Abstract
Introduction
Prader-Willi syndrome (PWS) is a complex neurodevelopmental genetic disorder. No definitive clinical signs of antenatal PWS have been identified.
Case
A healthy, nulliparous, 29-year-old woman demonstrated polyhydramnios at 27 weeks of gestation. Cardiotocography (CTG) showed an absence of foetal heart rate (FHR) acceleration and moderate FHR variability. Daily CTG demonstrated an absence of FHR acceleration. A male newborn was delivered by caesarean section, weighing 2492 g, which is appropriate for gestational age; the Apgar scores at 1 and 5 min were 6 and 6, respectively, and the umbilical artery pH was 7.295. The newborn exhibited marked hypotonia, lack of sucking, and cryptorchidism. FISH analysis performed due to severe hypotonia showed 46, XY. Ish del (15) (q11. 2q 11.2), which led to the diagnosis of PWS.
Discussion
Polyhydramnios and abnormal FHR patterns may be associated with feeding difficulty and hypotonia. These signs may be an indication for antenatal molecular genetic testing to diagnose PWS.
Keywords: Prader-Willi syndrome, Polyhydramnios, Abnormal foetal heart rate patterns, Molecular genetic testing
Highlights
-
•
Prader-Willi syndrome (PWS) is a complex neurodevelopmental genetic disorder.
-
•
Polyhydramnios and abnormal foetal heart rate patterns may be associated with feeding difficulty and hypotonia.
-
•
These signs may be an indication for antenatal molecular genetic testing to diagnose Prader-Willi syndrome.
1. Introduction
Prader-Willi syndrome (PWS) is a complex neurodevelopmental genetic disorder that was first described by Prader, Labhart, and Willi in 1956 [1]. PWS is caused by loss of a critical area on paternal chromosome 15q11-q13, which occurs due to a paternal microdeletion, unimaternal disomy, or defects in the imprinting centre [2,3]. The human small nuclear ribonucleoprotein polypeptide N (SNRPN) gene is a member of a gene family which encodes proteins involved in pre-mRNA splicing; this gene maps to the smallest deletion region in PWS, specifically within chromosome 15q11-q13 [4]. The major clinical features of neonates with PWS are marked hypotonia and feeding difficulty, which are central in origin.
Antenatal diagnosis of PWS is desirable to rule out lethal chromosomal abnormalities (e.g., myotonic dystrophy), improve neonatal outcomes, and provide early counselling to families to prepare them for neonatal care [5]. Because G-banding cannot detect chromosomal microdeletions, unimaternal disomy, or defects in the imprinting centre, antenatal PWS diagnosis is achieved through molecular genetic testing, such as DNA-methylation analysis [4] followed by fluorescence in situ hybridization (FISH) or chromosomal microarray testing to detect microdeletions, and single nucleotide polymorphism analysis to detect unimaternal disomy and imprinting defects [6]. Only a high suspicion of PWS can lead obstetricians to perform antenatal chromosome studies.
Importantly, few studies have reported the antenatal clinical diagnosis of PWS [7], and no definitive clinical signs have been identified. Although several reports have identified clinical features of foetuses with PWS, including polyhydramnios, diminished foetal movement, and abnormal foetal presentation, these are not specific to PWS [5,[8], [9], [10]]. Here we report a case of PWS with polyhydramnios and abnormal foetal heart rate (FHR) patterns, suggesting dysfunction of the foetal central nervous system (CNS) and chromosomal abnormalities.
2. Case Presentation
A healthy, nulliparous, 29-year-old woman was referred to an obstetric unit 35 weeks into a naturally conceived pregnancy because of polyhydramnios, which was identified at 27 weeks of gestation.
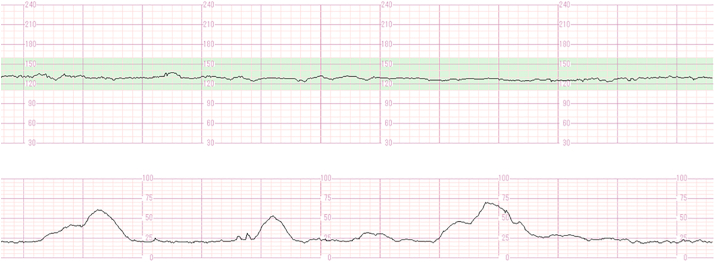
Ultrasonography (US) showed an amniotic fluid index of 31. The foetus demonstrated active movement, and was in a cephalic position with an appropriate estimated body weight and no structural anomalies. However, cardiotocography (CTG) showed an absence of FHR acceleration, moderate FHR variability, and no deceleration with uterine contractions (Fig. 1). Although CTG demonstrated a non-reactive foetal heart rate pattern, daily confirmation of moderate FHR variability, lack of decelerations, and active foetal movement allowed for prolongation of the pregnancy. Due to suspicion of a foetal CNS disorder, genetic amniocentesis was offered to the patient, but it was declined. The cause of polyhydramnios was not identified, and the pregnancy progressed uneventfully.
Fig. 1.
Cardiotocography shows the absence of FHR acceleration, moderate FHR variability, and no deceleration with uterine contractions.
An elective caesarean section was performed at 38 weeks of gestation, and a male newborn was delivered, weighing 2492 g, which is appropriate for gestational age; the Apgar scores at 1 and 5 min were 6 and 6, respectively. The umbilical artery pH was 7.295, pCO2 51.2 mmHg, pO2 18.7 mmHg and base excess −2.6 mmol.
The newborn exhibited marked hypotonia, lack of sucking, and cryptorchidism. Although G-banding showed a normal karyotype, FISH analysis performed due to severe hypotonia showed 46, XY. Ish del (15) (q11. 2q 11.2), which led to the diagnosis of PWS (Fig. 2). The infant was treated by nasal feeding, without any complications.
Fig. 2.
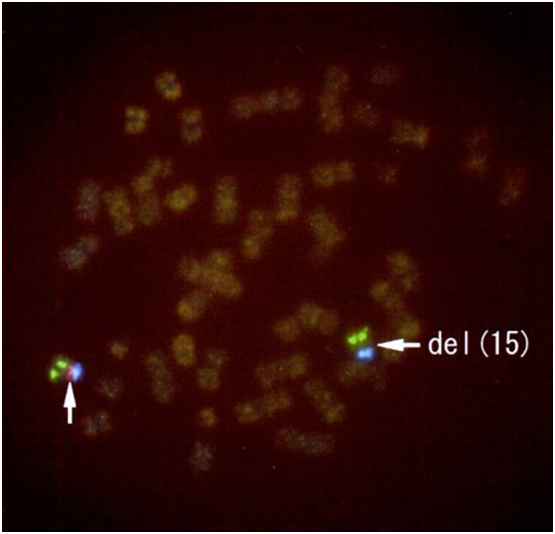
Neonatal fluorescence in situ hybridization (FISH) analysis shows 46, XY. Ish del (15) (q11. 2q 11.2). SNRPN, the D15Z1 region, and the PML region are labelled by red, blue, and green fluorescence, respectively. Neonatal FISH analysis shows deletion of a red signal (right arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
No definitive clinical features of foetuses with PWS have been reported, and obstetricians still require a means of diagnosing PWS antenatally. This may be possible by detecting polyhydramnios and abnormal FHR patterns; these signs are associated with feeding difficulty and hypotonia, respectively, and they indicate foetal CNS disorders. These findings may be indications for antenatal chromosome analysis [11,12].
Polyhydramnios, which is caused by dysfunctional foetal swallowing, was reported to be a characteristic US finding in PWS [13]. However, polyhydramnios may indicate the presence of several medical conditions, including foetal CNS disorders. Since US has limited utility in diagnosing PWS, additional clinical tools may be necessary for evaluating foetal conditions.
FHR monitoring is commonly used to evaluate foetal conditions during the antepartum and intrapartum periods [14]. Regarding FHR patterns in foetuses with PWS, prolonged inactive periods and short active periods correspond well with diminished foetal movement [8,9]. FHR accelerations are thought to represent sympathetic activity and are frequently associated with foetal movement [15], so prolonged inactive periods on CTG can indicate foetal CNS disorders.
Polyhydramnios and abnormal FHR patterns can indicate the presence of foetal CNS disorders. When the origin of these disorders is unknown, chromosome analysis is required [12]. Because PWS and other microdeletion syndromes may cause foetal CNS disorders [16], it is necessary to perform antenatal molecular genetic testing that can detect microdeletion syndromes and other chromosomal abnormalities.
In conclusion, polyhydramnios and abnormal FHR patterns may be characteristic clinical features in foetuses with PWS, and may even be indications for antenatal molecular genetic testing. Because early diagnosis of PWS may help rule out lethal chromosomal abnormalities and improve neonatal prognosis, clinicians should attempt to diagnose PWS antenatally.
Acknowledgments
Contributors
All authors were involved in the clinical care of the patient and contributed to the conception, drafting, review, and revision of the manuscript. All authors read and approved the final version of the paper and take full responsibility for the work.
Conflict of Interest
The authors have no potential conflicts of interest to declare.
Funding
This work did not receive any specific grants from funding agencies.
Patient Consent
Informed consent was obtained from the patient for publication of this work.
Provenance and Peer Review
This case report was peer reviewed.
References
- 1.Prader A., Labhart A., Willi H. Ein Syndrom von Adipositas, Kleinwuchs, Kryptorchismus und Oligophrenie nach myotonieartigem Zustand im Neugeborenalter. Schweizerische Medizinische Wochenschrift. 1956;86:1260–1261. [Google Scholar]
- 2.Donaldson M.D., Chu C.E., Cooke A., Wilson A., Greene S.A., Stephenson J.B. The Prader-Willi syndrome. Arch. Dis. Child. 1994;70:58–63. doi: 10.1136/adc.70.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy S.B., Driscoll D.J. Prader-Willi syndrome. Eur. J. Hum. Genet. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glenn C.C., Saitoh S., Jong M.T., Filbrandt M.M., Surti U., Driscoll D.J., Nicholls R.D. Gene structure, DNA methylation, and imprinted expression of the human SNRPN gene. Am. J. Hum. Genet. 1996;58:335–346. [PMC free article] [PubMed] [Google Scholar]
- 5.Bar C., Diene G., Molinas C., Bieth E., Casper C., Tauber M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet Journal of Rare Diseases. 2017;12 doi: 10.1186/s13023-017-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubota T., Sutcliffe J.S., Aradhya S., Gillessen-Kaesbach G., Christian S.L., Horsthemke B., Beaudet A.L., Ledbetter D.H. Validation studies of SNRPN methylation as a diagnostic test for Prader-Willi syndrome. Am. J. Med. Genet. 1996;66:77–80. doi: 10.1002/(SICI)1096-8628(19961202)66:1<77::AID-AJMG18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Bigi N., Faure J.M., Coubes C., Puechberty J., Lefort G., Sarda P., Blanchet P. Prader-Willi syndrome: is there a recognizable fetal phenotype? Prenat. Diagn. 2008;28:796–799. doi: 10.1002/pd.1973. [DOI] [PubMed] [Google Scholar]
- 8.Hiroi H., Kozuma S., Hayashi N., Unno N., Fujii T., Tsutsumi O., Okai T., Taketani Y. A fetus with Prader-Willi syndrome showing normal diurnal rhythm and abnormal ultradian rhythm on heart rate monitoring. Fetal Diagn. Ther. 2000;15:304–307. doi: 10.1159/000021026. [DOI] [PubMed] [Google Scholar]
- 9.Fong B.F., De Vries J.I. Obstetric aspects of the Prader-Willi syndrome. Ultrasound Obstet. Gynecol. 2003;21:389–392. doi: 10.1002/uog.90. [DOI] [PubMed] [Google Scholar]
- 10.Geysenbergh B., De Catte L., Vogels A. Can fetal ultrasound result in prenatal diagnosis of Prader-Willi syndrome? Genet. Couns. 2011;22:207–216. [PubMed] [Google Scholar]
- 11.Sagi-Dain L., Maya I., Reches A., Frumkin A., Grinshpun-Cohen J., Segel R., Manor E., Khayat M., Tenne T., Banne E., Shalata A., Yonath H., Berger R., Singer A., Ben-Shachar S. Chromosomal microarray analysis results from pregnancies with various Ultrasonographic anomalies. Obstet. Gynecol. 2018;132:1368–1375. doi: 10.1097/AOG.0000000000002975. [DOI] [PubMed] [Google Scholar]
- 12.Miller D.T., Adam M.P., Aradhya S., Biesecker L.G., Brothman A.R., Carter N.P., Church D.M., Crolla J.A., Eichler E.E., Epstein C.J., Faucett W.A., Feuk L., Friedman J.M., Hamosh A., Jackson L., Kaminsky E.B., Kok K., Krantz I.D., Kuhn R.M., Lee C., Ostell J.M., Rosenberg C., Scherer S.W., Spinner N.B., Stavropoulos D.J., Tepperberg J.H., Thorland E.C., Vermeesch J.R., Waggoner D.J., Watson M.S., Martin C.L., Ledbetter D.H. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liehr T., Brude E., Gillessen-Kaesbach G., König R., Mrasek K., von Eggeling F., Starke H. Prader-Willi syndrome with a karyotype 47,XY,+min(15)(pter->q11.1:) and maternal UPD 15--case report plus review of similar cases. European Journal of Medical Genetics. 2005;48:175–181. doi: 10.1016/j.ejmg.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists ACOG practice bulletin no. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet. Gynecol. 2009;114:192. doi: 10.1097/AOG.0b013e3181aef106. [DOI] [PubMed] [Google Scholar]
- 15.Krebs H.B., Petres R.E., Dunn L.J., Jordaan H.V., Segreti A. Intrapartum fetal heart rate monitoring. I. Classification and prognosis of fetal heart rate patterns. Am. J. Obstet. Gynecol. 1979;133:762–772. doi: 10.1016/0002-9378(79)90113-3. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg P. An update on common chromosome microdeletion and microduplication syndromes. Pediatr. Ann. 2018;47:e198–e203. doi: 10.3928/19382359-20180419-01. [DOI] [PubMed] [Google Scholar]