Abstract
Coronavirus disease 2019 (COVID-19) caused by the novel coronavirus has become a Public Health Emergency of International Concern. Among the various treatment proposals for COVID-19 infection, passive immunotherapy using plasma from recovering patients - "convalescent plasma" (CP)- could be a promising option in the treatment of SARS-CoV-2 infections. Immune (i.e. "convalescent") plasma refers to plasma that is collected from individuals, following resolution of infection and development of antibodies. Passive antibody administration through transfusion of convalescent plasma may offer the only short-term strategy to confer immediate immunity to susceptible individuals. According to the World Health Organization (WHO), the use of plasma therapy is permitted when faced with «serious diseases for which there are no effective pharmacological treatments». Several clinical trials are underway to test the effectiveness of hyperimmune plasma at various stages of SARS-CoV2.The Food and Drug Administration (FDA), the U.S. regulatory authority, has approved the use of CP for compassionate use in the treatment of patients with a critical COVID-19 infection. Below are the general indications for drawing up clinical protocols for the integral management of "COVID-19-convalescent plasma" for which the validation and approval of the Ethics Committees is still necessary.
Keywords: Covid -19, Hyperimmune plasma, Passive immunotherapy
1. Background
SARS CoV-2 is a new viral strain of Coronavirus, previously never identified in humans. It was first reported in Wuhan, China, in December 2019.Its diffusivity and epidemic/pandemic potential is linked to the absence of an immune reactive response by the population which, having never come into contact this strain, has not developed an immune response and an immunological memory. SARS CoV-2 shares 79% of the gene sequence of the SARS coronavirus and penetrates the cells by binding to the same ACE-2 [1] receptor. In the last 20 years, the SARS-CoV-2 outbreak is the third caused by the Coronavirus family, after the 2002–2003 SARS-CoV-1 and the 2013 MERS outbreak. The new SARS-CoV-2 infection is much more contagious than the previous two, but with a lower lethality rate [2,3]. Among the various treatment proposals for COVID-19 infection, passive immunotherapy using plasma from recovering patients - "convalescent plasma" (CP)-could be a promising option in the treatment of SARS-CoV-2 infections [4].This would make it possible to exploit the humoral immunity developed by these patients against the virus to treat and prevent a worsening of the condition of patients with active phase infection. Plasma infusions for convalescents are a method already used and authorized by the World Health Organization (WHO) to treat a range of diseases, such as polio, measles, mumps, Ebola, SARS, Mers and H1N1.The used therapy protocols differ in the following aspects: dosage, antibody titer and timing of administration (Table 1 ).CP contains antibodies, which could be valuable in fighting COVID-19 infection [5,6].According to the WHO, the use of plasma therapy is permitted when faced with «serious diseases for which there are no effective pharmacological treatments». Several clinical trials are underway to test the effectiveness of hyperimmune plasma at various stages of SARS-CoV2.The Food and Drug Administration (FDA), the U.S. regulatory authority, has approved the use of CP for compassionate use in the treatment of patients with a critical COVID-19 infection. It has now become necessary to start controlled and randomized clinical trials to consolidate the data obtained from the sporadic clinical experiences. Below are the general indications for drawing up clinical protocols for the integral management of "COVID-19-convalescent plasma" for which the validation and approval of the Ethics Committees is still necessary.
Table 1.
Dosing of convalescent plasma in sundry coronavirus outbreaks.
| Disease | Location | Dose of CP | Titer | Summary finding |
|---|---|---|---|---|
| SARS1 | Hong Kong, China | Mean volume 279.3 ± 127.1 ml(range, 160–640 ml) |
|
|
| SARS1 | Taipei, Taiwan | 500 mL |
|
|
| SARS1 | Hong Kong, China | 200mL |
|
|
| SARS1 | Shenzhen, China | 2 units of 250 ml each (total 500 mL); transfused12 h apart |
|
|
| MERS | Seoul, South Korea | 4 transfusions of CP to 3 patients; volumes not stated |
|
|
| MERS | Riyadh, Saudi Arabia |
|
Of 196 individuals with suspected or confirmed MERSCoV:
|
|
| MERS | Seoul, South Korea | 250 mL |
|
|
| COVID-19 | Wuhan, China | 200 mL |
|
|
| COVID-19 | Shenzhen, China | 2 consecutive transfusions of 200-250 ml (400 ml total) |
|
|
Abbreviations: CP-Convalescent plasma TRALI- Transfusion related acute lung injury ELISA- Enzyme Linked Immunosorbent Antibody assay PRNT-plaque reduction neutralization assay IFA- Indirect fluorescent antibody testing MN- Microneutralization assay.
2. Passive immunotherapy-convalescent plasma (CP)- hyperimmune plasma
2.1. Definition
Currently amenable immunotherapy modes can be divided into two types: passive and active. The active type strengthens the immune system's response to various pathogenic attacks by activating both humoral immunity and cell-mediated immunity, using the adaptive response. Passive antibody therapy involves the administration of antibodies against a given agent to a susceptible individual obtained from Convalescent Plasma (CP) from subjects who have recovered from an infection, monoclonal antibodies (mAbs),or preparations generated by pharmaceutical company producing purified preparations containing a high titer of neutralizing antibodies [42].Passive immunotherapy can prevent the clinical development of the infection or reduce the severity of the infection in those subjects recently exposed to various pathogens. Its effectiveness is higher if administered for preventive purposes or at an early stage from the onset of the symptoms [7,8]. A further use of CP is the development of a polyclonal "hyperimmune globulin" concentrate obtained from a pool of plasma donors called "hyperimmune plasma ",collected through apheresis from patients treated after an infection and who have developed high serum titers of neutralizing antibodies [43].
2.2. Mechanism of action
During plasma apheresis of SARS-Cov-2 healed donors, in addition to neutralizing antibodies (NAbs), other proteins such as anti-inflammatory cytokines, coagulation factors, antibodies, defensins, pentraxins and other undefined proteins are collected [8].Therefore, CP transfusion has an immunomodulatory action improving the severe inflammatory response triggered by the infection [9].In Sars-CoV-2 an excessive activation of the immune system with the development of a systemic hyperinflammation or " cytokine storm " linked to the release of IL-1β, IL-2, IL-6, IL-17, IL-8, TNF and CCL-2 has been shown; this framework determines the development of a Systemic Inflammatory Response Syndrome (SIRS) with enhanced damage on pulmonary ventilatory capacity that results in severe fibrosis [10,11].
2.3. Characteristics of anti SARS−COV-2 antibodies
In patients with COVID-19 disease it is possible to observe an initial antibody response “early” and a delayed antibody response “late” [20]. “Early" antibodies are mainly anti Spike protein (Ab-S) and enhance virus induced apoptosis; "late" antibodies are mainly anti nucleocapsid (Ab-N) and have a neutralizing action [21,22].It is of considerable interest that serum conversion in COVID 19 + patients occurs 12 days after infection but is not followed by rapid decline in viral load [23]. Such evidence is consistent with the finding of a non-optimal initial endogenous antibody response. Therefore, overall, these results suggest that the absence of reported serious adverse effects associated with hyperimmune plasma infusion may be, at least in part, related to the different quality of antibodies in convalescent patients at different stages of the disease. For this reason, both a proper evaluation of the antibody response in convalescent patients and an adequate titer of neutralizing SARS-CoV-2 antibodies present in the collected plasma are essential. The peak of IgG anti-Sars-CoV-2 and neutralizing antibodies is detectable up to approximately 4 months, after which there is a decline of up to 25-6% IgG antibodies and 16-1% Neutralizing antibodies in 36 months [24].The average antibody titer of neutralizing antibodies 1/60 (1/12 to 1/512) is almost similar to day + 35 and + 180 from the onset of infection symptoms [25,26]. In addition, the antibody level in convalescent patients has been correlated with the initial viral load and independently associated with the severity of the disease in both H1N1+ and MERS-CoV + patients [27,28].For such evidence it would be preferable to collect the plasma of convalescent patients from a significant Sars-CoV-2 disease.
3. Requirements for anti-Covid-19 hyperimmune plasma donor candidates
Candidates for hyperimmune plasma donation are individuals with documented SARS-CoV-2 infection who voluntarily and in an informed manner adhere to plasma apheresis donation dedicated to the therapy of complicated SARS-CoV-2 infections. In addition, they must have recently recovered from COVID-19 and have been previously hospitalized. This also includes asymptomatic individuals in fiduciary quarantine at home and under active supervision following a positive test, provided that they meet the following eligibility criteria:
-
-
age between 18- and 65-years old weight not < 50 Kg [12,13];
-
-
at least 14 days after clinical recovery (resolution of symptoms) and negative documentation of two Nucleic Acid Tests (NAT) on nasopharyngeal swab and
-
-
Reverse transcriptase-polymerase chain reaction (RT-PCR) plasma/serum performed 24 h apart immediately prior to patient discharge (if hospitalised);
-
-
possible documentation of negativity of a NAT test on nasopharyngeal swab and RT-PCR plasma/serum 14 days after recovery (if in fiduciary quarantine) (request not common to all protocols examined);
-
-
negativity to testing for Covid-19 repeated at donation timing and after 48 h;
-
-
appropriate serum titer of specific antibodies (> 160 by Enzyme Immunoassay (EIA) or equivalent value by other method, based on other published case studies [[14], [15], [16]];
-
-
absence of Human Leukocyte Antigen (HLA) antibodies.
It is recommended to verify the absence of Sars-Cov2 Rna on plasma collected by RT-PCR [17].This target population provides for the collection of a blood component with some exceptions to the standards defined by the selection criteria of Ministerial Decree(MD) 2.11.2015 [18].These exceptions may concern the age of the donor, a short clinical recovery interval (less than twice the incubation period, as recommended by the “Guide for the preparation, use and quality assurance of blood components” published by the Council of Europe - EDQM) [19] and the clinical use of donors not previously tested and therefore without a previous sampling history that qualifies their safety profile. On the other hand, the other criteria for the selection of the donor referred to in the mentioned of MD 2.11.2015 must be respected, especially the exclusion of individuals undergoing transfusion therapy and/or with previous pregnancies. In addition to these convalescent patients, regular blood donors should be considered if the specific antibody titer in their serum is >160 by EIA (or equivalent value by another method), these persons fall into the category of donors that can be used for the production of hyperimmune plasma because:
-
-
they already meet the requirements of regular blood donors [18];
-
-
a time equal to or greater than twice the incubation period (i.e. at least 28 days) has elapsed since the clinical recovery of the disease, as recommended by the “Guide for the preparation, use and quality assurance of blood components” published by the Council of Europe - EDQM [19].
4. Product requirements
Apheresis is a recommended procedure for obtaining plasma. This procedure is based on continuous centrifugation of whole blood with separation and collection of plasma. The effectiveness of this technique is to obtain about 400–800 mL of plasma from single aphaeretic donation.
The Transfusion Service has premises suitable for the intended use, in order to limit the risk of errors, as well as cleaning and maintenance operations to minimize the risk of contamination. For apheresis activities, cellular separators must be used (e.g.in total we have two cellular separators) with performance characteristics that guarantee the highest degree of safety for the donor, as well as the quality of the final products, by meeting the requirements for the collection of apheresis hemocomponents as provided for by the current legislation. For the sealing of the connection circuits of the sampling systems we have soldering systems suitable to prevent the risk of microbial contamination during the collection and production of blood components and we are in possession of a device designed to freeze the plasma produced, to ensure compliance with the specifications defined by the regulations in force. The collected plasma units will be subjected to the biological qualification tests provided for by MD 2/11/2015 and therefore must be negative for the following viral markers: - HBV- HIV−HCV serology and molecular, Syphilis serology [18].
As this is convalescent plasma from non-periodic donors, HAV and Parvovirus B19-DNA (<105 copies/ml) tests must also be performed.
-the content of Immunoglobulins (IgG, IgM, IgA),
-the specific antibody titer (by EIA must be >160 or equivalent by another method).
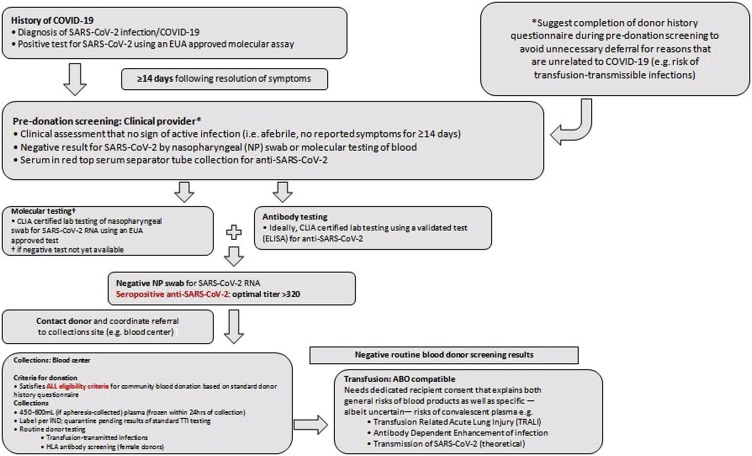
The effectiveness of this therapy has been associated with the concentration of neutralizing Ab in the plasma of donors [33]. Solvent/detergent (S/D) treatment for viral plasma inactivation is a matter of discussion [4,40] of literature data, as the treatment is not effective for encapsulated viruses such as HAV and Parvovirus B19 and therefore the procedure is not mandatory. Blood transfusion services should adapt their serum banks to the storage of sera, while requesting the informed consent of donors, allowing for subsequent epidemiological investigations of the blood donor population (Fig. 1 ).
Fig. 1.
Anti-SARS-CoV-2 hyperimmune plasma workflow.
Abbreviations: EUA-Emergency Use Authorization, CLIA-Clinical Laboratory Improvement Amendments, TRALI-Transfusion-Related Acute Lung Injury, ADE-antibody dependent enhancement, HLA: Human Leucocyte Antigens.
5. Directions and methods of clinical use
Hyperimmune plasma has, as already reported in the background, been successfully used in previous coronavirus epidemics:Sars-1 in 2003 and MERS in 2012 [29].In this Covid-19 epidemic there are several reports in which this therapy has been used successfully [[30], [31], [32]].The results suggest that the administration of plasma to convalescents is safe, reduces the viral load and improves the clinical course [4] (Table 1).Controlled clinical trials are necessary to define the most suitable treatment protocols in the various stages of Covid-19 infection, to test the safety and effectiveness of this passive immunotherapy. Based on Chinese reports, the use of high-dose intravenous immunoglobulins (Ivig-HD) also seems promising; however, the obtained data are few and therefore confusing.
The effectiveness and safety of plasma immunotherapy with convalescents' plasma is tested mainly in Asia with 5 types of clinical trials:
-
-
Use of anti-Sars-Cov-2 plasma for post exposure prophylactic use (Post-exposure prophylaxis)
-
-
Use of anti-Sars-Cov-2 plasma in patients with mild disease (Mild disease)
-
-
Use of anti-Sars-Cov-2 plasma in patients with moderate disease (Moderate ill patients)
-
-
Use of anti-Sars-Cov-2 plasma as salvage therapy (Rescue therapy)
-
-
Use of anti-Sars-Cov-2 plasma in high-risk pediatric patients (High risk pediatric patients) [33]
On March 24, 2020, the Federal Drug Administration (FDA) of the United States (USA) published guidelines for the use of the plasma of convalescents−COVID-19 [34] and outlined three pathways for therapeutic access to CP :
1. Compassionate use
2. Clinical Trials
3. Therapy protocols
The use for prophylactic purposes is not allowed.
The eligibility criteria are as follows:
-
•
COVID-19 infection confirmed with NAT on nasopharyngeal swab and RT-PCR plasma/serum.
-
•Severe or life-threatening Covid disease:
-
oSevere illness is manifested with one or more of the following symptoms:
-
▪dyspnea,
-
▪respiratory rate≥ 30/min,
-
▪SpO2 ≤ 93%,
-
▪partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, PaO2/FiO2 < 300 mm Hg in oxygen,
-
▪lung infiltrates > 50% within 24–48 hours after infection
-
▪
-
oLife-threatening disease is defined by one or more of the following symptoms:
-
▪Respiratory failure,
-
▪Septic shock,
-
▪Multi-organ failure (MOF)
-
▪
-
o
-
•
Informed consent of the patient or legal guardian.
-
•
Positive PCR with seronegativity [35].
5.1. Volume and posology
The volume and posology of administration of hyperimmune plasma are variable and anecdotal because they are based on clinical experience gained in previous epidemics and comparison with Chinese clinical trials on patients affected by Sars-Cov-2 [32].
Based on the Sars-Cov-1studies, therapeutic hyperimmune plasma was used at a dosage of 5 ml/kg with an Ab neutralizing titer of 1: 160 [16].
It should also be considered that 3.125/mL kg of plasma have an antibody titer >1.64, therefore 5 ml/kg of plasma corresponds to a titer of hyperimmune globulins 1:160. If we consider a typical patient of about 80 Kg the volume of plasma to infuse will be 250 ml (3.125 ml/kg x 80 Kg = 250 ml > 1.64 [31]).
The timing and therapeutic dosage to be used according to the various clinical experiences is as follows: 250–300 ml of hyperimmune plasma administered to each of the admitted patients for a maximum of 3 times within 5 days [32].
The maximum administered volume must not exceed 600 ml as the use of higher volumes of plasma would be contraindicated due to the risk of overload [41].
5.2. Timing of administration
Timely administration is strongly recommended, it is optimal in the first 7 days, good efficacy within 14 days,certainly not indicated beyond three weeks after the onset of the disease. It is important to carry out the therapy during the viral replication phase [[31], [32], [33], [34]].
5.3. Interaction with other medicines
The possible synergistic associations or advantages of this product compared to the simultaneous use of other drugs are not defined or studied. In the absence of such data, the product should be assigned on the basis of the approved clinical protocol.
5.4. Adverse effects and contraindications
Passive immunotherapy, according to studies conducted on Ebola virus and Mers-Cov, does not involve severe adverse events except in 2% of cases in the form of Transfusion related acute lung injury (TRALI). In most cases mild adverse events were found in 8% of patients treated, 5% of whom had a febrile reaction and 4% an urticarial reaction [[28], [29], [30], [31], [32], [33], [34], [35]].
Studies performed on animal models (Rhesus Macaques) have highlighted the risk of antibody-dependent enhancement (ADE).Rhesus Macaques immunized with vaccines containing Covid-19 Spike protein develop a high anti-Spike protein titer; it has been noted that infection with Sars-Cov -2 of these guinea pigs causes severe lung damage (severe acute lung injury- ALI),compared to non-immunized controls, despite a low viral load [21].
This phenomenon is likely linked to early seroconversion prior to virus clearance at lung level with an enhancement of macrophage dependent inflammatory damage by these non-neutralizing antibodies [36].
The tendency to ADE could be genetically determined, therefore in these clinical trials on hyperimmune plasma, a small unknown percentage of genetically susceptible patients could develop a worsening paradoxical symptomatology [37,38]. Finally, this product is subject to all precautions regarding undesirable effects and contraindications common to human plasma therapy; these include in particular:
- the absolute contraindication to its administration in complete IgA deficits, (which is why the dosage of IgA is advised before the onset of therapy)
- caution against circulatory overload (TACO) [39].
6. Conclusions
Hyperimmune plasma is a possible and powerful weapon against Sars-Cov-2 by Covid -19.Further thorough studies are needed to test its safety and the efficacy to make the best use of convalescent plasma on an appropriate patient target.
Authorship contributions
All Authors contributed equally to the study
Funding
This article was not funded.
Declaration of Competing Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
All Authors declare no organizations that funded our research, including grant numbers.
References
- 1.Lu R., Zhao X., Li J., Niu P. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie Q.-H., Luo X.-D., Hui W.-L. Advances in clinical diagnosis and treatment of severe acute respiratory syndrome. World J Gastroenterol. 2003;9:1139–1143. doi: 10.3748/wjg.v9.i6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., Van Boheemen S., Bestebroer T.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Chen L., Xiong J., Bao L. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):P398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoy Soo, Cheng Y., Wong R. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mair-Jenkins J., Saavedra-Campos Maria, Kenneth Baillie J. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A., Pirofski L.A. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24(9):474–478. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 8.Garraud O., Heshmati F., Pozzetto B. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23:39–44. doi: 10.1016/j.tracli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lünemann J.D., Nimmerjahn F., Dalakas M.C. Intravenous immunoglobulinin neurology--mode of action and clinical efficacy. Nat Rev Neurol. 2015;11 doi: 10.1038/nrneurol.2014.253. 80–9. [DOI] [PubMed] [Google Scholar]
- 10.McGonagle D., Sharif K., O’Regan A. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(June(6)) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan S., Yi Q., Fan S. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 doi: 10.1101/2020.02.10.20021832. Posted February 12. [DOI] [Google Scholar]
- 12.Duan K., Liu B., Li C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiberghien P., de Lambalerie X., Morel P. Collecting and evaluating convalescent plasma forCOVID-19 treatment: why and how. Vox Sang. 2020 doi: 10.1111/vox.12926. [DOI] [PubMed] [Google Scholar]
- 14.Shchukina V.N., Sia Loginova, Borisevich S.V. Experience with empirical treatment of severe acute respiratory syndrome due to coronavirus, genotype IV. Antibiot Khimioter. 2011;56(7-8):42–46. PMID: 22359870[Article in Russian]. [PubMed] [Google Scholar]
- 15.Arabi Yaseen, Balkhy Hanan, Ali H. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy forpatients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springer plus. 2015;19(November (4)):709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y., Wong R., Soo Y.O. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan L., Xu D., Ye G. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. (Pubmed ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GazzettaUfficiale I.T. 2015. Disposizioni relative ai requisiti di qualita’ e sicurezza del sangue e degli emocomponenti.https://www.gazzettaufficiale.I.T./eli/id/2015/12/28/15A09709/sg [Accessed 28 December 2015] [Google Scholar]
- 19.European Directorate for the Quality of Medicines & HealthCare (EDQM) Council of Europe . 19th ed. Council of Europe; 2017. Guide to the preparation, use and quality assurance of blood components.https://www.edqm.eu/sites/default/files/list_of_contents_19th_ed-blood-quality.pdf [Printed at the Council of Europe] [Google Scholar]
- 20.Wang S.F., Tseng S.P., Yen C.H. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L., Wei Q., Lin Q. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q., Zhang L., Kuwahara K. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human Primates. ACS Infect Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woelfel R., Comran V.M., Guggemos W. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. MedRxiv. 2020 03.05.20030502 (not peer-reviewed yet) [Google Scholar]
- 24.Cao W.C., Liu W., Zhang P.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 25.Hung I.F., To K.K., Lee C.K. Effect of clinical and virological parameters on the level of neutralizing antibody against pandemic influenza A virus H1N1 2009. Clin Infect Dis. 2010;51:274–279. doi: 10.1086/653940. [DOI] [PubMed] [Google Scholar]
- 26.Ko J.H., Muller M.A., Seok H. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn Microbiol Infect Dis. 2017;89:106–111. doi: 10.1016/j.diagmicrobio.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung I.F., To K.K., Lee C.K. Effect of clinical and virological parameters on the level of neutralizing antibody against pandemic influenza A virus H1N1 2009. Clin Infect Dis. 2010;51:274–279. doi: 10.1086/653940. [DOI] [PubMed] [Google Scholar]
- 28.Ko J.H., Muller M.A., Seok H. Serologic responses of 42 MERS-coronavirus- infected patients according to the disease severity. Diagn Microbiol Infect Dis. 2017;89:106–111. doi: 10.1016/j.diagmicrobio.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.S., Chen J.T., Liu Y.X., Zhang Z.S., Gao H., Liu Y.X. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77(2):147–150. doi: 10.1002/jmv.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xinhua N.E.T. 2020. China puts 245 COVID-19 patients on convalescent plasma therapy Xinhua.http://www.xinhuaN.E.T.com/english/2020-02/28/c_138828177.htm [accessed 28 February 2020] [Google Scholar]
- 31.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan K., Liu B., Li C. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020 doi: 10.1101/2020.03.16.20036145. [DOI] [Google Scholar]
- 33.Bloch Evan M., Bailey JeffreyA., Tobian Aaron A.R. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.2020. US food & drug administration development & approval process (CBER) investigational COVID-19 convalescent plasma - emergency INDs.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma [accessed 24 March 2020] [Google Scholar]
- 35.WHO . 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks.https://apps.who.int/iris/bitstream/handle/10665/135591/WHO_HIS_SDS_2014.8_eng.pdf;jsessionid=AED599D4070B0B37D7FD54414CFD9F99?sequence=1 2020[accessed 20 February 2020] [Google Scholar]
- 36.Jaume M., Yip M.S., Cheung C.Y. Anti-severeacute respiratory syndrome coronavirus spike antibodies trigger infection of humanimmune cells via a pH- and cysteine protease-independent Fc gamma R pathway. JVirol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan F.F., Tanner J., Chan P.K. Influence of Fc gamma RIIA and MBL polymorphisms on severe acute respiratory syndrome. TissueAntigens. 2005;66:291–296. doi: 10.1111/j.1399-0039.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming Andrew B. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of anti body dependent Enhancement. J Clin Virol. 2020;127(June) doi: 10.1016/j.jcv.2020.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SIMTI /SIDEM; 2020. SIMTI /SIDEM “Position paper” sulla produzione di plasma iperimmune da utilizzare nella terapia della malattia da SARS-CoV2.https://aiceonline.org/wp-content/uploads/2020/04/SIMIT-SIDEM-convalescent-plasma.pdf [Accessed 26 March 2020] [Google Scholar]
- 40.Xue X., Zhu C., Huang S. Effect of heat inactivation of blood samples on the efficacy of three detection methods of SARS-CoV-2 antibodies. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(March (3)):316–320. doi: 10.12122/j.issn.1673-4254.2020.03.03. [Article in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.2020. Clinicaltrials.gOv hyperimmune plasma for critical patients with COVID-19 (COV19 PLASMA)https://clinicaltrials.gov/ct2/show/NCT04321421 [accessed 25 March 2020] [Google Scholar]
- 42.Walker Laura M., Burton Dennis R. Passive immunotherapy of viral infections:’ super-antibodies’ enter the fray. Nat Rev Immunol. 2018;18(5):297–308. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casadevall Arturo, Pirofski Liise-anne. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]