Abstract
Bowman’s layer lies immediately posterior to the epithelial basement membrane (EBM) and anterior to the stroma proper in humans, chickens, quail, zebra fish, deer, giraffe, antelope, California sea lions, guinea pig and several other species. It is not found in dog, wolf, cat, tiger, lions, rabbit, pigs, cows, goats, or horses. Developmental anomalies of Bowman’s layer are rare, but acquired damage to Bowman’s layer, or even complete destruction, is frequently seen in advanced bullous keratopathy or Fuchs’ endothelial dystrophy. No detrimental effects of removal of Bowman’s layer over the central 6 to 7 mm of central cornea have been noted in millions of patients who’ve had photorefractive keratectomy (PRK). Recent studies have suggested the randomly-oriented collagen fibrils that make up Bowman’s layer do not have a significant barrier function in modulating the passage of moderate-to large-sized proteins. It is hypothesized that Bowman’s layer develops in the corneas of those species that have one because of cytokine-mediated interactions occurring between corneal epithelial cells and underlying keratocytes, including negative chemotactic and apoptotic effects on the keratocytes by low levels of cytokines such as interleukin-1α that are gradually released as epithelial cells die and slough during their normal development. A “Bowman’s like layer” can generate around stromal epithelial plugs after radial keratotomy, and possibly beneath the central corneal epithelial basement membrane many years after PRK.
Keywords: cornea, Bowman’s layer, chemotaxis, histopathology, wound healing
Sir William Bowman, a British ophthalmologist, anatomist and histologist (Galst, 2007) first reported the subepithelial collagenous layer that came to be initially termed “Bowman’s membrane” in the London Medical Gazette in 1847. More recently, the layer has been more accurately referred to as Bowman’s layer.
1. Bowman’s layer structure and species variation
This layer that lies immediately posterior to the epithelial basement membrane (EBM) and anterior to the stroma proper in species that have one (Fig. 1). In the adult human, Bowman’s layer is 8 to 12 μm thick, and is acellular and composed of randomly-oriented collagen fibrils (Dewitt, 1931; Germundsson et al., 2013). It has been reported to decrease in thickness with age (Hay, 1979). Jacobsen and coworkers (1984) reported, using frozen resin cracking, that the collagen fibrils of Bowman’s layer in human corneas were only half or two-thirds of the diameter fibrils in the deeper stroma. The normally smooth anterior surface of Bowman’s layer is exposed by debridement of the epithelium and EBM with a scalpel. The posterior surface of Bowman’s layer merges with the highly-organized collagen lamellae of the stroma (Hay, 1979; Jacobsen et al., 1984; Kayes and Holmberg, 1960). Tisdale and coworkers (1988) showed that the development of the anchoring fibrils that run between the basal epithelial cells and anterior stroma was the same in humans with Bowman’s layer and rabbits without Bowman’s layer. In humans, Bowman’s layer can be detected between 13 and 19 weeks of gestation (Tisdale et al., 1988; Sevel Disaacs, 1988). Two studies have noted that keratocytes are present in Bowman’s layer early in development of the cornea (Bach and Seefelder, 1912; Ozanics and Jakobiec, 1982). Apple et al. (1984) noted in the cornea from a patient with congenital corneal opacification secondary to Bowman’s layer dysgenesis, that keratocytes were retained in the layer and there was marked thickening of Bowman’s layer. Even rabbits (which do not have a Bowman’s layer in the adult), have a Bowman’s-like acellular layer in the anterior cornea early in development (Cintron et al., 1983).
Fig. 1.
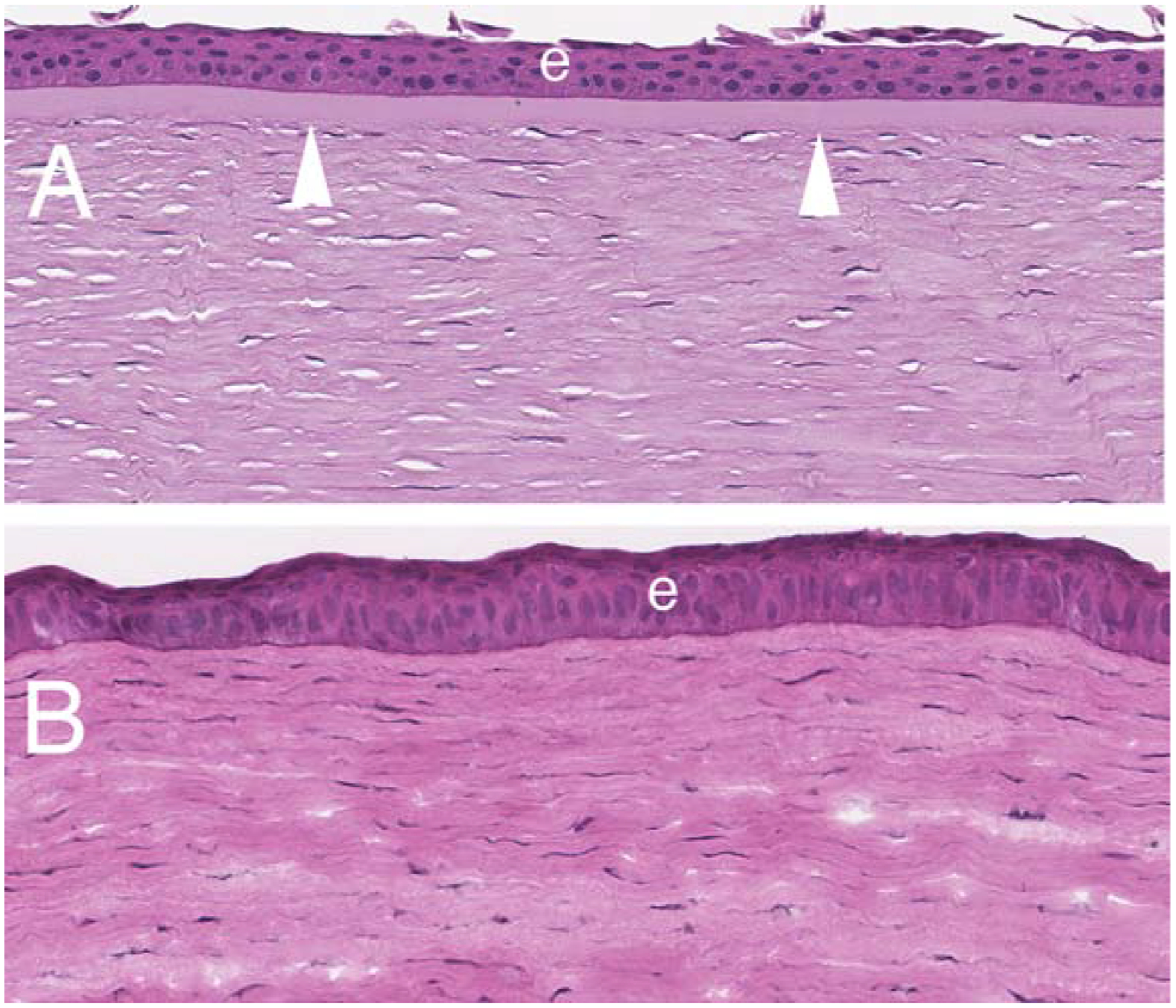
Hematoxylin and eosin staining of a human cornea (A) with Bowman’s layer (arrowheads) and a rabbit cornea without Bowman’s layer. Mag. 200X.
Some studies hypothesized that the epithelium contributes to the formation of Bowman’s layer. Thus, Tisdale et al (1988) suggested that Bowman’s layer develops from a “palisade of filaments” that extend from the basal lamina into the anterior stroma beginning at approximately the 13th week of gestation. Epithelial contributions to Bowman’s layer has also been suggested by Gordon et al (1994). Their studies showed that smaller collagen fibrils in Bowman’s layer, compared to the underlying stroma, were attributable to greater levels of collagen type V and they suggested that the epithelium was the source of collagen type V in Bowman’s layer.
Collagen type I is the is the major component of Bowman’s layer (Merandana et al., 2001; Hayashi et al., 2002), but collagens type III, V, and XII collagens are also found in these fibrils (Jacobsen et al., 1984; Hayashi et al., 2002; Marshall et al., 1991a,b; Zimmerman et al., 1988; Gealy et al., 2009: Marchant et al., 2002). Gordon and colleagues (1984) suggested that collagen type XII in the fibrils of Bowman’s layer contributes to the stability of these fibrils. Streeten and coworkers (1999) reported that Bowman’s layer has a higher concentration of the beta Ig-h3-coded protein keratoepithelin compared with the underlying stroma. Little is known regarding differences in other extracellular matrix components between Bowman’s layer and the stroma.
Bowman’s layer is found in humans (Dewitt, 1931; Germundsson et al., 2013) and nearly all non-human primates—excepting only the lemur of all primates examined (Merandana et al., 2001; Hayashi et al, 2002). It has also been noted in chickens, quail, zebra fish, deer, giraffe, antelope, California sea lions, guinea pig and several other species, but not found in dog, wolf, cat, tiger, lions, rabbit, pigs, cows, goats, or horses (Merandana et al., 2001; Hayashi et al, 2002; Gealy et al., 2009; Marchant et al., 2002; Streeten et al., 1999; Nautscher et al., 2016; Miller et al., 2010; Gonçalves et al., 2016; Merayo-Lloves et al., 2001: Cafaro et al., 2009; Merindano et al, 2002). There is disagreement in the literature as to whether mice have Bowman’s layer, even using transmission electron microscopy, with some studies suggesting it is present in mice with strains C3H and ddY (Hayashi et al., 2002), and others using BALB/c and C57 BL/6 mice reporting it is not present. (Wilson et al., 1996). This could be related to strain differences often found for mice, but I personally have never seen a Bowman’s layer in any strain of mice imaged with TEM. Figure 2 shows definitively that C57 Bl/6 mice do not have Bowman’s layer.
Fig. 2.
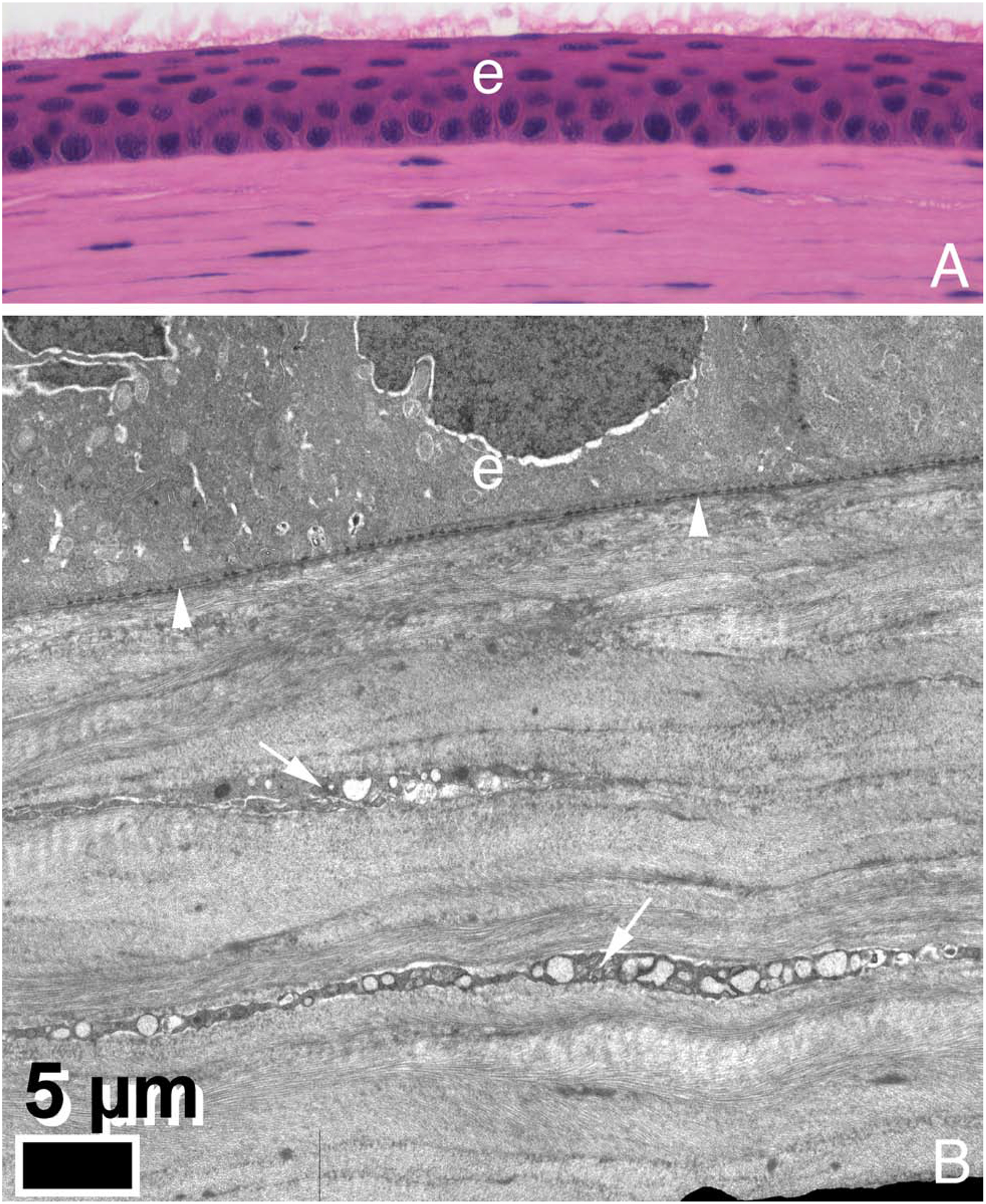
Hematoxylin and eosin staining (A, 200X) and transmission electron microscopy (B) of a mouse cornea. There is no Bowman’s layer in this C57 Bl/6 strain of mice. Images graciously provided by Paul FitzGerald, PhD, Dept. of Cell Biology and Human Anatomy, UC Davis School of Medicine.
2. Developmental and disease-related alterations in Bowman’s layer
Developmental anomalies of Bowman’s layer are rare. Congenital absence of Bowman’s layer has been reported in association with Peter’s anomaly (Marshall et al., 1991a,b), osteogenesis imperfecta type II (Chan et al., 1982) and III (Mietz et al., 1997), and sclerocornea (Petroutsos et al., 1983). Kasner et al. (1993) reported the congenital absence of Bowman’s layer in both eyes of three patients with otherwise normal corneas and normal vision—with no other ocular or systemic abnormalities. Congenital absence of Bowman’s layer and associated hypercellularity of the corneal stroma was also noted in three infants with trisomy 18 (Velzeboer et al., 1989). Congenital corneal opacification associated with dysgenesis of Bowman’s layer in an otherwise normal infant was reported (Ozanics and Jakobiec, 1982). In that patient, both primary corneas and a corneal graft that failed within a few months had a thickened Bowman’s layer that was three to four times the thickness of the layer in normal corneas. There were also cells that appeared to be keratocytes within Bowman’s layer of each of these corneas and irregular bundles of collagen that were thought to be produced by these ectopic cells. Abnormalities of Bowman’s layer were reported in patients with fetal alcohol syndrome (Edward et al., 1993)—with alterations varying from complete absence of Bowman’s layer to thickening associated with stromal edema.
Acquired abnormalities of Bowman’s layer have been associated with various corneal diseases, injuries and infections. Breakage or disruption of Bowman’s layer is commonly seen in histological examination of corneas with keratoconus (Scroggs and Proia, 1992; Kaas-Hansen, 1993; Kremer et al., 1995; Kim et al., 1999). It remains unknown whether these defects are attributable to the underlying pathophysiology of keratoconus or merely secondary changes that develop as the disease progresses. Stromal haze at the level of Bowman’s layer has been noted in Ehlers-Danlos syndrome (May and Beauchamp, 1987). Breakage or disruption of Bowman’s layer is also found in Salzmann’s nodular degeneration (Vannas et al., 1975), and could be related to the pathophysiology of this disorder where ectopic fibrous tissue develops anterior to Bowman’s layer and produces epithelial surface irregularity.
A likely instructive change is noted in Bowman’s layer in advanced cases of bullous keratopathy (Ljubimov et al., 1996) or Fuchs’ corneal dystrophy (Wilson and Bourne, 1988). Stromal edema occurs as the endothelial dysfunction progresses. Bowman’s layer is normal in the early stages of either disease. As the disease and stromal edema advances, however, the epithelium becomes increasingly edematous and dysfunctional. At the late stages of these diseases, changes occur in Bowman’s layer. At some point, keratocytes, or their progeny corneal fibroblasts and myofibroblasts, are no longer confined to the underlying stroma, but enter Bowman’s layer. Corneal fibroblasts eventually advance to the immediate subepithelial region (Fig. 3) and may differentiate into alpha-smooth muscle actin+ myofibroblasts. Thus, the subepithelial fibrous pannus that is a very late finding in either bullous keratopathy (Ljubimov et al., 1996) or Fuchs’ corneal dystrophy (Wilson and Bourne, 1988), likely develops from keratocytes/corneal fibroblasts that are no longer repulsed by factors normally produced by the healthy epithelium, such as constitutively produced IL-1α. In some cases, the keratocyte-derived cells even enter into the epithelium itself and form partitions within the epithelial layer (Fig. 3). Eventually, Bowman’s layer is completely destroyed (Ljubimov et al., 1996; Wilson and Bourne, 1988). Thus, this histopathological change noted in bullous keratopathy or Fuchs’ dystrophy supports the hypothesis that Bowman’s layer is actively maintained during adult life by regulatory systems that are compromised by the epithelial dysfunction associated with these diseases late in their courses. The cytokines, growth factors or other modulators that comprise this regulatory system likely modulate keratocyte phenotype, localization and viability.
Fig. 3.
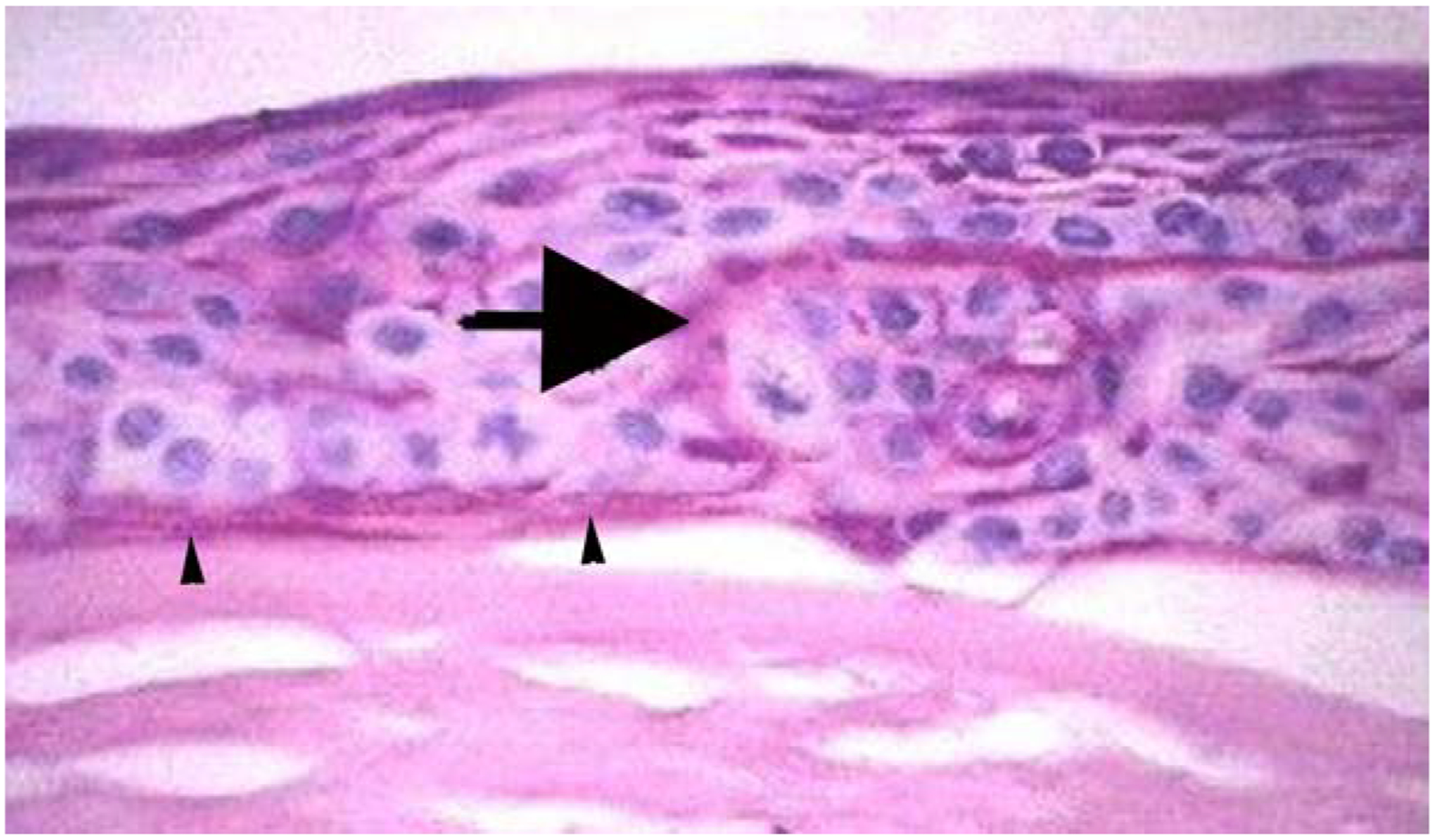
Destruction of Bowman’s layer in bullous keratopathy. A human cornea with advanced bullous keratopathy stained with hematoxylin and eosin. Ectopic fibrosis has extended through Bowman’s layer, into the epithelium (large arrow), and immediately beneath the epithelium (arrowheads). Mag 200X.
3. Chemotactic hypothesis of Bowman’s layer development and persistence
All sorts of magical properties have been attributed to Bowman’s layer over the decades. Those who hypothesize a critical function for Bowman’s layer must account for the apparent lack of detrimental effects of removal of the layer over the central 6 to 7 mm of central cornea in millions of patients since 1988 using photorefractive keratectomy (PRK). One of the most prominent properties ascribed to Bowman’s layer is that it provides some sort of barrier function against the passage of macromolecules. There is no evidence for this and a great deal of evidence to the contrary. First, modulators of stromal-epithelial interactions between the epithelium and keratocytes, that occur even after simple epithelial abrasion injuries (Wilson et al., 1996; Mohan et al., 1997; Wilson et al., 1993), could not function if Bowman’s layer blocked passage of cytokines and growth factors such as interleukin-1α, hepatocyte growth factor and keratinocyte growth factor. The structure that provides barrier function to these macromolecules, and others like transforming growth factor-β1, is the epithelial basement membrane, which must be injured or removed for these growth factors and cytokines to pass back and forth between the epithelium, Bowman’s layer, and the underlying stroma (Torricelli et al., 2013). There is direct evidence of the passage of macromolecules such as perlecan, a 470 kilodalton heparan sulfate proteoglycan (Fig. 4). After epithelial scrape injury to the normal human cornea, with removal of the epithelium and epithelial basement membrane, of an eye enucleated for choroidal melanoma, perlecan penetrates Bowman’s layer (Torricelli et al., 2015). Whether this perlecan comes from the scraped corneal epithelium, or as these studies suggested, it comes from keratocytes that begin producing perlecan when the epithelium is injured (which is our hypothesis supported by several publications), this large macromolecule most definitely penetrated Bowman’s layer after the injury in these human corneas. Thus, the randomly-oriented collagen fibrils that make up Bowman’s layer do not have a significant barrier function modulating the passage of moderate-to large-sized proteins.
Fig. 4.
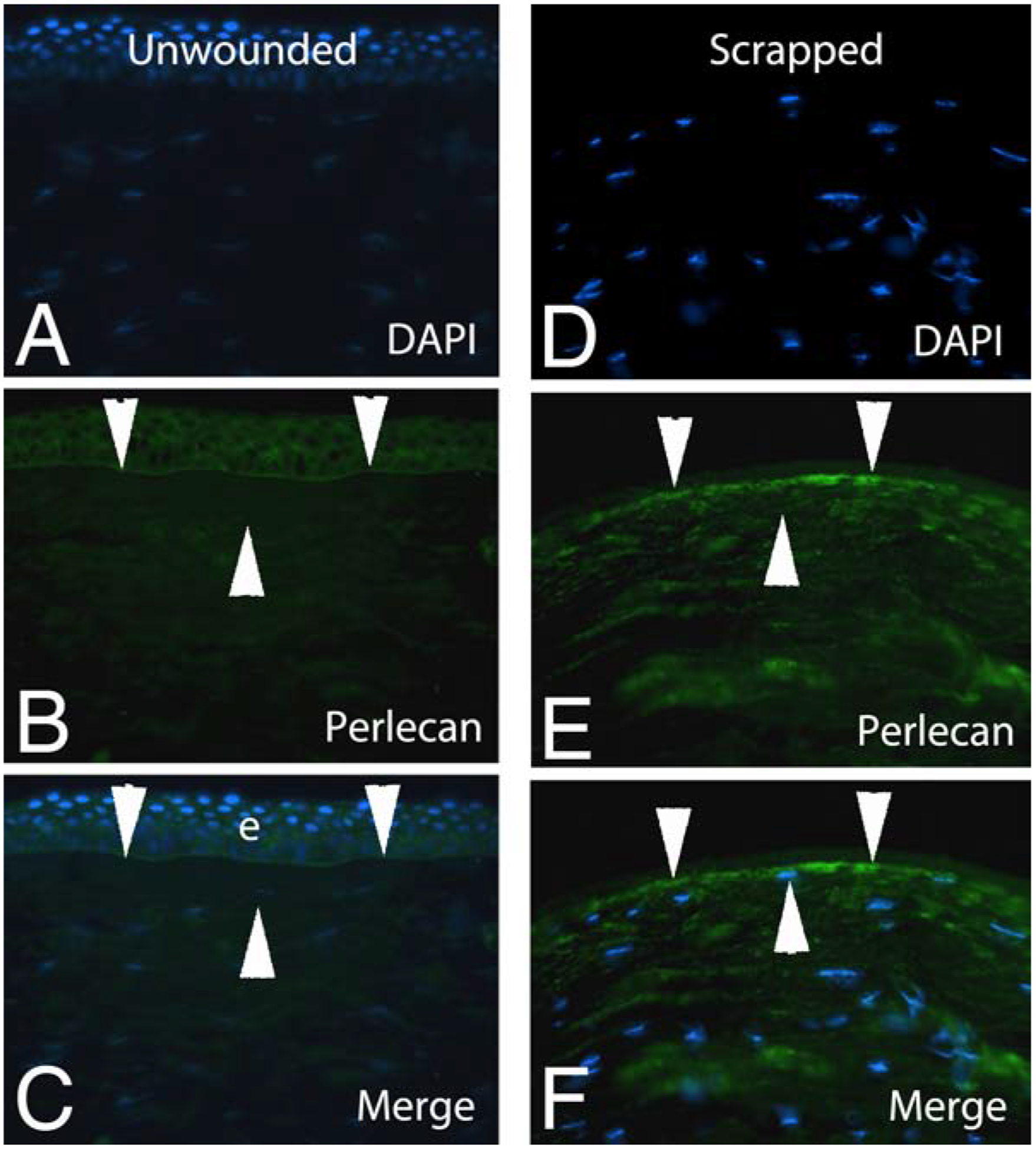
Immunohistochemistry for 470 kilodalton protein perlecan (an epithelial basement membrane component) in a normal human unwounded cornea (A–C) or a normal human cornea at 30 minutes after epithelial debridement with removal of the epithelial basement membrane (D–F) in eyes enucleated for choroidal melanoma. It can be seen that perlecan penetrates the full thickness of Bowman’s layer (area between the two superior and one inferior arrowheads in each panel) either from keratocytes that have upregulated perlecan production (seen throughout stroma in E and F) or from the scraped epithelial cells as they were removed, or both. As a part of the wound healing response, some stromal cells have moved anterior where they are nearer or even in Bowman’s layer in the scraped cornea (F). Blue is DAPI staining of cell nuclei in all panels. e is epithelium. Mag 400x. Republished with permission from Torricelli et al. Exp Eye Res., 2015;134:33–8.
Another property that some have contributed to Bowman’s layer is that it is responsible for a substantial portion of the biomechanical rigidity of the cornea. However, Seiler and coworkers (Seiler et al., 1992) demonstrated convincingly that this is not the case.
What then is the function or significance of Bowman’s layer? We hypothesized (Wilson and Hong, 2000) that Bowman’s layer develops in the corneas of those species that have one because of cytokine-mediated interactions occurring between corneal epithelial cells and underlying keratocytes, including negative chemotactic and apoptotic effects on the keratocytes by low levels of cytokines such as interleukin-1 that are gradually released as epithelial cells die and slough during their normal development. In experiments where 40 ng of mouse IL-1α in 1μl of vehicle was microinjected into the BALBc mouse stroma (a strain of mice that does not have detectable Bowman’s layer seen with H&E staining or transmission electron microscopy, Fig. 5), the negative chemotactic effects of IL-1α (in addition to apoptotic effects directly at the site of injection where the IL-1α concentration is greatest) triggered keratocytes, and perhaps corneal fibroblasts that develop from keratocytes in response to the cytokine, to redistribute in the stroma (Wilson et al., 1996). This redistribution occurred such that many of the keratocytes line up approximately 10 μm posterior to the corneal epithelium (Fig. 5)—as though they were trapped between the negative chemotactic effect of the injected IL-1α depot and negative chemotactic effects of epithelial modulators (that likely also include IL-1α). There must be poorly characterized systems in place that modulate the organization of the adult cornea, for example, by preventing keratocytes, and their progeny corneal fibroblasts, from moving anterior to disrupt epithelial physiology. The existence of these systems is supported by disruption of the histology and function of the cornea in advanced bullous keratopathy and Fuchs’ dystrophy (Ljubimov et al., 1996; Wilson and Bourne, 1988). Further evidence for the presence of these systems that modulate corneal tissue organization is provided by the response of human corneas to radial keratotomy surgery. In radial keratotomy, nearly full-thickness incisions are made through the epithelium and deep into the corneal stroma approaching Descemet’s membrane. Epithelial plugs often extend into these incisions and remain indefinitely within the stromal incisions following healing. Melles et al (1995) showed that a “Bowman’s-like layer” frequently develops in the stroma surrounding the ectopic epithelial tissue when corneas are examined histopathologically 5 to 10 years after radial keratotomy. This Bowman’s-like layer does not develop around epithelial plugs in rabbits, a species without Bowman’s layer, that have deep incisions into the corneal stroma (Marino et al., 2017). A Bowman’s-like layer was noted beneath the epithelial basement membrane ten years after PRK in a cornea rejected for transplantation due to a history of prior surgery (Wilson SE, unpublished data, 2002).
Fig. 5.
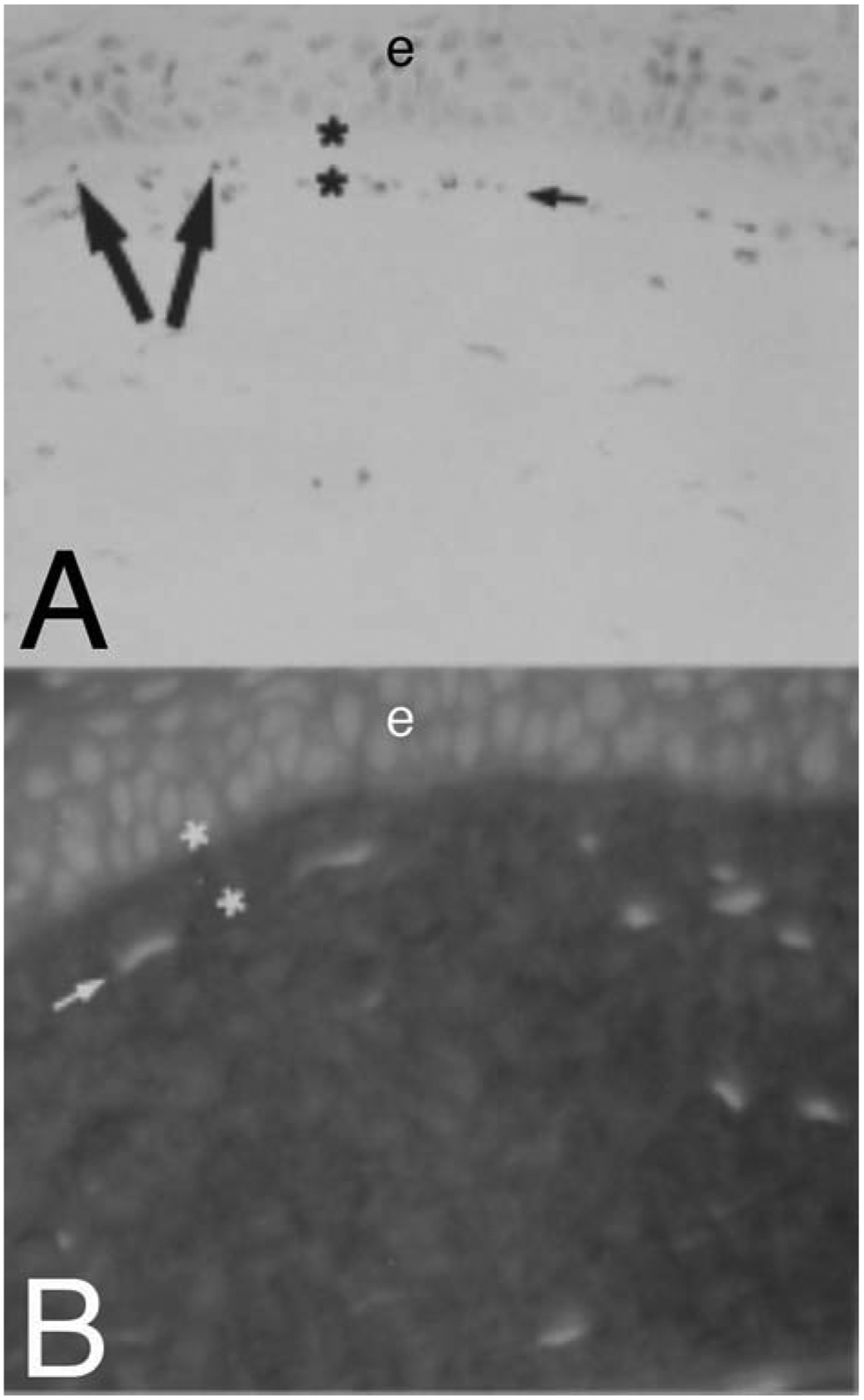
Evidence for negative chemotactic effects of epithelium on keratocytes/corneal fibroblasts in the cornea. When 40 ng in one microliter of mouse interleukin-1 alpha (IL-1a) is microinjected into a normal BALB/c mouse corneas (a strain of mice without Bowman’s layer visible on transmission electron microscopy28), many keratocytes at the injection undergo apoptosis.28 More peripheral keratocytes, that survive, redistribute in the stroma. Those anterior to the injection move more anterior. At 4 hours after the injection, staining with H&E (A) or propidium iodide (B), shows redistribution of stromal cells. The injection occurred inferior to the large arrows, which indicate the direction of redistribution of cells that were anterior to the depot of IL-1α. The small arrows in A and B indicate lines of stromal cells that have left an area of the most anterior stroma (between the asterisks) free of stromal cells. Mag 400X. Republished with permission from Wilson SE et al. Exp Eye Res 1996;62:325–8.
Bowman’s layer remains a fascinating corneal structure. It’s origin and function will be potentially fruitful area of research for decades to come.
Bowman’s layer is present in some species and absent in others
Bowman’s layer is not a barrier to passage of large molecules
A Bowman’s-like layer may regenerate after corneal surgery
Bowman’s layer likely maintained by ongoing epithelial-stromal interactions
Funding
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
“RIGHTS & PERMISSIONS
Author Rights
Authors of IOVS, JOV, and TVST articles retain the right to reuse material from their own articles in their future work, with proper attribution. Permission does not need to be obtained from ARVO.”
Proprietary interest statement
The author doesn’t have any commercial or proprietary interests in this study.
References
- Apple DJ, Olson RJ, Jones GR, Carey JC, Van Norman DK, Ohrloff C, Philippart M, 1984. Congenital corneal opacification secondary to Bowman’s layer dysgenesis. Am. J. Ophthalmol 98, 320–8. [DOI] [PubMed] [Google Scholar]
- Bach LB, Seefelder R, 1912. Atlas Zur Entwicklungsgeschichte Des Menschlichen Auges. Wilhelm Engelmann Verlag, Leipzig, Germany. [Google Scholar]
- Cafaro TA, Ortiz SG, Maldonado C, Espósito FA, Croxatto JO, Berra A, Ale OL, Torrealday JI, Urrets-Zavalía EA, Urrets-Zavalía JA, Serra HM, 2009. The cornea of guinea pig: structural and functional studies. Vet. Ophthalmol 12, 234–41. [DOI] [PubMed] [Google Scholar]
- Chan CC, Green WR, Cruz ZC, Hillis A, 1982. Ocular findings in osteogenesis imperfecta congenita. Arch. Ophthalmol 100, 1459–63. [DOI] [PubMed] [Google Scholar]
- Cintron C, Covington H, Kublin CL, 1983. Morphogenesis or rabbit corneal stroma. Invest. Ophthalmol. Vis. Sci 24, 543–56. [PubMed] [Google Scholar]
- Dewitt EN, 1931. The histopathology of Bowman’s membrane. Trans. Am. Ophthalmol. Soc 29, 461–85. [PMC free article] [PubMed] [Google Scholar]
- Edward DP, Li J, Sawaguchi S, Sugar J, Yue BY, Tso MO, 1993. Diffuse corneal clouding in siblings with fetal alcohol syndrome. Am. J. Ophthalmol 115, 484–93. [DOI] [PubMed] [Google Scholar]
- Galst JM, 2007. “Sir William Bowman (1816–1892). Arch. Ophthalmol 125, 459. [DOI] [PubMed] [Google Scholar]
- Gealy C, Hayes AJ, Buckwell R, Young RD, Caterson B, Quantock AJ, Ralphs JR 2009. Actin and type I collagen propeptide distribution in the developing chick cornea. Invest. Ophthalmol. Vis. Sci 50, 1653–8. [DOI] [PubMed] [Google Scholar]
- Germundsson J, Karanis G, Fagerholm P, Lagali N, 2013. Age-related thinning of Bowman’s layer in the human cornea in vivo. Invest. Ophthalmol. Vis. Sci 54, 6143–9. [DOI] [PubMed] [Google Scholar]
- Gonçalves GC, Pérez-Merino P, Martínez-García MC, Barcía A, Merayo-Loves J, 2016. Comparison of the characteristics in hen and quail corneas as experimental models of refractive surgery. Arch. Soc. Esp. Oftalmol 91, 310–5. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Birk DE, Fitch JM, Linsenmayer TF, 1994. Type V collagen and Bowman’s membrane. J. Biol. Chem 269, 24959–66. [PubMed] [Google Scholar]
- Hay ED, 1979. Development of the vertebrate cornea. Int. Rev. Cytol 1979, 63:263–322. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Osawa T, Tohyama K, 2002. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol 254, 247–58. [DOI] [PubMed] [Google Scholar]
- Jacobsen IE, Jensen OA, Prause JU, 1984. Structure and composition of Bowman’s membrane. Study by frozen resin cracking. Acta Ophthalmol. (Copenh.) 62, 39–53. [DOI] [PubMed] [Google Scholar]
- Kaas-Hansen M, 1993. The histopathological changes of keratoconus. Acta Ophthalmol. 71, 411–4. [DOI] [PubMed] [Google Scholar]
- Kasner L, Mietz H, Green WR, 1993. Agenesis of Bowman’s layer. A histopathological study of four cases. Cornea 12, 163–70. [DOI] [PubMed] [Google Scholar]
- Kayes J, Holmberg A, 1960. The fine structure of Bowman’s layer and the basement membrane of the corneal epithelium. Am. J. Ophthalmol 50, 1013–21. [DOI] [PubMed] [Google Scholar]
- Kim W-J, Rabinowitz YS, Meisler DM, Wilson SE, 1999. Keratocyte apoptosis associated with keratoconus. Exp. Eye Res 69, 475–81. [DOI] [PubMed] [Google Scholar]
- Kremer I, Eagle RC, Rapuano CJ, Laibson PR, 1995. Histologic evidence of recurrent keratoconus seven years after keratoplasty. Am. J. Ophthalmol 119, 511–2. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Wu RR, Ninomiya Y, Sado Y, Maguen E, Nesburn AB, Kenney MC, 1996. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest. Ophthalmol. Vis. Sci 37, 997–1007. [PubMed] [Google Scholar]
- Marchant JK, Zhang G, Birk DE, 2002. Association of type XII collagen with regions of increased stability and keratocyte density in the cornea. Exp. Eye Res 75, 683–94. [DOI] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE, 2017. Regeneration of defective epithelial basement membrane and restoration of corneal transparency after photorefractive keratectomy. J. Ref. Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GE, Konstas AG, Lee WR, 1991a. Immunogold fine structural localization of extracellular matrix components in aged human cornea. I. Collagen types I–IV and laminin. Graefes Arch. Clin. Exp. Ophthalmol 229, 157–63. [DOI] [PubMed] [Google Scholar]
- Marshall GE, Konstas AG, Lee WR 1991b, Immunogold fine structural localization of extracellular matrix components in aged human cornea. II. Collagen types V and VI. Graefes Arch. Clin. Exp. Ophthalmol 229, 164–71. [DOI] [PubMed] [Google Scholar]
- May MA, Beauchamp GR, 1987. Collagen maturation defects in Ehlers-Danlos keratopathy. J. Pediatr. Ophthalmol. Strabismus 24, 78–82. [DOI] [PubMed] [Google Scholar]
- Melles GR, Binder PS, Moore MN, Anderson JA, 1995. Epithelial-stromal interactions in human keratotomy wound healing. Arch. Ophthalmol 113, 1124–30. [DOI] [PubMed] [Google Scholar]
- Merandana Ma.D., Costa J, Canals M, Potau JM, Ruano D, 2001. A comparative study of Bowman’s layer in some mammals: Relationships with other constituent corneal structures. Eur. J. Anat 6, 133–139. [Google Scholar]
- Merayo-Lloves J, Yáñez B, Mayo A, Martín R, Pastor JC, 2001. Experimental model of corneal haze in chickens. J. Refract. Surg 17, 696–9. [DOI] [PubMed] [Google Scholar]
- Merindano MD, Costa J, Canals M, Potau JM, Ruano D 2002. A comparative study of Bowman’s layer in some mammals: Relationships with other constituent corneal structures. Eur. J. Anat 6, 133–139. [Google Scholar]
- Mietz H, Kasner L, Green WR, 1997. Histopathologic and electron-microscopic features of corneal and scleral collagen fibers in osteogenesis imperfecta type III. Graefes Arch. Clin. Exp. Ophthalmol 235, 405–10. [DOI] [PubMed] [Google Scholar]
- Miller SN, Colitz CM, Dubielzig RR, 2010. Anatomy of the California sea lion globe. Vet. Ophthalmol 13 Suppl, 63–71. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim W-J, Helena MC, Baerveldt F, Wilson SE, 1997. Apoptosis in the cornea: further characterization of Fas-Fas ligand system. Exp. Eye Res 65, 575–89. [DOI] [PubMed] [Google Scholar]
- Nautscher N, Bauer A, Steffl M, Amselgruber WM, 2016. Comparative morphological evaluation of domestic animal cornea. Vet. Ophthalmol 19, 297–304. [DOI] [PubMed] [Google Scholar]
- Ozanics V, Jakobiec FA, 1982. Biomedical Foundations of Ophthalmology. Harper and Row, Philadelphia: pp. 8–14. [Google Scholar]
- Petroutsos G, Patey A, Savoldelli M, Pouliquen Y, 1983. Sclero-cornee: etude ultrastructurale et morphologique. J. Fr. Ophtalmol 10, 769–75. [PubMed] [Google Scholar]
- Scroggs MW, Proia AD, 1992. Histopathological variation in keratoconus. Cornea 11, 553–9. [DOI] [PubMed] [Google Scholar]
- Seiler T, Matallana M, Sendler S, Bende T, 1992. Does Bowman’s layer determine the biomechanical properties of the cornea? Refract. Corneal Surg 8, 139–42. [PubMed] [Google Scholar]
- Sevel Disaacs R, 1988. A re-evaluation of corneal development. Trans. Am. Ophthalmol. Soc 86, 178–207. [PMC free article] [PubMed] [Google Scholar]
- Streeten BW, Qi Y, Klintworth GK, Eagle RC Jr., Strauss JA, Bennett K, 1999. Immunolocalization of beta Ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch. Ophthalmol 117, 67–75. [DOI] [PubMed] [Google Scholar]
- Tisdale AS, Spurr-Michaud SJ, Rodrigues M, Hackett J, Krachmer J, Gipson IK, 1988. Development of the anchoring structures of the epithelium in rabbit and human fetal corneas. Invest. Ophthalmol. Vis. Sci 29, 727–36. [PubMed] [Google Scholar]
- Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE, 2015. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp. Eye Res 134, 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Santhiago MR, Wilson SE, 2013. The corneal epithelial basement membrane: Structure, function and disease. Invest. Ophth. Vis. Sci, 54, 6390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannas A, Hogan MJ, Wood I, 1975. Salzmann’s nodular degeneration of the cornea. Am. J. Ophthalmol 79, 211–9. [DOI] [PubMed] [Google Scholar]
- Velzeboer CM, van der Harten JJ, Koole FD, 1989. Ocular pathology in trisomy 18. A histopathological report of 3 cases. Ophthalmic Paediatr. Genet 10, 263–9. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Bourne WM, 1988. Fuchs’ dystrophy. Cornea 7, 2–18. [PubMed] [Google Scholar]
- Wilson SE, He Y-G, Weng J, Li Q, McDowall AW, Vital M, Chwang EL, 1996. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res 62, 325–8. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Hong J-W, 2000. Bowman’s layer structure and function: Critical or dispensable to corneal function? A hypothesis. Cornea 19, 417–420. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Walker JW, Chwang EL, He Y-G, 1993. Hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), their receptors, FGF receptor-2, and the cells of the cornea. Invest. Ophthalmol. Vis. Sci 34, 2544–61. [PubMed] [Google Scholar]
- Zimmerman DR, Fisher RW, Winterhalter KH, Witmer R, Vaughn L, 1988. Comparative studies of collagens in normal and keratoconus corneas. Exp. Eye Res 46, 431–42. [DOI] [PubMed] [Google Scholar]


