Abstract
Gliomas are the most common primary central nervous system tumors occurring in children and adults with neurofibromatosis type 1 (NF1). Over the past decade, discoveries of the molecular basis of low-grade gliomas (LGGs) have led to new approaches for diagnosis and treatments. However, these new understandings have not been fully applied to the management of NF1-associated gliomas. A consensus panel consisting of experts in NF1 and gliomas was convened to review the current molecular knowledge of NF1-associated low-grade “transformed” and high-grade gliomas; insights gained from mouse models of NF1-LGGs; challenges in diagnosing and treating older patients with NF1-associated gliomas; and advances in molecularly targeted treatment and potential immunologic treatment of these tumors. Next steps are recommended to advance the management and outcomes for NF1-associated gliomas.
Keywords: gliomas, immunotherapy, molecular-targeted therapy, neurofibromatosis type 1, pilocytic astrocytomas
Over the past decade remarkable advances have been made in the understanding of the molecular pathogenesis of low-grade gliomas (LGGs), especially those occurring in childhood. With the discovery in 2007 that the majority of pediatric pilocytic astrocytomas were driven by identifiable aberrations of genes encoding proteins of the RAS–mitogen-activated protein kinase (MAPK) pathway, there was a new conceptualization and understanding of these tumors.1,2 Furthermore, with the recognition that different types of genetic alterations in this pathway may underlie these LGGs, including tumors that are considered World Health Organization (WHO) grade II or infiltrating lesions, biopsies for the molecular characterization of sporadic LGGs have become more routine and essentially mandatory in many situations.3 Neurofibromatosis type 1 (NF1), an autosomal-dominant condition with a prevalence of approximately 1 in 3000, has a myriad of manifestations, including the development of LGGs.3 Separation of non-NF1-associated pediatric low-grade tumors by mutational status, such as BRAF fusion and V600E mutation determination, was recommended by a consensus conference held with experts in the field.3 The increased molecular characterization of sporadic pediatric LGGs revealed that other less common fusions and mutations may occur and, in total, compose nearly 20% of all genetic alterations in these tumors.2–5
Histologically unconfirmed presumed LGGs, which can occur in as many as 20% of children with NF1, are often diagnostically and therapeutically managed differently than their sporadic counterparts.3,6 Given the erratic and often indolent natural history of such tumors coupled with the lack of need of antitumor treatment for many patients, biopsy and/or surgical removal is performed less frequently than for sporadic tumors. The molecular characterization of these tumors is less likely to be performed. Even in those tumors that require treatment, therapy may be different due to the relative responsivity of these lesions compared with non-NF1-associated tumors, as well as the tendency of some NF1-associated gliomas, especially those of the visual pathway, to stabilize, senesce, or even involute without treatment.
Prior consensus conference recommendations have suggested that biopsies were not required for the majority of NF1-associated presumed LGGs,3,6 as most will be pilocytic astrocytomas and treatment can be based predominantly on clinical and radiographic features. However, this recommendation has been called into question with the findings of one recent large-scale genomic study which revealed that the molecular genetics of gliomas in children and young adults with NF1 are more complex than initially realized.7 Some of these findings are consistent with the molecular alterations identified by other researchers in a study of “anaplastic astrocytoma with piloid features,” a tumor type often harboring NF1 gene mutations in addition to other mutations not typically seen in pilocytic astrocytomas or sporadic high-grade gliomas (HGGs).8 Additional molecular abnormalities which may occur, in addition to NF1 loss, include concomitant mutations in cyclin-dependent kinase inhibitor (CDKN)2A/B and alpha thalassemia/mental retardation syndrome X-linked (ATRX). The concept of transformed LGGs in children and especially young adults with NF1 has taken on greater credence, with “transformed” tumor showing a more aggressive phenotype; a phenotype that is driven by a more complex mutational pattern and may be pathogenic in the majority of HGGs occurring in this patient population, especially in adults.
Given these new complexities, a consensus conference with experts in NF1 and LGGs was held, reviewing the biology of pediatric and adult gliomas in patients with NF1, the status of preclinical information available concerning such tumors including animal modeling and the results of therapeutic studies. Specific recommendations were made regarding the management of NF1-LGGs and the necessary next steps to improve the understanding and ultimately outcome of affected patients.
Diagnosis
Historically, biopsy has been deferred for children with NF1-LGGs, given the typical imaging appearance, concern for functional decline following biopsy in a sensitive location such as the optic pathway or brainstem, and the assumption that no additional molecular abnormalities beyond the loss/mutation of both NF1 alleles would be found.3,4 Although emerging experience suggests that biopsy of a brainstem mass can be performed with minimal permanent risk to function,9 there continue to be concerns about the risk of visual decline with biopsy of the optic nerve or chiasm.
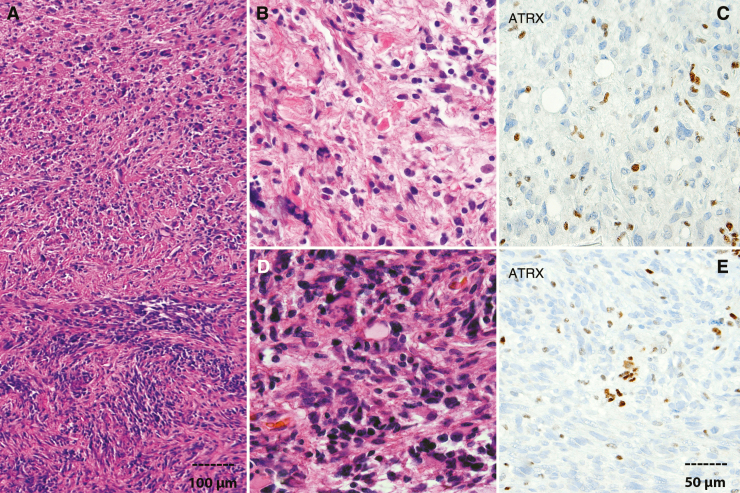
Routine histologic evaluation allows for the classification and grading of most gliomas, although a subset of gliomas, predominantly low grade, developing in the context of NF1 in older patients, remain difficult to classify. However, the development of anaplasia in tumors with pilocytic histology is increasingly recognized, especially in adults, and associated with more aggressive behavior in adults and NF1-associated gliomas.10 In a recent study, Reinhardt et al identified a group of aggressive gliomas termed anaplastic astrocytoma with piloid features as a molecular subgroup based on methylation profiling.8 Many of these tumors correspond histologically to pilocytic astrocytomas with anaplasia, although not all tumors in the molecular subgroup satisfy histologic criteria for anaplasia, which has traditionally been applied to tumors with overt features of pilocytic astrocytoma (at least in part), including Rosenthal fibers, eosinophilic granular bodies, concurrent with aggressive features typical of conventional HGGs, particularly brisk mitotic activity and necrosis11 (Fig. 1). Clinical evidence of NF1 or NF1 mutations are overrepresented in these groups, but sporadic tumors and other MAPK pathway alterations may also have these molecular features and histologic characteristics. However, it is not possible to identify the NF1-associated tumors based on morphologic features alone.
Fig. 1.
Anaplastic astrocytoma with piloid features (pilocytic astrocytoma with anaplasia) arising in the cerebellum of a 29-year-old NF1-patient. Well-differentiated pilocytic astrocytoma component (top a, b) and sharp interface with an anaplastic component characterized by high cellularity and brisk mitotic activity (bottom a, d). ATRX loss detected by immunohistochemistry in well-differentiated (c) and anaplastic (e) components. Positive cells in the stroma and vessels serve as an important internal control (scale bar representing 100 µm applicable to panel a; scale bar representing 50 µm applicable to panels b–e).
Of interest, loss of ATRX expression is associated with NF1-associated diffuse LGGs and HGGs, and it is also a feature of pilocytic tumors with anaplasia.10 This marker may be tested through immunohistochemistry during routine diagnostic workup, with the caveat that a small number of tumors with ATRX mutations, and presumed loss of function, have intact protein levels. Despite this limitation, testing for ATRX loss is advisable in NF1-associated gliomas believed atypical enough to biopsy, as loss of ATRX function may be associated with a more aggressive natural history than suggested by histologic features alone. These data can be particularly informative in histologically low-grade tumors where there is clinical concern for more aggressive behavior. Conversely, dedicated testing for IDH1 and IDH2 mutations is not a priority when there is a limited sample, as mutations in these genes, with few exceptions and in contrast to sporadic gliomas, are not a feature of NF1-associated gliomas. In addition to ATRX, CDKN2A/p16 inactivation is an important event in the process of malignant conversion in NF1-associated tumors. This has been best studied in NF1-associated peripheral nerve sheath tumors such as atypical neurofibromas and malignant peripheral nerve sheath tumors (MPNSTs).11–13 This is less well established in the setting of NF1 glioma.14
Biopsy specimens obtained from individuals with NF1 are often small, and it is imperative that they be triaged appropriately for diagnostic accuracy. This is important particularly for tumors involving areas of difficult access, including the optic pathway and brainstem. Priority should be given to formalin-fixed paraffin-embedded (FFPE) tissue for an initial accurate pathologic diagnosis and grading, which frequently requires routine hematoxylin and eosin stained sections and immunohistochemistry. Ancillary molecular testing can also be routinely performed on FFPE sections using a variety of commercial or in-house next-generation sequencing platforms. If enough tissue is available at the time of surgery after the sample for clinical diagnostics has been selected, it is helpful to immediately (snap) freeze a portion and keep it at −80°C for more comprehensive molecular testing if required, which may be guided by current or future clinical trial enrollment criteria.
Recommendations:
Histologic diagnosis, although not indicated for all NF1-associated LGGs, especially in children, is required in potentially “transformed” gliomas of childhood becoming symptomatic or enlarging in adulthood, possibly in those tumors that progress despite empiric therapy, in all tumors first discovered in adulthood, and possibly in atypical, “aggressive” tumors in childhood.
Biopsy specimens of presumed gliomas in individuals with NF1 must be evaluated judiciously prioritizing immunohistochemical assessment of genes frequently mutated (such as ATRX and CDKN2A/B) rather than genes more commonly mutated in sporadic low-grade and anaplastic gliomas.
The impacts on management and outcome of biopsy results in patients with NF1-LGGs should be studied prospectively and evidence-based guidelines developed for indications for biopsy in both children and adults.
Molecular Understandings and Diagnostic/Therapeutic Implications
Recent comprehensive molecular profiling studies of a large number of pediatric NF1-LGG samples confirmed that most pediatric NF1-LGG contain only inactivating alterations of both NF1 alleles, as previously described.15 However, a subset (<15%) of patients harbored additional mutated genes,8 which does not increase in frequency in older children. In contrast, gliomas in adults with NF1 are more likely to exhibit high-grade histology (~75% of tumors), as well as additional genetic aberrations beyond biallelic NF1 inactivation.7
The first study from the LANDING (genomic LANDscape In NF1-mutant Glioma) Consortium examined the molecular alterations in pediatric and adult LGG and HGG from patients with NF1.7 In this analysis, high- but not low-grade NF1 gliomas harbor loss-of-function genetic alterations of ATRX, CDKN2A, and TP53, similar to a previous report demonstrating co-mutation of NF1, ATRX, and CDKN2A/B in a molecularly defined subset of isocitrate dehydrogenase (IDH) wildtype HGG (anaplastic astrocytoma with piloid features).8 Recently, DNA methylation profiling has emerged as an important tool for the accurate classification and identification of molecular subtypes of brain tumors in children and adults.14,16 The application of DNA methylation profiling by The Cancer Genome Atlas originally classified NF1 glioma (both low and high grade tumors) within the LGm6 IDH wildtype cluster of sporadic glioma.14 In this context, an important role of ATRX inactivation was shown, with ATRX mutations predicting poor clinical outcome among WHO grade III sporadic tumors as well as in the NF1-glioma cohort.7 When examining these data more closely, however, a further subdivision can be found. In the “pilocytic astrocytoma (PA)–like” subset of LGm6, for example, of sporadic pilocytic astrocytomas it has been shown that some tumors indeed closely match reference pilocytic astrocytoma samples and some to glioblastoma (GBM), but a further substantial proportion matches the methylation signature for anaplastic pilocytic astrocytomas.8
Particularly for pediatric tumors, further work will be required to confirm DNA methylation–based subgrouping. In this regard, preliminary data from the Synodos for NF1 Pediatric Glioma Consortium suggests that while pediatric NF1-gliomas mostly resemble their sporadic PA counterparts, some tumors more closely resemble other classes, including dysembryoplastic neuroepithelial tumor, anaplastic astrocytoma with piloid features, or rosette-forming glioneuronal tumor (D.T.W.J., S.M.P., M.J.F., D.H.G., unpublished data). Interestingly, these subgroups also appeared to be linked with certain co-occurring mutations. For example, NF1-associated tumors with a methylation profile resembling rosette-forming glioneuronal tumors were found to harbor co-mutation of FGFR1 and PIK3CA, as recently reported for their sporadic counterparts.17 These data suggest that a more precise molecular classification of NF1-associated tumors may elucidate their specific molecular drivers.
Genetic alterations of ATRX, CDKN2A, and TP53 characterize tumors histologically diagnosed as pilocytic astrocytoma in adolescents and young adults with NF1 (or occurring as sporadic tumors), but classified as anaplastic astrocytomas with piloid features or by DNA methylation profile (LGm6 subgroup). These tumors exhibit a more aggressive clinical behavior. With additional analysis, the application of DNA methylation profiling and next-generation sequencing to gliomas in patients with NF1, and particularly in those with non–optic pathway gliomas (OPGs), or occurring in adolescents and adults will be of utmost importance to inform the optimal clinical follow-up.
With the application of genomic analyses to NF1-associated LGGs and HGGs, additional targets for therapeutic drug design may emerge.8 These future therapeutically actionable nodes include cooperating genetic alterations that impact on cancer cell cycle progression (eg, TP53, CDKN2A), chromatin remodeling/telomere maintenance (eg, ATRX, alternative lengthening of telomeres [ALT]), and mitogenic growth control pathways that intersect with neurofibromin loss/RAS hyperactivation (eg, FGFR1, PTPN11, PIK3CA).
An additional notable finding from the study reporting the molecular landscape of NF1 glioma was the identification of a high-immune subgroup of NF1-LGGs characterized by abundant CD3 and CD8-positive T-lymphocyte infiltration and mutation-derived neoantigens.8 These CD8 T-lymphocyte infiltrates exhibited positivity for granzyme B, the key cytolytic effector that is upregulated upon CD8+ T-cell activation and productive responses to immunotherapies.18 These findings suggested that a group of LGGs in NF1 patients may have retained the ability to restrict tumor growth through an active immune response and possibly prevent progression toward more aggressive glioma subtypes. Coupled with experimental evidence in preclinical murine models supporting a role for infiltrating T lymphocytes in the pathogenesis of NF1-associated LGGs,19,20 such information provides critical evidence to guide immunologic therapeutic interventions.19,20 Furthermore, NF1 patients with the high-immune subgroup of NF1-LGGs might uniquely benefit from immune therapy approaches such as checkpoint inhibitors.
However, the clinical relevance of T-lymphocyte infiltrates and mutation-derived neoantigens in NF1 glioma will have to be established as it remains unknown whether such infiltrates or neoantigens are associated with a more indolent course of the disease and actually prevent glioma progression. In particular for pediatric patients, where the overall mutational burden (and hence neoantigen load) is extremely low, more data are required as to the significance of these findings. Furthermore, the cellular complexity of the immune microenvironment reported in the mouse models of LGGs sustained by loss of NF119 indicates that future studies will have to determine at the single cell level the full molecular and clinical significance of the immune microenvironment of gliomas occurring in NF1 patients. Such analyses will probably require the deconvolution of individual immune cells from global transcriptome analyses and the direct interrogation of the range of cellular states that characterize the tumor microenvironment of gliomas in NF1 patients by single cell functional genomics resolution and other methods such as flow cytometry staining (fluorescence activated cell sorting) and immunostaining.
Still another aspect of NF1 glioma pathogenesis that is poorly understood is senescence. It is well recognized that LGGs in pediatric patients may cease to grow or even involute as a child ages; this is especially true in visual pathway gliomas in children with NF1 after age 5 or 6.3,6 However, the biologic underpinnings of LGG senescence, be they molecular, immunologic, or by other means, are essentially unknown.
Recommendations:
Molecular diagnostics, or at a minimum appropriate surrogate immunohistochemical markers for identification of concomitant mutations, in addition to NF1, are required for full characterizations of NF1-associated anaplastic piloid tumors and glial tumors in adults, as well as atypical aggressive LGGs in children.
In particular ATRX, CDKN2A, and TP53 testing should be conducted in each circumstance of pediatric LGGs in which progression to a more aggressive behavior might be suspected, as well as all gliomas arising in adults with NF1; testing of FGFR1 and PIK3CA should also be considered for all pediatric NF1-associated tumors.
Such molecular findings should be considered in developing treatment and surveillance plans.
Further study of the immunologic milieu of NF1-associated HGGs and LGGs are indicated.
The biologic underpinnings of NF1-LGG-associated senescence require study.
Modeling
Mouse Modeling of Nf1 Optic Glioma
Given the paucity of human surgical specimens available for study and the difficulties encountered when attempting to establish patient-derived xenograft models, much of our understanding of the pathogenesis of NF1-associated optic gliomas has derived from the use of genetically engineered mouse models. Numerous murine models of Nf1 optic glioma have been established that couple a germline Nf1 gene mutation with somatic Nf1 loss in neuroglial progenitor cells during embryonic development (Supplementary Table 1).21,22 Using these strains, the impact of the germline Nf1 mutation,23 additional genomic alterations,24 cell of origin,25,26 timing of somatic Nf1 loss,27 and immune cell infiltration (microglia, T cells)19,28–30 on optic glioma pathogenesis have been demonstrated. In this regard, mice with different germline Nf1 gene mutations exhibit different tumor penetrance and proliferation, while those with additional genomic alterations (eg, Pten mutation) have increased tumor proliferation and volumes.20,23,24 Similarly, mice with different cells of origin (eg, neural stem cells, astrocytes) or timing of somatic Nf1 loss have different tumor penetrance and latency.25–28 In addition, these different mouse strains have revealed that glioma growth is dictated in large part by the ability of tumor cells to recruit T cells and microglia and create a supportive tumor microenvironment.20 These mice models have afforded extraordinary opportunities to define the temporal course of cellular and molecular events that culminate in optic glioma development, progression, and glioma-associated vision loss.31
Mouse Modeling of Nf1 High-Grade Glioma
In contrast to LGG, neurofibromin loss alone is not sufficient to induce NF1-associated HGGs in either patients or mouse models. Instead, the accumulation of additional genetic alterations, particularly in the CDKN2A/p53 pathway, is required for high-grade gliomagenesis. Consistent with mathematical modeling results in human HGGs,32 genetic studies using mouse models have demonstrated that a sequential loss of p53 and Nf1 are required for generating HGGs, including GBMs,33–36 sometimes promoted by Pten loss.37,38 In addition, the microenvironment created by GBMs, important for their maintenance, is partly dictated by the spectrum of cancer-associated genetic changes in the cancer cells.39
In general, 2 types of models have been developed based on the cell type(s) targeted with GBM-associated mutations (including NF1) or “cell-of-mutation” (Supplementary Table 2). One is to use a tissue-specific promoter—for example, hGFAP-cre (human glial fibrillary acidic protein promoter to direct Cre-dependent recombination), which targets both embryonic and adult neural stem cells as well as mature astrocytes (Supplementary Table 2). The other is to employ an inducible strategy that targets neural stem or progenitor cells in the adult brain (Supplementary Table 2). Despite critical differences in targeting strategies, all these models suggest that neural stem cells or their closest progeny—immature progenitors in the subventricular zone (SVZ) are the cells-of-origin for NF1-associated HGGs and GBMs. It should be noted that in contrast to an earlier study, cells of a relatively mature neuronal lineage are resistant to HGG and GBM formation. A recent study provides the evidence that GBM founder mutations were observed in the SVZ of human brains with IDH-wildtype GBMs.
Preclinical Translation
The availability of Nf1 low-grade and malignant glioma mouse strains provides tractable experimental platforms for preclinical translation. As such, Nf1 optic glioma mice have also been critical for the identification and preclinical evaluation of promising therapies prior to their translation to children with NF1-OPG,40 including mechanistic target of rapamycin (mTOR), mitogen-activated extracellular signal-regulated kinase (MEK), and phosphatidylinositol-3 kinase (PI3K) as targets for biologically based therapies.41–43 Moreover, additional molecular targets for future therapeutic drug design have been discovered using these strains, including microglia and microglia-produced tumor growth factors.19,29 Similarly, Nf1 optic glioma mice have been instructive in elucidating the mechanisms underlying optic glioma–induced vision loss,44–46 including why girls with NF1-OPG are more likely to experience visual impairment from their tumors relative to their male counterparts. In Nf1 genetically engineered mice, this sexually dimorphic effect is partly due to estrogen activation of microglia, which results in retinal ganglion cell death and vision loss in Nf1 optic glioma mice.47,48 Similar studies using malignant glioma strains may reveal other molecular and cellular dependencies amenable to therapeutic intervention, including growth factor and chemokine signaling networks.44–46 Future deployment of these genetically engineered mice for effective clinical translation should consider the incorporation of numerous strains that better represent the spectrum of clinical heterogeneity seen in patients with NF1, the design of preclinical trials that more accurately mirror those used for human clinical trials, co-targeting the neoplastic and non-neoplastic cells that control glioma growth, and a greater emphasis on attenuating vision loss in NF1-OPG.49 Moreover, the use of patient-derived tumor xenografts, human-induced pluripotent stem cell tumor models, and other experimental optic glioma models50 from other species is likely to increase knowledge of molecular and immunologic mechanisms of tumor development and growth.
While general preclinical modeling has been hampered by the difficulty in establishing immune competent models, immunocompetent murine models of NF-associated CNS tumors exist; these mice harbor Nf1 heterozygosity with somatic mutation in neuroglial progenitors leading to OPG development.9 This resource enables the potential generation of robust preclinical modeling in the pursuit of appropriate and effective immunotherapy in NF. However, it should be appreciated that while these preclinical models share some similarities with their human counterparts (ie, location within the optic nerves and chiasm, associated vision loss), they lack the characteristic pilocytic histological features (ie, eosinophilic granular bodies, Rosenthal fibers) and develop later than typically seen in children with NF1-OPG (based on equivalent species ages).
Recommendations:
Mouse models of childhood NF1-associated gliomas should be further refined and explored to better delineate pathogenesis and to identify stromal factors which cooperate in glial tumor development.
Models of anaplastic “piloid” and transformed LGGs arising in adulthood are needed.
Models better representing the genetic heterogeneity of NF1 “anaplastic” gliomas, including those derived directly from tumor tissue, need to be developed and interrogated.
Immunocompetent models of NF1-associated gliomas are needed, especially to better inform the immunologic pathophysiology underlying tumor development, growth, and possibly senescence.
Clinical Management and Therapeutic Approaches
General Aspects
In the pediatric and adolescent age group, NF1-associated LGG is often an indolent disease. In general, children with NF1 and MRI findings consistent with LGG receive tumor-directed therapies if they experience symptomatic progression such as functional deterioration, most commonly visual decline associated with OPG.51,52 However, most clinical trials to date have included patients with radiographically documented growth and/or lesions considered likely to threaten neurologic function or vision on study without objective documentation of functional loss. Because of the 15–20% incidence of OPGs in children with NF1, screening via ophthalmologic examinations is recommended annually until age 8 years (the age of highest risk), and then every other year until 18 years of age, after which the development of OPGs is exceedingly rare.53 Screening for LGG in other locations is not performed routinely, but rather prompted by suspicion based on history, physical examination findings, or symptoms. There is consensus that newly diagnosed NF1-LGG should be monitored with both imaging and ophthalmology for optic pathway tumors; however, there is limited data regarding the ideal frequency of such monitoring.53
There are mixed data regarding the behavior of extra-optic gliomas in adults with NF1.54,55 However, most pediatric studies suggest that such lesions in the brainstem and other regions of the brain respond similarly to chemotherapy as visual pathway lesions. As noted previously, HGGs in adults with NF1 have been shown to have a more aggressive course than their low-grade counterparts; however, there is some evidence indicating that adults with NF1 and HGG may have better outcomes than HGGs in the general population.56 Conversely, increasing experience suggests that pilocytic astrocytomas and other LGGs behave more aggressively in adults with NF1 than their histology would predict.57 In retrospective series, extra-optic location, age greater than 18 years, and progressive symptoms were associated with shorter survival independently of low-grade histology.54,58 Recent series evaluating the impact of ALT and loss of ATRX suggest that these alterations are predictive of aggressive tumor behavior. Specifically, ALT is present in 60% of histologically defined HGGs, but only 19% of histologically defined LGG. Interestingly, only 2/26 “classic” NF1-associated PA had ALT versus 21/44 non-classical NF1-associated PA gliomas and ALT status was a major determinant of survival (median overall survival 18 mo ALT positive vs normal telomere length not reached, or 69 mo for long telomeres even when adjusted for patient age and histological grade).26 In the future, routine assessment of molecular diagnostics in samples from adults with NF1 and glioma and in children, who because of growth rate, unusual location, or lack of response to therapy have undergone biopsy or resection, hold the promise of better characterization of molecular features that may distinguish aggressive gliomas from more indolent lesions. These findings may be the critical data needed to comfortably recommend surveillance versus aggressive management, and ultimately may provide novel therapeutic targets for the aggressive gliomas associated with very short survival data.
Currently, the mainstay of initial therapy for children with NF1-LGG is classic chemotherapy using carboplatin, most often carboplatin + vincristine (CV); vinblastine monotherapy59,60 has also been used. Surgical resection may be warranted in symptomatic NF1-associated LGG; however, this is not usually feasible or safe in patients whose tumors are located within the optic pathway or brainstem. Radiotherapy is avoided in these children given the risk of late effects such as secondary malignancy, neurocognitive decline and moyamoya syndrome.61 However, it should be noted that the relative enhanced risk of secondary tumors or treatment-related malignant transformation has not been demonstrated by prospective studies. Retrospective studies have demonstrated a somewhat higher incidence of new malignancies in children with NF1 receiving radiotherapy, compared with those not treated with radiotherapy.62 Alkylating chemotherapeutic agents have also been avoided given the risk of secondary malignancy.
For adults, there is no NF1-specific treatment paradigm and patients are generally treated according to the histological pathology; as such, patients with pilocytic astrocytomas are often managed with surveillance. Those with diffuse LGG may be offered radiation therapy and individuals with HGG are usually offered conventional radiation, at times with concurrent temozolomide (personal communication, J.B.). However, there is tremendous variability in practice given the uncertain prognosis with both low and high grade histologies, the incompletely documented toxicity and mutagenesis of radiation therapy in adults with NF1, and the emerging molecular diagnostic and treatment landscape for NF1 tumors.
Chemotherapy
The regimen of CV is associated with a 5-year event-free survival of 69% ± 4%60 in children with NF1-associated LGGs. Although there are limited prospective data on functional outcomes following chemotherapy, a retrospective review of visual acuity (VA) in NF1-associated OPG using carboplatin-containing regimens in previously chemotherapy-naïve patients revealed that at the completion of chemotherapy, VA improved in 32%, remained stable in 40%, and declined in 28%.63 A variable correlation was found between tumor response by imaging and visual outcome, indicating that each endpoint should be incorporated into future trials, using standardized assessment methods and clearly defined measurements of vision.63 There is still a proportion of patients who progress during or after completing classic chemotherapy or have vision deterioration requiring alternate treatment strategies.3,6
Molecularly Targeted Therapy
Based on the knowledge that alterations in the MAPK pathway including the interaction with the aberrations common to NF1 lead to LGG propagation,3,6 numerous novel agents are now in clinical trials targeting the MAPK pathway, such as direct MEK or BRAF inhibitors. BRAF inhibitors have not been studied extensively in NF1 patients given that the initial agents were developed to target the BRAF V600e mutation (which is not typically seen in NF1-LGG) and the theoretic concern, demonstrated to likely be true by one small series using sorafenib, that such agents would have a paradoxical effect and increase the growth of tumors in those with NF1 or BRAF fusions.3,6,64 In contrast, MEK inhibitors have been studied extensively in NF1. Selumetinib (AZD6244), a selective and oral small molecule inhibitor of MEK1/2, has the most robust data to date for NF1-LGG with a 40% response rate, stable to improved VA in visual pathway glioma patients and 2-year progression-free survival (PFS) of 96% demonstrated in a phase II trial.65 Based on these results, the Children’s Oncology Group has opened a randomized clinical trial comparing selumetinib with CV in patients with chemotherapy-naïve NF1- LGG. Other MEK1/2 inhibitors, including trametinib and binimetinib (MEK-162), also show promise for refractory/recurrent pediatric LGG in early-phase trials; however, the numbers of children with NF1 on these trials are currently too few to make definitive conclusions.66–68 The response rate may be similar to selumetinib based on the agents’ similar mechanism of action (ATP non-competitive inhibitor of MEK1/2), but this needs to be substantiated by prospective trials.
Other molecularly targeted therapies and “biologic agents” have shown some degree of efficacy in children with NF1-associated LGGs. The anti-angiogenic drug bevacizumab has been investigated in patients with recurrent disease, singly or coupled with irinotecan, has demonstrated a 40–60% radiographic response rate, and has resulted in clinical improvement (including visual improvement in acuity and field) of over 40% of those treated.69–71 Response may only be transient and deterioration may occur after stoppage of drug; although some patients have maintained their clinical benefit for months to years after treatment. There is an ongoing study enrolling newly diagnosed patients with LGGs, including those with NF1, on treatment with vinblastine alone or vinblastine plus bevacizumab. The goals of treatment of this study are to determine not only the radiographic response, but also the clinical benefit to patients. The hypotheses underlying this treatment approach are that bevacizumab will result in a greater occurrence of visual and neurologic improvement while the vinblastine will result in longer disease control after the 6 months of treatment compared with the historical experience with bevacizumab alone. It should be noted that the latter experience was obtained only in children with recurrent NF1-LGGs.
The use of mTOR inhibitors has been explored in pediatric LGGs including in those children with NF1.72 Overall radiographic response rate was less (<20%) compared with that observed with other molecular agents; however, some patients with recurrent disease experienced long-term disease control while on and after completion of treatment.
In an attempt to build on the early successes with molecularly targeted therapy, there is great interest in combining effective agents for children with recalcitrant LGGs, especially those that have failed standard therapy or therapy with a single molecular drug. The addition of an epidermal growth factor receptor inhibitor (Tarceva) to an mTOR inhibitor (rapamycin) did not result in any obvious clinical advantage.73 The combination of MEK inhibitor and mTOR inhibitors are under study in children and adults with NF1 and MPNSTs. A similar trial, if the combination is found to be tolerable, is planned in pediatrics for children with progressive LGGs. Additionally, trials are planned attempting to combine MEK inhibitors with bevacizumab, as well as standard cytotoxic agents such as vinblastine.
There has been some reluctance to move ahead with combination trials due to the demonstrable high degree of efficacy, as noted by imaging response, of MEK inhibitors used as single agents in children with LGGs. However, studies comparing the durability of response of single agent therapy with combination therapy are needed. Another concern is the potential of overlapping toxicities when using multiple agents concurrently; especially in children with LGGs that usually act as chronic conditions. As example, both the MEK inhibitors and the mTOR inhibitors may cause skin, mucous membrane, and gastrointestinal toxicities. Similarly, the use of MEK inhibitors with anti-angiogenesis agents, such as bevacizumab, may increase the risk of possible hemorrhage or other vascular toxicities as MEK inhibitors may also have anti-angiogenic properties.
As all these studies continue, the long-term benefits of treatment with molecularly targeted therapy must be assessed, not only for toxicity and durability of response, but for late relapse. The impact of molecularly targeted therapy on senescence, a phenomenon that may underlie the tendency of pediatric LGGs not to progress years after cessation of treatment or in some patients without treatment, must be carefully evaluated.
The management of the more aggressive anaplastic piloid astrocytomas in older children and adults with NF1 remains unsettled.7 There are anecdotal reports of efficacy of conventional radiation plus temozolomide in newly diagnosed higher-grade NF1-associated gliomas in adults; however, these therapies have been associated with substantial short- and long-term toxicity in the setting of NF1, and hence, management of treatment-associated injury/inflammation is a major factor.74 Based on these observations, there has been interest in deferring alkylator therapy in favor of targeted therapies for all adult gliomas. Limited experience, to date, is mixed, with some adults having as long as 12 months of disease control on single agent MEK inhibitor and others having progression within two cycles (J.O.B., personal communication). Work is ongoing to describe the outcomes of adults with NF1-associated gliomas to better define the natural history after surveillance alone, as well as response to conventional and molecularly targeted therapies (J.O.B., personal communication). There is also significant interest in utilizing combination molecularly targeted therapies including MEK inhibitors, mTOR inhibitors, and possibly the CDK4/6 inhibitors (attempting to possibly target concomitant mutations such as CDKN2A) in adult NF1-associated gliomas.
Immunotherapeutic Approaches
Based on emerging preclinical animal model data for NF1-LGG, and to a lesser extent the sequencing and molecular data derived from NF1-LGG clinical specimens, it is becoming increasingly clear that the tumor microenvironment and dysregulation of the immune system play a key role in NF1-LGG, and thus immunotherapy is another promising strategy.57,74,75
Neurofibromin gene defects are associated with myeloid progenitor dysregulation,76 and preclinical models are often augmented by inflammatory pressure.77 NF1-associated tumors have increased serum concentrations of pro-inflammatory cytokines, such as interferon-γ and tumor necrosis factor-α.78 A recent characterization of NF-associated tumor immunophenotypes revealed that most NF1-associated neurofibromas contain programmed cell death ligand 1 (PD-L1)–positive tumor cells. PD-L1 positivity was higher in plexiform neurofibromas in which PD-L1+ cells approached 100%. Interestingly, PD-L1 positivity was associated with CD8+ tumor-infiltrating lymphocyte density, thus suggesting that the combination of the two parameters may provide predictive biomarkers for immunotherapy with checkpoint inhibitors.79 Expression of PD-L1 and CD8 immune infiltrates has also been observed in MPNST. More specifically, 57% of MPNST had cytotoxic T-cell infiltrates in the tumor microenvironment.80,81 The presence of an inflamed tumor microenvironment with increased T-cell infiltration has been viewed as a crucial component for the antitumor immune responses to programmed cell death protein 1 blockade recently reported in patients with MPNST.82,83
Another analysis of non-CNS NF1-associated tumors examined human leukocyte antigen and PD-L1 expression and characterized infiltrating T cells, also demonstrating variability in the immunophenotype of NF1 tumors, although some tumors seemed primed for immunologic modulation.84 On the other hand, in NF1-associated CNS tumors, the majority of non-neoplastic cells in the microenvironment supporting tumor growth consist of macrophages/microglia, which are evident early in tumorigenesis and have been demonstrated to support glioma growth via production of paracrine factors and chemokines increasing NF1-deficient glial proliferation.28,85,86 Although complex immunophenotypic analysis has not been robustly completed in NF-associated CNS tumors, these data suggest that immune modulation potentially may serve as a compelling therapy.
Several vaccine-based and other interferon-based immunotherapeutic early clinical trials that include patients with NF1-LGGs have been conducted,87,88 and future plans to expand such investigations are under way. It will be imperative to begin understanding the detailed molecular landscape of both the responders and non-responders so as to better prognosticate and tailor therapies more appropriately at the outset of treatment. In one trial testing poly-ICLC, 2 patients with NF1-related LGGs were enrolled. Efficacy data were not reported, although one of these patients exhibited surprisingly significant toxicity.89 In a different recently completed phase II study of poly-ICLC, 5 patients with NF1-associated progressive LGGs were treated; all tolerated therapy well and 4 demonstrated objective tumor response and ongoing stability through completion of 24 months of therapy (T.J.M., personal communication).
The orthogonal approach is a novel technique to display the antibodies in the patient, disclosing the “immunosignature” (IMS) of the tumor.90 The underlying premise to this methodology is that tumors will elicit antibodies to their components and the profile of the antibodies will be clinically useful. An advantage of this strategy is that antigen-activated B cells amplify their signal. A preliminary application of the IMS strategy to NF1 samples seemed promising. Blood from the patients was applied to 0.5 cm2 chips on which 125K peptides had been in situ synthesized. The peptides were ~11aa and had been selected from random sequence space to maximize chemical diversity. Two hundred peptides were chosen that appeared to separate 21 NF1 individuals (non-affected carriers, LGG, and neurofibroma) from age-matched controls (S.J., personal communication).
A second type of peptide array was also applied to these samples. These arrays contained 400K, 15aa peptides corresponding to the 220K possible frameshift peptides (FSPs) arising from missplicing of RNA in the tumor. It has been previously shown that these FSPs are highly immunogenic and provide protection in mouse tumor models. These arrays were also able to separate the 21 NF1 samples from non-NF1 controls, as well or better than the IMS arrays. Though the sample set was small (7 LGG, 5 plexiform neurofibroma), these arrays also may be able to distinguish these two classes, which is not possible radiographically. The FSP arrays have the advantage over the IMS arrays in that it may be possible to directly design a therapeutic vaccine based on the reactive FSPs in common across the tumor type. For example, it is theoretically possible to design a vaccine for any child with LGG.
Measures of Outcomes
There has been a paradigm shift regarding the goals of therapy in children with NF1- LGG. Both the NF1 and neuro-oncology scientific communities now clearly recognize that response rate and tumor shrinkage are not as important as PFS and functional outcomes, especially given the excellent overall survival for NF1-LGG in children. To this end, future studies including the upcoming Children’s Oncology Group trial will focus upon visual acuity as a primary outcome measure in patients for OPG in addition to PFS.68 Other functional outcomes such as patient reported outcomes, quality of life assessments, and motor outcomes will also be evaluated.91,92
As the precision oncology era unfolds for children with NF1- LGG, it will be important to not only focus on functional outcomes but also better understand the length of therapy required, the duration of response/stability, the impact of therapy on senescence, and the late effects with these agents—all of which remain unanswered questions at this juncture. In particular, since NF1 is associated with neurocognitive deficits that can impact everyday functioning, it will be imperative to investigate the long-term neurocognitive outcomes in untreated, chemotherapy, and molecularly targeted NF1-LGG individuals to better understand how newer targeted therapies for NF1-LGG comparatively impact this critical outcome.68,92
Recommendations:
Therapeutic interventions for NF1-associated LGGs, especially those of the visual pathway, should focus as much, if not more, on functional outcomes, such as vision, rather than solely on imaging measures tumor control or response.
Precision oncology, utilizing drugs such as the MEK inhibitors, should be used earlier in the disease process, compared with standard chemotherapeutic approaches, and be assessed for efficacy based on not only radiographic imaging response and survival, but also functional outcomes.
Therapeutic studies, based preferably on robust preclinical data, should be developed for transformed, anaplastic piloid, anaplastic, and/or gliomas occurring in adults.
The long-term impact of novel therapies on the phenomena of NF1-LGG associated senescence should be carefully evaluated.
Summary
Clearly the molecular and neuroimmunologic understandings of NF1-associated gliomas are actively evolving. The molecular genetic of NF1-associated gliomas is more complex than previously appreciated, especially in young adults, and the presence of likely transformed LGGs raises important challenges. Biopsy is increasingly indicated for patients with NF1-associated gliomas not only for histologic confirmation of the type of tumor present and its histologic grade, but also for, at least, focused testing for mutations, such as ATRX, CDKN2A, and TP53, which have been identified in a sizable portion of transformed or higher-grade tumors. Further, the immunologic makeup of these tumors is complex and another important avenue for discovery and possibly therapeutic intervention. Mouse modeling has already been shown to be an important component of the development of new therapies for NF1-related LGGs and additional mouse models, especially of “transformed” LGGs and NF-related higher-grade gliomas, will be required to identify and evaluate new therapies. Chemotherapy remains the predominant form of treatment for children with LGGs and often results in radiographic response or stabilization; however, functional outcome may not be as optimal as MRI may suggest. For older children and adults with higher-grade or transformed gliomas, the benefits of chemotherapy are much less well substantiated. Molecularly targeted therapy, especially treatment with the MEK inhibitors, has been a dramatic addition to the armamentarium of those children with NF-LGGs requiring treatment; however, the long-term benefits and safety of MEK inhibitor therapy, its benefit compared with chemotherapy, and its utility in older patients are essentially unknown. Agents that target the immune circuitry may also be of utility in the near future. Functional outcome measures must be incorporated in future clinical trials to assess the true benefits of all these interventions. These new understandings of NF1-associated LGGs have changed the way these tumors should be approached diagnostically and possibly therapeutically and have led to many new questions that must be rigorously addressed for management and treatment to be optimized.
Supplementary Material
Acknowledgments
We would like to thank the Gilbert Family Foundation for its support.
Funding
Financial support has been provided by the Gilbert Family Foundation (EIN number 81-0810541; Bridge number 0912232251).
Conflict of interest statement
Jaishri O. Blakeley, MD—No disclosures directly related. Consulting (unpaid), Exelixis, AstraZeneca, Springworks; paid consulting—Abbie. Michael J. Fisher, MD—Advisory Board, AstraZeneca. David H. Gutmann, MD, PhD—Tuberous Sclerosis Alliance, license fee. Antonio Iavarone, MD—Has received research grants from AstraZeneca and Taiho Pharmaceuticals and is a member of the SAB of AIMED Bio. Stephen A. Johnston, PhD—Is an inventor of technologies discussed (immunosignature/frameshift) and co-founded two companies, Calviri and Health Tell that are developing the technologies. Roger J. Packer, MD—Advisory Board, AstraZeneca; Advisory Board, Novartis. No other authors have conflicts of interest.
References
- 1. Pfister S, Janzarik WG, Remke M, et al. . BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones DT, Kocialkowski S, Liu L, et al. . Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Packer RJ, Pfister S, Bouffet E, et al. . Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones DT, Hutter B, Jäger N, et al. ; International Cancer Genome Consortium PedBrain Tumor Project Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones DTW, Kieran MW, Bouffet E, et al. . Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Angelo F, Ceccarelli M, Tala, et al. The molecular landscape of glioma in patients with neurofibromatosis 1. Nat Med. 2019;25(1):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reinhardt A, Stichel D, Schrimpf D, et al. . Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136(2):273–291. [DOI] [PubMed] [Google Scholar]
- 9. Puget S, Beccaria K, Blauwblomme T, et al. . Biopsy in a series of 130 pediatric diffuse intrinsic pontine gliomas. Childs Nerv Syst. 2015;31(10):1773–1780. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez FJ, Brosnan-Cashman JA, Allen SJ, et al. . Alternative lengthening of telomeres, ATRX loss and H3-K27M mutations in histologically defined pilocytic astrocytoma with anaplasia. Brain Pathol. 2019;29(1):126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beert E, Brems H, Daniëls B, et al. . Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50(12):1021–1032. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am J Pathol. 1999;155(6):1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutmann DH, James CD, Poyhonen M, et al. . Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology. 2003;61(10):1397–1400. [DOI] [PubMed] [Google Scholar]
- 14. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gutmann DH, McLellan MD, Hussain I, et al. . Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capper D, Jones DTW, Sill M, et al. . DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sievers P, Appay R, Schrimpf D, et al. . Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019;138(3):497–504. [DOI] [PubMed] [Google Scholar]
- 18. Tumeh PC, Harview CL, Yearley JH, et al. . PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan Y, Xiong M, Chen R, et al. . Athymic mice reveal a requirement for T-cell-microglia interactions in establishing a microenvironment supportive of NF1 low-grade glioma growth. Genes Dev. 2018;32(7–8):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro Oncol. 2019;21(10):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajenaru ML, Hernandez MR, Perry A, et al. . Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63(24):8573–8577. [PubMed] [Google Scholar]
- 22. Zhu Y, Harada T, Liu L, et al. . Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132(24):5577–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toonen JA, Anastasaki C, Smithson LJ, et al. . NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet. 2016;25(9):1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaul A, Toonen JA, Gianino SM, Gutmann DH. The impact of coexisting genetic mutations on murine optic glioma biology. Neuro Oncol. 2015;17(5):670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee DY, Gianino SM, Gutmann DH. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez FJ, Graham MK, Brosnan-Cashman JA, et al. . Telomere alterations in neurofibromatosis type 1-associated solid tumors. Acta Neuropathol Commun. 2019;7(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solga AC, Toonen JA, Pan Y, et al. . The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget. 2017;8(29):47206–47215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. [DOI] [PubMed] [Google Scholar]
- 29. Solga AC, Pong WW, Kim KY, et al. . RNA sequencing of tumor-associated microglia reveals Ccl5 as a stromal chemokine critical for neurofibromatosis-1 glioma growth. Neoplasia. 2015;17(10):776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toonen JA, Ma Y, Gutmann DH. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. 2017;19(6):808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Way GP, Allaway RJ, Bouley SJ, Fadul CE, Sanchez Y, Greene CS. A machine learning classifier trained on cancer transcriptomes detects NF1 inactivation signal in glioblastoma. BMC Genomics. 2017;18(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu C, Sage JC, Miller MR, et al. . Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alcantara Llaguno SR, Wang Z, Sun D, et al. . Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell. 2015;28(4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alcantara Llaguno S, Chen J, Kwon CH, et al. . Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Y, Guignard F, Zhao D, et al. . Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, et al. . Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun. 2015;6:7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon CH, Zhao D, Chen J, et al. . Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68(9):3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deguchi T, Nakane K, Yasuda M, Maeda S. Emergence and spread of drug resistant Neisseria gonorrhoeae. J Urol. 2010;184(3):851–858; quiz 1235. [DOI] [PubMed] [Google Scholar]
- 40. Hegedus B, Banerjee D, Yeh TH, et al. . Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. [DOI] [PubMed] [Google Scholar]
- 41. Dasgupta B, Yi Y, Hegedus B, Weber JD, Gutmann DH. Cerebrospinal fluid proteomic analysis reveals dysregulation of methionine aminopeptidase-2 expression in human and mouse neurofibromatosis 1-associated glioma. Cancer Res. 2005;65(21):9843–9850. [DOI] [PubMed] [Google Scholar]
- 42. Banerjee S, Gianino SM, Gao F, Christians U, Gutmann DH. Interpreting mammalian target of rapamycin and cell growth inhibition in a genetically engineered mouse model of Nf1-deficient astrocytes. Mol Cancer Ther. 2011;10(2):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 2015;17(6):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herting CJ, Chen Z, Pitter KL, et al. . Genetic driver mutations define the expression signature and microenvironmental composition of high-grade gliomas. Glia. 2017;65(12):1914–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan Y, Smithson LJ, Ma Y, Hambardzumyan D, Gutmann DH. Ccl5 establishes an autocrine high-grade glioma growth regulatory circuit critical for mesenchymal glioblastoma survival. Oncotarget. 2017;8(20):32977–32989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Z, Feng X, Herting CJ, et al. . Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77(9):2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diggs-Andrews KA, Brown JA, Gianino SM, et al. . Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014;75(2):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J Exp Med. 2017;214(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Freret ME, Gutmann DH. Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1. J Neurosci Res. 2019;97(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Isakson SH, Rizzardi AE, Coutts AW, et al. . Genetically engineered minipigs model the major clinical features of human neurofibromatosis type 1. Commun Biol. 2018;1:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khatua S, Gutmann DH, Packer RJ. Neurofibromatosis type 1 and optic pathway glioma: molecular interplay and therapeutic insights. Pediatr Blood Cancer. 2018;63(3). [DOI] [PubMed] [Google Scholar]
- 52. Campen CJ, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1. J Child Neurol. 2018;33(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Blank PMK, Fisher MJ, Liu GT, et al. . Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol. 2017;37(Suppl 1):S23–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guillamo JS, Créange A, Kalifa C, et al. ; Réseau NF France Prognostic factors of CNS tumours in neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–160. [DOI] [PubMed] [Google Scholar]
- 55. Ullrich NJ, Raja AI, Irons MB, Kieran MW, Goumnerova L. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007;61(4):762–766; discussion 766. [DOI] [PubMed] [Google Scholar]
- 56. Theeler BJ, Ellezam B, Yust-Katz S, Slopis JM, Loghin ME, de Groot JF. Prolonged survival in adult neurofibromatosis type I patients with recurrent high-grade gliomas treated with bevacizumab. J Neurol. 2014;261(8):1559–1564. [DOI] [PubMed] [Google Scholar]
- 57. Strowd RE 3rd, Rodriguez FJ, McLendon RE, et al. . Histologically benign, clinically aggressive: progressive non-optic pathway pilocytic astrocytomas in adults with NF1. Am J Med Genet A. 2016;170(6):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Byrne S, Connor S, Lascelles K, Siddiqui A, Hargrave D, Ferner RE. Clinical presentation and prognostic indicators in 100 adults and children with neurofibromatosis 1 associated non-optic pathway brain gliomas. J Neurooncol. 2017;133(3):609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lassaletta A, Scheinemann K, Zelcer SM, et al. . Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2016;34(29):3537–3543. [DOI] [PubMed] [Google Scholar]
- 60. Ater JL, Xia C, Mazewski CM, et al. . Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Blank P, Bandopadhayay P, Haas-Kogan D, Fouladi M, Fangusaro J. Management of pediatric low-grade glioma. Curr Opin Pediatr. 2019;31(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharif S, Ferner R, Birch JM, et al. . Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. [DOI] [PubMed] [Google Scholar]
- 63. Fisher MJ, Loguidice M, Gutmann DH, et al. . Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karajannis MA, Legault G, Fisher MJ, et al. . Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16(10):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. . Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bouffet E, Kieran M, Hargrave D, et al. . Trametinib therapy in pediatric patients with low-grade gliomas (LGG) with BRAF gene fusion; a disease-specific cohort in the first pediatric testing of trametinib. Neuro Oncol. 2018;20:i114. [Google Scholar]
- 67. Robison N, Pauly J, Malvar J, et al. . A phase I dose escalation trial of the MEK1/2 inhibitor MEK162 (binimetinib) in children with low-grade gliomas and other RAS/RAF pathways-activated tumors. Neuro Oncol. 2018;20:i114. [Google Scholar]
- 68. Fangusaro JR, Onar-Thomas A, Young-Poussaint T, et al. . A phase II prospective study of selumetinib in children with recurrent or refractory low-grade glioma (LGG): A Pediatric Brain Tumor Consortium (PBTC) study. J Clin Oncol. 2017;35(suppl; abstr 10504:10504). [Google Scholar]
- 69. Packer RJ, Jakacki R, Horn M, et al. . Objective response of multiply recurrent low-grade gliomas to bevicizumab and irinotecan. Ped Blood Cancer. 2009;52:791–795. [DOI] [PubMed] [Google Scholar]
- 70. Gururangan S, Fangusaro J, Poussaint TY, et al. . Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of visition in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmo. 2014;132(1):111–114. [DOI] [PubMed] [Google Scholar]
- 72. Kieran MW, Yao X, Macy M, et al. . A prospective multi-institutional phase II study of everolimus (RAD001), an mTOR inhibitor, in pediatric patients with recurrent or progressive low-grade glioma. A POETIC Consortium trial. Pediatr Blood Cancer. 2013;60(Suppl.3):19–20. [Google Scholar]
- 73. Yalon M, Rood B, MacDonald T, et al. . A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade gliomas (LGG). Ped Blood Cancer. 2013;1:71–76. [DOI] [PubMed] [Google Scholar]
- 74. Karmakar S, Reilly KM. The role of the immune system in neurofibromatosis type 1-associated nervous system tumors. CNS Oncol. 2017;6(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. [DOI] [PubMed] [Google Scholar]
- 76. Zhang YY, Vik TA, Ryder JW, et al. . Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med. 1998;187(11):1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Staser K, Yang FC, Clapp DW. Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression. Annu Rev Pathol. 2012;7:469–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park SJ, Sawitzki B, Kluwe L, Mautner VF, Holtkamp N, Kurtz A. Serum biomarkers for neurofibromatosis type 1 and early detection of malignant peripheral nerve-sheath tumors. BMC Med. 2013;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang S, Liechty B, Patel S, et al. . Programmed death ligand 1 expression and tumor infiltrating lymphocytes in neurofibromatosis type 1 and 2 associated tumors. J Neurooncol. 2018;138(1):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Farid M, Demicco EG, Garcia R, et al. . Malignant peripheral nerve sheath tumors. Oncologist. 2014;19(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shurell E, Vergara-Lluri ME, Li Y, et al. . Comprehensive adipocytic and neurogenic tissue microarray analysis of NY-ESO-1 expression—a promising immunotherapy target in malignant peripheral nerve sheath tumor and liposarcoma. Oncotarget. 2016;7(45):72860–72867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Davis LE, Nicholls LA, Babiker HM, Liau J, Mahadevan D. PD-1 inhibition achieves a complete metabolic response in a patient with malignant peripheral nerve sheath tumor. Cancer Immunol Res. 2019;7(9):1396–1400. [DOI] [PubMed] [Google Scholar]
- 83. Patnaik A, Kang SP, Rasco D, et al. . Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–4293. [DOI] [PubMed] [Google Scholar]
- 84. Haworth KB, Arnold MA, Pierson CR, et al. . Immune profiling of NF1-associated tumors reveals histologic subtype distinctions and heterogeneity: implications for immunotherapy. Oncotarget. 2017;8(47):82037–82048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Simmons GW, Pong WW, Emnett RJ, et al. . Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pollack IF. A Vaccine Trial for Low Grade Gliomas. 2015. NCT02358187. [Google Scholar]
- 88. Emory University. Phase II Pegylated Interferon (Peg Interferon). 2015. NCT02343224. [Google Scholar]
- 89. Hartman LL, Crawford JR, Makale MT, et al. . Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. J Pediatr Hematol Oncol. 2014;36(6):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stafford P, Cichacz Z, Woodbury NW, Johnston SA. Immunosignature system for diagnosis of cancer. Proc Natl Acad Sci U S A. 2014;111(30):E3072–E3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Garcia-Penas JJ. Learning disorders in neurofibromatosis type 1. Rev Neurol. 2017;64(s01):S59–S63. [PubMed] [Google Scholar]
- 92. Walsh KS, Janusz J, Wolters PL, et al. ; REiNS International Collaboration Neurocognitive outcomes in neurofibromatosis clinical trials: recommendations for the domain of attention. Neurology. 2016;87(7 Suppl 1): S21–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.