (Fig 1, B). Alternatively, categorizing the patients according to PEC < or ≥ 6 or 15/HPF, PEC-low patients had significantly fewer CD41-positive blood eosinophils than PEC-high patients (Fig E6).
FIG 1.
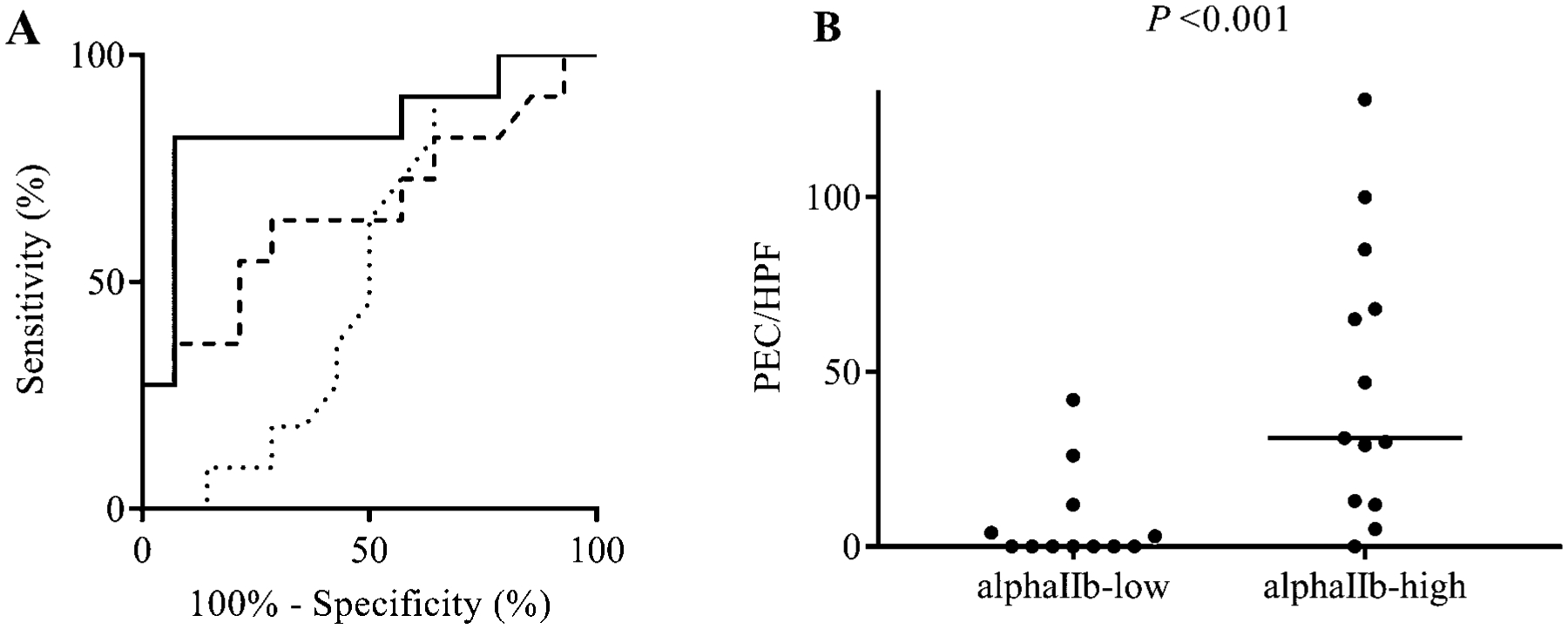
ROC curve for the ability of blood eosinophil activated β1 integrin (mAb N29), P-selectin (CD62P)- or αIIb integrin (CD41)-positive blood eosinophils to predict PEC at V2, and PEC at V2 in αIIb-low or –high patients. A, Dotted line, N29; dashed line, P-selectin (CD62P); solid line, αIIb (CD41). To predict PEC < 6/HPF, area under curve (AUC) = 0.52 for N29, 0.66 for P-selectin, and 0.84 for αIIb (P < 0.001); for criterion < 22.9% αIIb-positive cells, specificity = 93% and sensitivity = 82%. B, Patients were divided according to median αIIb-positive cells (26.7%) at V2; Bar, median PEC in each group (median in the αIIb-low group = 0).
In summary, our results indicate that a pathway of platelet activation and platelet-eosinophil association, likely also P-selectin-triggered α4β1 integrin activation and α4β1-mediated eosinophil arrest on VCAM-1 on activated endothelium in the esophagus, occurs in EoE, as suggested in the lung in asthma.5, 6 Such a scenario is consistent with evidence for platelet activation and platelet-eosinophil interactions, as visualized by immunofluorescence microscopy in vitro of αIIb/CD41-positive eosinophils,7 which we have also observed in vivo in a patient with EoE (Fig. E7), leading to eosinophil recruitment in allergic diseases.8 It is also consistent with upregulated VCAM-1 in the esophagus in EoE and association between response to treatment and decreased VCAM-1.9
PEC was our primary measure of disease status. PEC correlated strongly with the histology score, whereas the other correlations among scores were more modest. This is consistent with the struggles to identify an ideal metric for EoE disease activity, as there is a loose relationship among symptoms, pathology, and visualization of the esophagus. To date, PEC remains the primary metric for EoE diagnosis and monitoring. Limitations of the present study include the relatively small sample size, exclusion of children, and that subjects were recruited by EoE guidelines at the time of study design, i.e., subjects with PPI-responsive esophageal eosinophilia were excluded. To validate our findings and address these and other limitations, we plan to examine a larger cohort including patients with PPI-responsive esophageal eosinophilia and pediatric patients.
In conclusion, we demonstrate that αIIb integrin (CD41) associated with circulating eosinophils is a potential non-invasive biomarker for residual esophageal eosinophilic inflammation, after a period of recommended standard of care treatment. (Word count 1000)
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health (NIH; grant No. R21 AI122103 to SKM, EAG, and MWJ; grant No. T32 AI007635 to ELA; Program Project grant No. P01 HL088594 to NJ Jarjour, DFM, and SKM; Clinical and Translational Science Award grant No. UL1 RR025011 to MK Drezner, and the University of Wisconsin [UW] Carbone Cancer Center [CCC] support grant No. P30 CA014520 to HH Bailey). The funding source had no involvement in the study design; the data collection, analysis, or interpretation; the writing of the report; or the decision to submit the article for publication.
We thank Julia Bach and Ann Sexton for patient recruitment and screening; Dagna Sheerar, Lauren Nettenstrom, Kathryn Fox, Abigale Bleil, Alex Henkel, and Zach Stenerson for advice on flow cytometry; and Tina Palas for administrative help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: MWJ reports receiving a grant from Hoffmann-La Roche, having received a speaker fee and been an advisory board member for Genentech, and receiving consulting fees from Guidepoint Global, unrelated to this work. SKM reports receiving consulting and speaker fees from Astra-Zeneca, unrelated to this work; EMM, PSF, ELA, JLB, KEL, MDE, LDL, DFM, SMC, and EAG declare that they have no conflicts of interest to disclose.
REFERENCES
- 1.Collins MH, Capocelli K, Yang GY. Eosinophilic gastrointestinal disorders pathology. Front Med (Lausanne) 2017; 4:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018; 154:319–32 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Pawankar R, Ackerman SJ, Akin C, Clayton F, Falcone FH, et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ J 2016; 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hines BT, Rank MA, Wright BL, Marks LA, Hagan JB, Straumann A, et al. Minimally invasive biomarker studies in eosinophilic esophagitis: A systematic review. Ann Allergy Asthma Immunol 2018; 121:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson MW. Eosinophil activation status in separate compartments and association with asthma. Front Med (Lausanne) 2017; 4:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson MW, Mosher DF. Integrin activation states and eosinophil recruitment in asthma. Front Pharmacol 2013; 4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson MW, Mosher DF. Activation of beta1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol 2011; 45:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SA, Page CP, Pitchford SC. Platelet-eosinophil interactions as a potential therapeutic target in allergic inflammation and asthma. Front Med (Lausanne) 2017; 4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nhu QM, Aceves SS. Tissue remodeling in chronic eosinophilic esophageal inflammation: Parallels in asthma and therapeutic perspectives. Front Med (Lausanne) 2017; 4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


