Abstract
Background
The optimal radiation dose for adult supratentorial low-grade glioma is unknown. The aim of this study was to provide a final update on oncologic and cognitive outcomes of high-dose versus low-dose radiation for low-grade glioma.
Methods
Between 1986 and 1994, 203 patients with supratentorial low-grade glioma were randomized (1:1) to 50.4 Gy in 28 fractions versus 64.8 Gy in 36 fractions after any degree of resection.
Results
For all patients, median overall survival (OS) was 8.4 years (95% CI: 7.2–10.8). Median progression-free survival (PFS) was 5.2 years (95% CI: 4.3–6.6). Median follow-up is 17.2 years for the 33 patients still alive. High-dose radiation did not improve 15-year OS (22.4%) versus low-dose radiation (24.9%, log-rank P = 0.978) or 15-year PFS (high dose, 15.2% vs low dose, 9.5%; P = 0.7142). OS was significantly better for patients with preoperative tumor diameter <5 cm and baseline Mini-Mental State Examination (MMSE) >27 and who underwent gross total resection. PFS was improved for patients with oligodendroglioma versus astrocytoma, preoperative tumor diameter <5 cm, patients who had gross total resection, and patients with baseline MMSE >27. For patients who had normal MMSE at baseline, at 7 years only 1 patient (5%) had a clinically significant decrease in MMSE from the previous time point, with the remainder (95%) stable. None had decrease in MMSE at 10, 12, or 15 years.
Conclusions
Long-term follow-up indicates no benefit to high-dose over low-dose radiation for low-grade gliomas. Cognitive function appeared to be stable after radiation as measured by MMSE.
Keywords: clinical trials, cognition following radiation, glioma, neurosurgery, radiation therapy
Key Points.
1. High-dose radiation therapy did not improve OS or PFS in adult low-grade glioma.
2. Late toxicity or cognitive decline was uncommon following treatment.
Importance of the Study.
Radiation therapy is well established for low-grade glioma, though the long-term efficacy and toxicity of treatment has not been well described. This study (NCCTG 86-72-51/RTOG 9110) is one of the largest prospective randomized trials of low-grade glioma completed to date, in which patients were randomized to high-dose versus low-dose radiation. The primary analysis was published in 2003 and showed no improvement in OS or PFS with high-dose radiation, consistent with the previously published European Organisation for Research and Treatment of Cancer 22844 trial. Because of the long natural history of low-grade glioma, this analysis with over 17 years median follow-up for surviving patients provides important insights into the late oncologic outcomes as well as the associated late toxicities and neurocognitive impacts of treatment with surgery and radiation.
Low-grade glioma (LGG) is a heterogeneous group of brain tumors comprising approximately 10% of central nervous system (CNS) tumors diagnosed annually in the United States.1 Diffuse astrocytoma and oligodendroglioma are 2 types of World Health Organization grade II LGG commonly diagnosed in young adults. The majority of these patients die prematurely from their disease.
Treatment for LGG typically includes maximal safe resection followed by adjuvant radiation and/or chemotherapy.2 Earlier retrospective studies indicated a potential benefit with higher doses of radiation and based on these findings two phase III dose escalation trials were conducted.3 The European Organisation for Research and Treatment of Cancer (EORTC) 22844 trial randomized 379 patients with LGG to 59.4 Gy in 33 fractions versus 45 Gy in 25 fractions, and did not show an OS or progression-free survival (PFS) benefit to high-dose radiation.4 Similarly, the initial report 86-72-51 of the North Central Cancer Treatment Group (NCCTG) in 2002 showed no benefit to high-dose over low-dose radiation for LGG.5 Still, the long-term impact of standard and dose-escalated radiotherapy on this patient population is unknown. Here we provide a final update on long-term oncologic outcomes, cognitive outcomes, and toxicities of low-dose versus high-dose radiation for LGG.
Methods
Intergroup NCCTG 86-72-51 was a prospective, multi-institution, phase III randomized controlled trial to determine whether high-dose radiation improves outcomes for supratentorial LGG compared with low-dose radiation. This trial was conducted by the NCCTG, the Eastern Cooperative Oncology Group (ECOG), and the Radiation Therapy Oncology Group (RTOG). NCCTG is now part of the Alliance for Clinical Trials in Oncology; ECOG is now part of ECOG‒American College of Radiology Imaging Network (ACRIN) Cancer Research Group; RTOG is now part of NRG Oncology. Our methods were previously described in the initial report.5
Patients and Treatments
Eligible patients were at least 18 years of age and had a centrally reviewed supratentorial LGG with a histologic diagnosis of astrocytoma, oligodendroglioma, or mixed oligoastrocytoma. For patients with oligoastrocytoma, the pathologist determined whether the dominant component was astrocytoma or oligodendroglioma. Patients underwent biopsy, subtotal resection (STR), or gross total resection (GTR) within 3 months of study entry. Extent of resection was defined by the surgeon at the time of surgery. Patients with other previous surgery, radiotherapy, or chemotherapy for intracranial CNS tumors were excluded.
Patients were enrolled between May 1986 and December 1994, and were stratified using a dynamic allocation randomization method by the NCCTG Randomization Office to ensure equal distribution between arms. Stratification factors included age (<40 y vs ≥40 y), preoperative CT or MRI tumor diameter (<5 cm vs ≥5 cm), histology (astrocytoma or oligoastrocytoma [astrocytoma dominant] vs oligodendroglioma or oligoastrocytoma [oligodendroglioma dominant]), Kernohan grade (1 vs 2), extent of resection (GTR vs STR vs biopsy only), and enrolling institution (Mayo vs other NCCTG institution vs ECOG vs RTOG). Each participant signed an institutional review board–approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Patients were randomized (1:1) to either low-dose radiation (arm A: 50.4 Gy in 28 fractions over 5.6 wk) or high-dose radiation (arm B: 64.8 Gy in 36 fractions over 7.2 wk). Radiation planning was MRI based in 164 patients, CT based in 32 patients, and unknown in the remaining 7. All patients received 50.4 Gy in 28 fractions of localized radiation to the preoperative tumor volume and edema, plus a 2-cm margin, as defined by CT with contrast or MRI using T1 (with gadolinium) and T2 sequences. Patients randomized to arm B received an additional 14.4 Gy in 7 fractions to the preoperative tumor volume plus a 1-cm margin. Radiation treatment plans and images were reviewed by the principal investigator (E.S.) after completion of radiation as a part of quality assurance. Patients did not receive chemotherapy as part of protocol therapy.
Endpoints, Design, and Assessments
The primary trial endpoint was OS, defined as time from date of randomization to date of death. Additional endpoints included (i) PFS, defined as time from date of randomization to progression or death without documented progression, (ii) physician-assessed toxicity, Neurologic Function Score (NFS), and (iii) Mini-Mental State Examination (MMSE) scores.
This phase III study was designed with a target accrual of 200 patients (corresponding to 152 events) to provide at least 82% power for detecting a 50% survival improvement in arm B (high dose), assuming 40% survival in arm A (low dose) at 5 years using a two-sided log-rank test with significance level of 0.05. Two interim analyses incorporating investigations of response and toxicity were performed after 100 and 150 patients were randomized.6 At that time, criteria for neither futility nor superiority were met. In 2002, the death and toxicity rates were noted to be higher in the high-dose arm, and the NCCTG Data Monitoring Committee recommended early publication based on the futility rule.7 Therefore, we published initial outcomes at that time, which showed no benefit to high-dose radiation.5 We continued to observe patients for at least 15 years or until death. This is the final report of outcomes.
Patients had physical exam including neurologic exam, MMSE, NFS examination, toxicity assessment, and CT or MRI every 4 months for years 1–2, every 6 months for years 3–5, and annually thereafter. Progression was defined as 25% increase in tumor size based on perpendicular diameter or clear increase in tumor size. Toxicity was assessed using Common Terminology Criteria for Adverse Events. We defined a long-term adverse event (AE) as any AE reported at 5 years from study entry or later. We have previously described MMSE and NFS assessments and initial results.8,9 NFS scores range from 0 (no neurologic symptoms, fully active at home and work without assistance) to 4 (severe neurologic symptoms, totally inactive and unable to work, requires complete assistance at home or in an institution). Any change in NFS was considered clinically significant.
Statistical Analysis
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on February 4, 2019.
For several continuous variables (tumor size, MMSE, age), categorical variables were defined to create clinically appropriate groups (ie, <5 cm vs ≥5 cm preoperative tumor size). OS and PFS were assessed using Kaplan–Meier curves, and compared using two-sided log-rank tests.10 Significance was defined as P < 0.05.
Results
Patients and Treatment
Of 211 patients enrolled and randomized, 5 were found to be ineligible due to incorrect histology, and 3 refused to begin treatment. Therefore, 203 patients began treatment, with 101 receiving low-dose radiation and 102 receiving high-dose radiation (Fig. 1). Baseline characteristics were well balanced between the 2 treatment arms (Table 1). Radiation plans and images were reviewed after completion of treatment by the principal investigator, who deemed most patients (81.8%) to have no deviations (Supplement 4).
Fig. 1.
CONSORT diagram for patient enrollment (treatment arm A: 50.4 Gy in 28 fractions; arm B: 64.8 Gy in 36 fractions).
Table 1.
Baseline characteristics by treatment arm. Patient characteristics by treatment arm (arm A: 50.4 Gy in 28 fractions; arm B: 64.8 Gy in 36 fractions)
| A (N = 101) | B (N = 102) | Total (N = 203) | |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 40.1 (11.5) | 40.7 (11.7) | 40.4 (11.6) |
| Median | 40.0 | 39.5 | 40.0 |
| Q1, Q3 | 33.0, 46.0 | 33.0, 49.0 | 33.0, 47.0 |
| Range | (18.0–73.0) | (18.0–71.0) | (18.0–73.0) |
| Age, y a | |||
| <40 | 50 (49.5%) | 51 (50.0%) | 101 (49.8%) |
| ≥40 | 51 (50.5%) | 51 (50.0%) | 102 (50.2%) |
| Sex | |||
| Female | 44 (43.6%) | 42 (41.2%) | 86 (42.4%) |
| Male | 57 (56.4%) | 60 (58.8%) | 117 (57.6%) |
| NFS | |||
| 0–1 | 82 (83.7%) | 78 (82.1%) | 160 (82.9%) |
| 2–3 | 16 (16.3%) | 17 (17.9%) | 33 (17.1%) |
| Missing | 3 | 7 | 10 |
| MMSE | |||
| N | 95 | 92 | 187 |
| Mean (SD) | 28.3 (2.7) | 27.4 (4.5) | 27.9 (3.7) |
| Median | 29.0 | 29.0 | 29.0 |
| Q1, Q3 | 28.0, 30.0 | 27.0, 30.0 | 27.0, 30.0 |
| Range | (10.0–30.0) | (2.0–30.0) | (2.0–30.0) |
| MMSE Score | |||
| 0–27 | 21 (22.1%) | 26 (28.3%) | 47 (25.1%) |
| 28–30 | 74 (77.9%) | 66 (71.7%) | 140 (74.9%) |
| Missing | 6 | 10 | 16 |
| Tumor Location | |||
| Temporal lobe | 6 (5.9%) | 15 (14.7%) | 21 (10.3%) |
| Frontal lobe | 33 (32.7%) | 45 (44.1%) | 78 (38.4%) |
| Parietal lobe | 23 (22.8%) | 9 (8.8%) | 32 (15.8%) |
| Occipital lobe | 1 (1.0%) | 2 (2.0%) | 3 (1.5%) |
| Other/mixed | 38 (37.6%) | 31 (30.4%) | 69 (34.0%) |
| Preoperative Tumor Diameter* | |||
| <5 cm | 37 (36.6%) | 35 (34.3%) | 72 (35.5%) |
| ≥5 cm | 64 (63.4%) | 67 (65.7%) | 131 (64.5%) |
| Extent of Surgery* | |||
| GTR | 13 (12.9%) | 17 (16.7%) | 30 (14.8%) |
| STR | 37 (36.6%) | 34 (33.3%) | 71 (35.0%) |
| Biopsy | 51 (50.5%) | 51 (50.0%) | 102 (50.2%) |
| Histologic Subtype* | |||
| Astrocytoma | 32 (31.7%) | 31 (30.4%) | 63 (31.0%) |
| Oligodendroglioma | 69 (68.3%) | 71 (69.6%) | 140 (69.0%) |
| Original Grade (Kernohan)* | |||
| 1 | 5 (5.0%) | 5 (4.9%) | 10 (4.9%) |
| 2 | 96 (95.0%) | 97 (95.1%) | 193 (95.1%) |
| Enrollment Time Period | |||
| 1986–1991 | 47 (46.5%) | 48 (47.1%) | 95 (46.8%) |
| 1992–1994 | 54 (53.5%) | 54 (52.9%) | 108 (53.2%) |
Abbreviations: NFS, Neurologic Function Score; MMSE, Mini-Mental Status Examination; GTR, gross total resection; STR, subtotal resection; NCCTG, North Central Cancer Treatment Group.
aStratification Factor
Survival and PFS
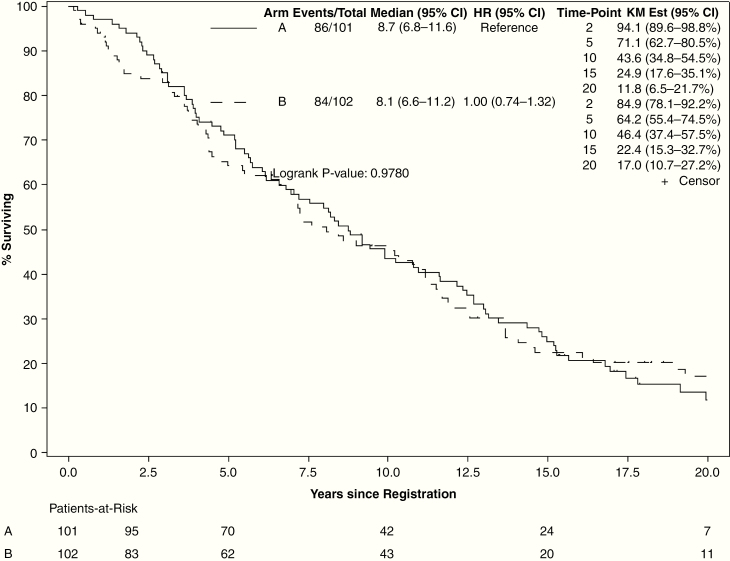
At final analysis, there were 33 patients alive (15 on the low-dose arm and 18 on the high-dose arm), with a median follow-up of 17.2 years (range, 0.4–29.5 y). For all evaluable patients, median OS was 8.4 years (95% CI: 7.2–10.8). High-dose radiation did not improve OS (hazard ratio [HR] = 1.00, 95% CI: 0.74–1.36; 15-y OS: 22.4% vs 24.9%, log-rank P = 0.98; Fig. 2). OS was significantly better for patients with preoperative tumor diameter <5 cm (HR = 0.47, 95% CI: 0.34–0.66; 15-y OS: 39.4% vs 15.2%, P < 0.001), baseline MMSE >27 (HR = 0.53, 95% CI: 0.37–0.77; 15-y OS: 27.3% vs 9.8%, P = 0.001), and for patients who underwent GTR (HR = 0.54, 95% CI: 0.33–0.90; 15-y OS: 39.3% GTR vs 16.4% subtotal resection vs 24.5% biopsy only, P = 0.012) (Supplement 1). Age (>40 vs ≤40 y) and sex were not associated with significant change in OS. For patients with astrocytoma (n = 63), OS was not improved with high-dose radiation (median OS: 6.9 y, 95% CI: 4.1–12.7) over low-dose radiation (median OS 5.4 y, 95% CI: 2.2–10.3, log-rank P = 0.60).
Fig. 2.
Overall survival by arm (arm A: 50.4 Gy in 28 fractions; arm B: 64.8 Gy in 36 fractions).
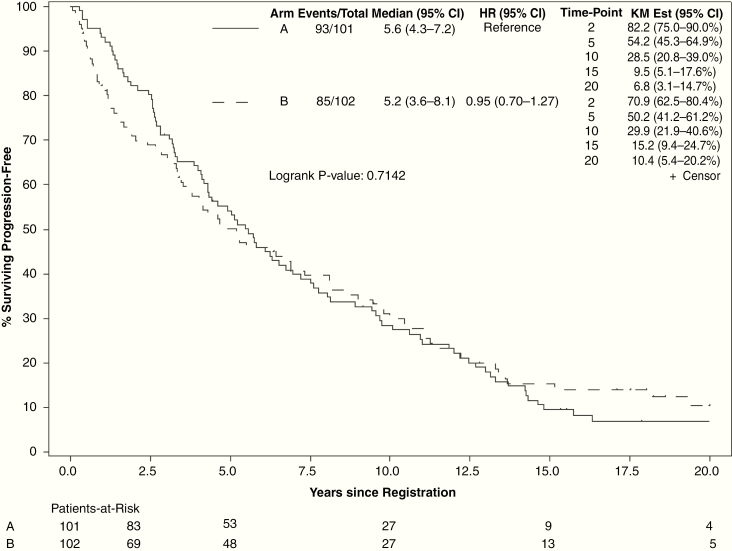
Median PFS for all evaluable patients was 5.2 years (95% CI: 4.3–6.6). At final analysis, 85 of 102 patients in the high-dose arm and 93 of 101 patients in the low-dose arm had progression events. High-dose radiation did not improve PFS (HR = 0.95, 95% CI: 0.70–1.27; 15-y PFS: 15.2% vs 9.5%, P = 0.71; Fig. 3). PFS was improved for patients with oligodendroglioma versus astrocytoma, preoperative tumor diameter <5 cm, patients who had GTR, and patients with baseline MMSE >27 (Supplement 2). Age >40 years and sex were not associated with significant change in PFS. For patients with histopathological astrocytoma, PFS was not improved with high-dose radiation (median PFS: 4.5 y, 95% CI: 3.1–7.2) over low-dose radiation (median PFS 1.7 y, 95% CI: 0.8–6.4, log-rank P = 0.67).
Fig. 3.
Progression-free survival by treatment arm (arm A: 50.4 Gy in 28 fractions; arm B: 64.8 Gy in 36 fractions).
Toxicity
We defined long-term toxicity as any AE reported at 5 years from study entry or later. Of 203 patients, 132 were alive for at least 5 years: 70 on the low-dose study arm and 62 on the high-dose study arm. Earlier toxicities (within the first 5 years after study entry) were reported previously.5
Seven patients on the low dose arm experienced at least one long-term AE, compared with 6 on the high dose arm. These events occurred from 5 to 14.9 years after study entry. Two patients on the low dose arm experienced long-term grade 3 or higher AEs, compared with 3 on the high dose arm. On the low dose arm, one patient had grade 4 serous otitis media from years 6 to 8.7, and another patient experienced grade 3 neuromotor and neurologic-CNS toxicities at the 10-year evaluation. Radiation necrosis was not reported for any patient on the low dose arm at year 5 or later. On the high dose arm, one patient experienced grade 3 radiation necrosis at 5.6 years, another patient experienced grade 4 neurologic-CNS toxicity at 5.3 years and grade II anorexia at 12.8 years, and a third patient developed grade 3 headache at 8 years.
MMSE
Of 203 patients, 187 had baseline MMSE testing. Of these, 140 had normal baseline MMSE (see Table 2). As demonstrated below, completion of the MMSE was poor over time, with less than 50% completion at all time points 5 years and beyond. For patients who had normal MMSE at baseline, at 7 years only 1 patient (5%) among those with an evaluation had a clinically significant decrease in MMSE from the previous time point, with the remainder (95%) stable. No patient with evaluations at the specified time points had a decrease in MMSE at 10, 12, or 15 years. Changes in NFS are reported in Supplement 3.
Table 2.
Long-term change in MMSE by treatment arm. Clinically significant change in MMSE score at key evaluations for patients without tumor progression
| Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | 7 | 8 | 10 | 12 | 15 | |
| Alive | 203 | 192 | 178 | 166 | 132 | 113 | 102 | 85 | 68 | 44 |
| Alive, baseline MMSE | 187 | 178 | 165 | 153 | 121 | 103 | 94 | 78 | 62 | 39 |
| Alive, prog-free, baseline MMSE | 187 | 162 | 141 | 126 | 91 | 69 | 63 | 45 | 34 | 18 |
| Abnormal baseline MMSE | 47 | 35 | 26 | 22 | 12 | 7 | 7 | 4 | 3 | 3 |
| n with MMSE | 47 | 25 | 19 | 16 | 7 | 2 | 4 | 2 | 1 | 1 |
| Decrease | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Increase | 10 | 9 | 6 | 1 | 1 | 2 | 1 | 0 | 0 | |
| Stable | 14 | 9 | 9 | 6 | 1 | 2 | 1 | 0 | 1 | |
| Normal baseline MMSE | 140 | 127 | 115 | 104 | 79 | 62 | 56 | 41 | 31 | 15 |
| n with MMSE | 140 | 97 | 87 | 69 | 42 | 20 | 20 | 12 | 3 | 1 |
| Decrease | 7 | 3 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | |
| Stable | 90 | 84 | 67 | 40 | 19 | 19 | 12 | 3 | 1 | |
Discussion
In this final report of high-dose versus low-dose radiation for adult supratentorial LGG, there was no significant difference observed between groups with respect to OS or PFS. This is consistent with our initial report, as well as with the findings of EORTC 22844.4,5 These findings support current guidelines that dose escalation is not needed for most patients with LGG.11
With a median follow-up of over 17 years for surviving patients, we are able to provide long-term outcomes. At 15 years, 22.4% of patients who received high dose radiation were alive, versus 24.9% of those who received low dose radiation (P = 0.98). Factors associated with improved OS included GTR (vs STR or biopsy only), baseline MMSE >27, and preoperative tumor diameter <5 cm. Similar to the analysis of EORTC trials, preoperative tumor diameter and baseline neurologic function were significantly associated with survival.12 Supporting the stratification utilized on RTOG 9802, GTR was a favorable factor.2
Long-term PFS also did not appear to differ between groups, with 15.2% of patients who received high-dose radiation free of disease progression at 15 years, versus 9.5% of patients who received low-dose radiation. At 20 years, 9 patients (5 on the high-dose arm and 4 on the low-dose arm) had follow-up and remained free of progression. At 25 years, 2 patients had follow-up and remained free of progression (both on the high-dose arm). It is unclear whether patients free of progression at these late time points may represent the possibility of “cure.”
Late toxicities (defined as occurring at 5 years after study entry or later) were relatively uncommon on either arm, with late grade 3+ toxicities occurring in approximately 10% of patients with adequate follow-up on both arms. In our initial analysis we demonstrated increased incidence of radiation necrosis with high-dose radiation.5 Only one patient (on the high-dose arm) developed late radiation necrosis at 5.6 years, with no radiation necrosis noted in any patient beyond that time point. Given the relatively low rates of toxicities described here and in the initial publication, underreporting of toxicity on this study is a possibility.
Long-term changes in NFS and MMSE did not differ significantly between groups at late follow-up. Because the compliance of long-term MMSE and NFS was incomplete, any conclusion from these data must be drawn with caution. Still, these results indicate that treatment-related toxicities and neurocognitive decline may be most likely to occur in the years immediately following treatment, and are less likely to develop years later for long-term survivors. For patients who had abnormal MMSE or NF scores before treatment, improvements in neurologic function appeared to be more likely than declines after treatment (Table 2 and Supplement 3). This demonstrates the potential of radiation therapy to provide long-term improvements in performance status for these patients.
This study has limitations. Patients were classified by histopathology and not molecular classifiers, which are integral to diagnostic and therapeutic decisions in modern practice. The absence of molecular data in this study may limit its applicability. Patients were treated in an era with older surgical and radiation techniques. Patients were not treated with adjuvant chemotherapy, which has since become standard after RTOG 9802 demonstrated that the addition of procarbazine, lomustine, and vincristine (PCV) to radiation improved OS in patients who had STR or were older than 40 years of age.2 In this study, cognitive decline was assessed using NFS and MMSE, which have limited sensitivity compared with more extensive cognitive batteries. Furthermore, compliance with completion of the MMSE was poor over time. This study did not contain a patient-reported quality of life component. EORTC 22844 did report on quality of life, and showed worse acute fatigue/malaise and insomnia, and worsening in leisure time and emotional functioning 7–15 months after randomization for patients receiving high-dose radiation.13
More recent and current ongoing trials are not using high-dose radiation for LGG, but are assessing optimal chemotherapy, modern radiotherapy techniques, and stratification by molecular factors. For example, the EORTC 22033–26033 intergroup clinical trial randomized patients with low-grade glioma to 50.4 Gy in 28 fractions as the standard treatment arm versus temozolomide alone, with patients prospectively stratified by molecular factors.14 The CODEL trial (ClinicalTrials.gov Identifier NCT00887146) is currently randomizing patients with 1p/19q codeletion to radiation followed by adjuvant PCV versus radiation with temozolomide followed by adjuvant temozolomide.15 NRG BN005 (ClinicalTrials.gov Identifier NCT03180502) randomizes patients with isocitrate dehydrogenase‒mutated LGG to proton versus photon therapy (both study arms receive adjuvant temozolomide) and prospectively assesses cognitive and quality of life outcomes.
In this phase III randomized clinical trial of high-dose versus low-dose radiation for adults with supratentorial low-grade glioma, no OS or PFS benefit was detected with dose escalation over a long-term follow-up. Patients with smaller tumors (<5 cm) and better baseline mental state (MMSE >27) and who had GTR at surgery had improved survival. Late toxicities and neurocognitive decline were uncommon.
Supplementary Material
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), https://acknowledgments.alliancefound.org; U10CA180790, U10CA180868 (NRG), CA-15083, CA-35415, and the Linse Bock Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement
No conflicts of interest exist for any of the authors.
Authorship statement
Analysis and interpretation: WGB, PDB, KVB, BPO, WJC, RAA, NNL, RL, EG, JCB, EGS. Data analysis: KSA, XWC. Experimental design: EGS.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckner JC, Shaw EG, Pugh SL, et al. . Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw EG, Daumas-Duport C, Scheithauer BW, et al. . Radiation therapy in the management of low-grade supratentorial astrocytomas. J Neurosurg. 1989;70(6):853–861. [DOI] [PubMed] [Google Scholar]
- 4. Karim AB, Maat B, Hatlevoll R, et al. . A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. [DOI] [PubMed] [Google Scholar]
- 5. Shaw E, Arusell R, Scheithauer B, et al. . Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20(9):2267–2276. [DOI] [PubMed] [Google Scholar]
- 6. O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 7. Wieand S, Schroeder G, O’Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med. 1994;13(13-14):1453–1458. [DOI] [PubMed] [Google Scholar]
- 8. Brown PD, Buckner JC, O’Fallon JR, et al. . Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination. J Clin Oncol. 2003;21(13):2519–2524. [DOI] [PubMed] [Google Scholar]
- 9. Daniels TB, Brown PD, Felten SJ, et al. . Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys. 2011;81(1):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 11. Central Nervous System Cancers, Version 1.2019. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2019; Version 1.2019:https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed July 30, 2019.
- 12. Pignatti F, van den Bent M, Curran D, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organisation for Research and Treatment of Cancer Radiotherapy Cooperative Group Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 13. Kiebert GM, Curran D, Aaronson NK, et al. . Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co-operative Group. Eur J Cancer. 1998;34(12):1902–1909. [DOI] [PubMed] [Google Scholar]
- 14. Baumert BG, Hegi ME, van den Bent MJ, et al. . Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaeckle K, Vogelbaum M, Ballman K, et al. . CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071, NCIC-CEC-2): phase III randomized study of RT vs. RT plus TMZ vs. TMZ for newly diagnosed 1p/19q-codeleted anaplastic oligodendroglial tumors. Analysis of patients treated on the original protocol design. Neurology. 2016;86(16):PL02.005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.