Coronavirus pandemic disease (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). At the time of this paper writing, over 2,5 million cases of COVID-19 have been reported, with more than 280,000 deaths all over the world. Italy is one of the first countries involved, with more than 200,000 reported infected people and 30,000 deaths. In the Basilicata south Italy region live 560,000 people, with 372 reported infected cases, 34 recovered in intensive care units (ICU) and 15 deaths [1]. There are no specific treatments for COVID-19 and the vaccine is still far away. Scientific community is trying to better define best shared therapeutic protocols in early and advanced clinical stages. Still, critical cases are about 6%, with serious illness rate of 14%, with involvement of sepsis, acute respiratory distress syndrome (ARDS), multiple organ failure, and vascular and thromboembolic findings [2,3]. Mortality rate is 2–3%, with higher values, more than double, for >65 years old patients [4]. Among ICU patients, almost 1 patient on 3 dies, with higher rates for older patients [5].
A systematic review and exploratory meta-analysis performed in 2014 identified 32 studies of SARS coronavirus infection and severe influenza [6]. These studies involved 699 treated patients and 568 untreated “controls”. The review revealed evidence for a consistent reduction in mortality upon plasma therapy. Furthermore, exploratory post hoc meta-analysis showed a significant reduction in the pooled odds of mortality following treatment, compared with placebo or no therapy [7]. As for SARS-CoV-2, many clinical trials are in course, FDA approved the procedure [8] and there are recent Chinese encouraging reports [[9], [10], [11], [12]].
In order to preliminarly evaluate the importance of convalescent plasma transfusions in intensive care units, authors compared mortality rates between two groups: patients untreated (group 1) and those treated with convalescent plasma (group 2). Group 1 includes all 34 Basilicata region patients recovered in COVID dedicated intensive care units (ICU), with mean age of 63 years old and comorbidity rate >70%. All patients were treated by means of invasive and not invasive O2 ventilation, tracheostomy when needed, antiretroviral agents, hydrossycloroquine (Plaquenil, Sanofi, Italy), steroids and not steroid antinflammatory agents, heparin, azithromycin and other wide spectrum antibiotics, tocilizumab (Roactemra, Roche, Italy). Group 2 is composed of all actually reported COVID-19 ICU patients in literature, included in three recently published papers [[10], [11], [12]], with a total number of 19 cases, mean age of 54 years old, with mean comorbidity rate similar to group 1, underwent to pharmaceutical and supporting treatments with these differences: no heparin, hydrossycloroquine and tocilizumab, but in addiction submitted to arbidol (Umifenovir, Pharmstandard, China) in 10 cases, and in all cases to convalescent plasma transfusion, starting from a single 200 ml dose, repeated in the majority of cases after 7–10 days and once again after the same time, until a total amount of 800–900 ml and in only one case, with multiple organ failure, of 2400 ml [10]. Clinical and instrumental improvements were detectable within 3 days after procedure and without any adverse reaction excepting an evanescent facial red spot on 1 patient. The donors had been well (asymptomatic) for at least 10 days, with an high serum SARS-CoV-2– specific ELISA antibody (more than 1:600), 400 ml of plasma were obtained with apheresis and routinely checked. The observation window for all patients (both groups) was lower than 40 days.
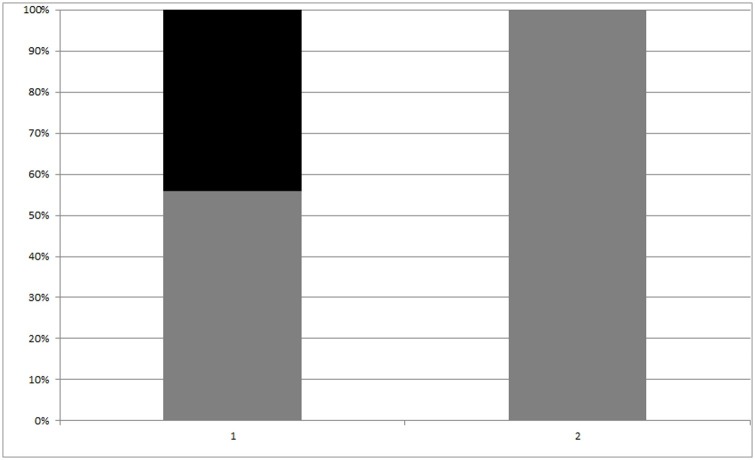
In Group 1 15 recipients died due to pulmonary or cardiovascular complications, with a mortality rate of 44,1%. No deaths were found in group 2. Proportions comparison showed a significant difference in death rate, despite the few number of patients, with 19,65-62,1% of 95% CI, Chi-square 9,612, p = 0,0019 (Fig. 1 ). Mortality with plasma transfusion odds ratio value is 0,03223 (95% CI 0,0018 – 0,5777, z = 2,333 and p = 0,0197).
Fig. 1.
Death rate (black bar) and survival one (grey bar) of ICU patients in both groups (p = 0,0019).
Coronaviruses are Coronaviridae family enveloped positive-stranded RNA viruses. An envelope-anchored spike protein promotes coronavirus entry into host cells and genome sequencing of viral RNA has revealed that the virus causing COVID-19 is phylogenetically related to the SARS-related coronaviruses. Angiotensin-converting enzyme 2 (ACE 2) is most probably used by the spike protein of the COVID-19 virus as a receptor similar to that SARS-CoV. Fortunately, it seems that mutational capacity of viral surface proteins is poor, encouraging immunology-based therapies and vaccine research [13,14]. Convalescent plasma therapy has been applied to the prevention and treatment of many infectious diseases for more than one century. It is a safe and routinary clinical procedure, with very low risk of complications. Plasma obtained from recovered SARS patients who had established humoral immunity against the virus, contains a large quantity of neutralizing antibodies capable of neutralizing and eradicating the pathogen from blood circulation and pulmonary tissues [15]. In a previous study with n = 50 SARS patients, mortality rate results were similar (0% vs 23.8%; P = 0.049, plasma treated 19 patients, untreated 21) [6]. Furthermore, metanalysis reports comparing plasma treated vs untreated SARS patients in terms of mortality rate are encouraging [7]. No similar studies on SARS-CoV-2 patients are available. Our report shows statistical significant strong mortality rate reduction for COVID-19 ICU patients treated with convalescent plasma vs untreated ones, in line with what shown in SARS previous papers. Still, many aspects should be pointed out. Of course, all patients evaluated in this paper were treated with multiple other agents (including antiviral medications), and it is not possible to determine whether differences observed could have been related to therapies other than plasma. Still, the only pharmaceutical agent used in group 2 and not in group 1 is arbidol, and this product seems not to improve significatively the overall survival rate in severe/critical patients [16]. One other point is the mean age, which is 10 years lower in group 2, and the low homogeneity of two groups: italians and chinese, with ethnic and health assistance differences, all that could play a role in final result, but many clinical reports don’t show so big variations in terms of mortality rate between 54 and 64 mean age [17] and the odds ratio high significancy we obtained can reasonably rule out a prevalence of other factors on plasma submission one. Comorbidity is another considerable point, despite it is difficult to evaluate in multicenter colleting and intensive care units, due to their own high comorbidity rate. Both groups patients were affected in not less than 70% of cases by other pathologies at time of recovering in ICU, mainly hypertension, in line with those reported recently in literature [4,5,18]. Nevertheless, further studies with high number of patients, more homogeneous groups, double blind case-control analysis, are mandatory in order to validate these interesting findings on COVID-19 convalescent plasma treatment efficacy in increasing COVID-19 ICU patients survival possibilities [19,20]; but in consideration of the very low risk of complications, the unexpected high mortality of the disease in some countries and the absence of specific treatments, it should be recommended a wide and fast procedure spreading.
Declaration of Competing Interest
Authors declares no conflict of interests, no financial relationships that could influence authors actions, no financial interest, relationships or affiliations relevant to the subject of the manuscript.
Acknowledgement
To Tonino, and all other victims, patients and colleagues. Science will win this war too, mainly for them.
References
- 1.Italian National Health Department, COVID-19 daily report (http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioNotizieNuovoCoronavirus.jsp?lingua=italiano&menu=notizie&p=dalministero&id=4451).
- 2.Chen J., Qi T., Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keith P., Day M., Perkins L. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care. 2020;24(128) doi: 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W.H., Wang W.J., Li C.C. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): A Nationwide Analysis of China. Eur Respir J. 2020 doi: 10.1183/13993003.00562-2020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G., Zangrillo A., Zanella A. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiberghien P., De Lambalerie X., Morel P. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how. Vox Sang. 2020 doi: 10.1111/vox.12926. in press. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins Tanne J. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020 doi: 10.1136/bmj.m1256. in press. [DOI] [PubMed] [Google Scholar]
- 9.Bloch E.M., Shoham S., Casadevall A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Investigation. 2020 doi: 10.1172/JCI138745. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B., Liu S., Tan T. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. CHEST. 2020 doi: 10.1016/j.chest.2020.03.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2002 doi: 10.1001/jama.2020.4783. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duana K., Liuc B., Lid C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020 doi: 10.1073/pnas.2004168117. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu D., Zhu C., Ai L. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J Mol Sci. 2020;21:2272. doi: 10.3390/ijms21072272. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marano G., Vaglio S., Pupella S. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L., Xiong J., Bao L. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seghatchian J., Lanza F. Convalescent plasma, an apheresis research project targeting and motivating the fully recovered COVID 19 patients: A rousing message of clinical benefit to both donors and recipients alike. Transfus Apher Sci. 2020;23(April) doi: 10.1016/j.transci.2020.102794. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanza F., Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID -19 infected patients. Br J Haematol. 2020 doi: 10.1111/bjh.16814. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]