Highlights
-
•
Incidence of grade ≥2 RP was 36% (including grade 3 RP: 5% and grade 5 RP: 3%).
-
•
Patient characteristics did not significantly differ between patient with grade ≥2 RP and grade ≤1 RP.
-
•
Dose-volume parameters were significantly higher among patients with grade ≥2 RP.
Keywords: Radiation pneumonitis, Dose-volume parameters, Durvalumab, Lung cancer
Abstract
Background and purpose
We investigated the incidence and dose-volume relationships of radiation pneumonitis (RP) after concurrent chemoradiotherapy (CCRT) followed by durvalumab for locally advanced non-small-cell lung cancer (LA-NSCLC).
Materials and methods
We retrospectively analyzed records of 36 patients with LA-NSCLC who underwent CCRT followed by durvalumab. Incidence of RP was analyzed for correlations with clinical factors and dose-volume parameters of lung in radiotherapy.
Results
All patients received 60 Gy in 30 fractions of radiotherapy with concurrent chemotherapy. Over a median follow-up period of 7 months, incidence of grade ≥2 RP was 36% (including grade 3 RP: 5% and grade 5 RP: 3%). Age, sex, Brinkman index, and blood test results did not significantly differ between patients with grade ≥2 RP and grade ≤1 RP. Dose-volume parameters (lung volumes that received 5 Gy, 10 Gy, 20 Gy, 30 Gy, 40 Gy, 50 Gy, and mean lung dose) were significantly higher among patients with grade ≥2 RP compared with patients with grade ≤1 RP.
Conclusion
Incidence of grade ≥2 RP was 36% after CCRT followed by durvalumab for LA-NSCLC, but did not significantly differ from those of patients treated with CCRT alone. Lung dose-volume parameters were significantly correlated with RP.
1. Introduction
Lung cancer is a major cause of cancer death worldwide [1], [2]. For locally advanced non-small-cell lung cancer (LA-NSCLC), concurrent chemoradiotherapy (CCRT), which combines platinum-based chemotherapy and radiotherapy (RT), is the standard treatment [3]. However, the 5-year survival rate for LA-NSCLC treated with CCRT is reportedly only 15–40% [4], [5], [6], [7]. Recently, CCRT followed by durvalumab was shown to improve overall survival significantly for patients with LA-NSCLC [8]. Durvalumab is a human IgG1 monoclonal antibody that blocks programmed death ligand-1 binding to the programmed death-1 receptor and CD80, thus increasing anti-tumor activity by T cells [9], [10]. However, possible adverse effects (AEs) due to immune system activation are major concerns. A major AE after CCRT followed by durvalumab is radiation pneumonitis (RP). RP is associated with the dose-volume parameter of lung. [11], [12]. If durvalumab increases susceptibility to RP, the relationship incidence of RP and dose-volume relationship will be different from that in patients treated with CCRT alone. Here, we retrospectively analyzed clinical data of LA-NSCLC patients treated by CCRT followed by durvalumab to clarify the incidence and dose-volume relationship of RP.
2. Materials and methods
2.1. Patients
We retrospectively analyzed records of patients with LA-NSCLC who were treated with CCRT followed by durvalumab at our hospital from May 2018 to October 2019. Although most tumors were pathologically diagnosed using biopsies, some patients could not receive biopsies because of comorbidity and were therefore diagnosed clinically. Clinical stage was determined using fluorodeoxyglucose-positron emission tomography, contrast-enhanced computed tomography (CT), and gadolinium-enhanced head magnetic resonance imaging and classified according to the Union for International Cancer Control (8th ed.) criteria. This study was approved by our hospital’s Institutional Review Board and was carried out in accordance with the Declaration of Helsinki.
2.2. Radiotherapy
Radiotherapy was performed using 10-MV X-rays with CT image simulation (1.25-mm thickness). Four-dimensional CT (4-D CT) was used to evaluate the tumor respiratory motion. For treatment planning, images of expiratory phase were used. Irradiation technique was conventional three-dimensional conformal radiotherapy and prescribed dose was 60 Gy in 30 fractions for all the patient. Gross target volume was defined in simulated CT images of the lung window. Internal target volume (ITV) was determined by tumor motion in 4-D CT images to encompass respiratory tumor motion. Clinical target volume (CTV) included a 5–10 mm margin in all directions from ITV and prophylactic lymph node area at the mediastinum. The planning target volume was defined as CTV with a 5-mm margin to compensate for any set-up error. Dose-volume parameters such as mean lung dose (MLD), normal lung volumes that received more than 5 Gy (V5), 20 Gy (V20), 30 Gy (V30), 40 Gy (V40), 50 Gy (V50), and 60 Gy (V60) were recorded prior to treatment. Dose constraints for organs at risk were <50 Gy to spinal cord and V20 of the lung should be <35%.
2.3. Chemotherapy
The chemotherapy regimens included daily carboplatin (CBDCA) alone, weekly CBDCA + paclitaxel (PTX), cisplatin (CDDP) + docetaxel (DTX), CDDP + TS-1 and CBDCA + DTX, and varied according to physicians’ decisions.
2.4. Durvalumab
Durvalumab was intravenously administered at 10 mg/kg every 2 weeks for 1 year. Diagnostic CTs were taken immediately after finishing CCRT to evaluate its efficacy and toxicity. If no abnormalities were seen on CT or blood tests, durvalumab was started. After initiating durvalumab, patients were required to make weekly hospital visits to monitor their conditions and take chest x-rays, complete blood cell counts and laboratory measurements. Chest CT images were also taken every 3 months for the first year and every 6 months thereafter. AEs were classified using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0. Administration of durvalumab was postponed for grade 2 lung AEs until the lung AEs was resolved, but canceled and never administered again for grade ≥3 lung AEs. We carefully judged pneumonia as RP with multidisciplinary discussion because it is sometimes difficult to differentiate radiation pneumonitis from other pneumonia. Our criteria to consider RP were such as abnormal shadows on X-ray or CT within the irradiated field and no evidence of bacterial nor viral infection such as positive urinary antigen detection of streptococcus pneumonia, legionella and positive antigen test of influenza.
2.5. Statistical analysis
Mean parameters in the two groups were compared with the Student’s t-test. Relationships between categorical data and RP were analyzed by chi-square test. P < 0.05 was considered significant. Receiver operating characteristic (ROC) curves and areas under the curve (AUC) were calculated to assess optimal cut-off values for dose-volume parameters and their ability to predict RP. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (SPSS Inc., Armonk, NY, USA).
3. Results
Between May 2018 to October 2019, 36 patients with LA-NSCLC were treated by CCRT followed by durvalumab at our hospital. During same period, 13 patients received CCRT but they could not receive durvalumab due to progression of disease, poor general condition or patient’s refusal. Their median age was 72 years (range: 51–80 years), and included 27 men and 9 women. Their histologic diagnoses were adenocarcinoma (n = 20), squamous cell carcinoma (n = 10), and mixed adenocarcinoma and squamous cell carcinoma (n = 1). Five patients were treated based on their clinical diagnoses because they could not receive biopsies for medical reasons. Their disease stages were IIB (n = 2), IIIA (n = 12), IIIB (n = 13) and IIIC (n = 2). Chemotherapy regimens included daily CBDCA (n = 16), weekly CBDCA + PTX (n = 12), CDDP + DTX (n = 4), CDDP + TS-1 (n = 3), and h CBDCA + DTX (n = 1; Table 1). Median number of cycles of durvalumab was 6.5 times. Among 36 patients, 7 patients accomplished administration of durvalumab for 1 year, 12 patients had been receiving durvalumab at last follow up and 17 patients could not continue administration of durvalumab due to adverse events including 8 patients with lung toxicity , 6 patients with progression of disease and 3 patients with other auto-immune related toxicity. Among 8 patients with discontinuation of durvalumab caused by lung toxicity, 4 patients received corticosteroid administration. Over a median follow-up period of 7 months, 16 patients (44%) developed grade 1 RP, 10 (28%) had grade 2 RP, 2 (5%) had grade 3 RP, and 1 (3%) had grade 5 RP. Median interval from first administration of durvalumab to occurrence of RP was 2 months. Patient characteristics such as age, Brinkman Index and other pre-treatment blood test results such as c-reactive protein, Sialylated carbohydrate antigen KL-6, lactate dehydrogenase, HbA1c and blood sugar, did not significantly differ in patients with grade ≥2 RP (Table 2) compared with patients with grade ≤1 RP. Patients’ sex, history of diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hyperlipidemia and specific regimen of chemotherapy were not associated with development of grade ≥2 RP (Table 3).
Table 1.
Patient and treatment characteristics (n = 36).
| Characteristic | ||
|---|---|---|
| Age, years, median (range) | 72 (51–80) | |
| Sex, n (%) | Male | 27 (75) |
| Female | 9 (25) | |
| Histo-pathological type, n (%) | Adenocarcinoma | 20 (56) |
| Squamous cell carcinoma | 10 (28) | |
| Mixed type | 1 (3) | |
| Not identified | 5 (13) | |
| *FEV1.0, L, mean (±standard deviation) | 2.2 (±0.4) | |
| PD-L1 expression, n (%) | Not examined | 7 (19) |
| ≦1% | 15 (42) | |
| 2–50% | 6 (17) | |
| ≥50% | 8 (22) | |
| Chemotherapy regimen, n (%) | Daily low dose CBDCA | 16 (44) |
| CBDCA + PTX | 12 (33) | |
| CDDP + DTX | 4 (11) | |
| CDDP + TS-1 | 3 (8) | |
| CBDCA + DTX | 1 (3) | |
| Interval between last day of RT and start of durvalumab, days, median (range) | 11 (1–39) | |
| Cycles of durvalumab, median (range) | 6.5 (1-24) | |
| Clinical stage, n (%) | IIB | 5 (13) |
| IIIA | 12 (33) | |
| IIIB | 13 (36) | |
| IIIC | 2 (6) | |
| Post-operative recurrence | 4 (11) | |
| Comorbidity, n (%) | Diabetes mellitus | 11 (31) |
| COPD | 3 (8) | |
| Hypertension | 16 (44) | |
| Hyperlipidemia | 11 (31) | |
*Data were taken from 27 patients who underwent respiratory function test.
FEV1.0; forced expiratory volume in one second, PD-L1; Programmed death-ligand 1, CBDCA; carboplatin, CDDP; cisplatin, COPD; chronic obstructive pulmonary disease, DTX; docetaxel, PTX; paclitaxel, RT; radiation therapy.
Table 2.
Comparison of factors and dosimetric parameters between patients with grade ≤ 1 radiation pneumonitis (RP) and those with grade ≥2 RP.
| Parameter | grade ≤ 1 RP group (n = 23) | grade ≧2 RP group (n = 13) | p-value |
|---|---|---|---|
| Patient factor | |||
| Brinkman Index | 968 (±1085) | 980 (±456) | 0.970 |
| KL-6 | 353 (±175) | 352 (±193) | 0.991 |
| CRP | 0.71 (±1.37) | 1.03 (±1.97) | 0.570 |
| LDH | 176 (±41) | 188 (±39) | 0.401 |
| HbA1c | 6.2 (±0.6) | 6.3 (±0.6) | 0.409 |
| Blood sugar | 116 (±26) | 123 (±36) | 0.491 |
| Age | 70.1 (±7.7) | 72.1 (±5.6) | 0.432 |
| FEV1.0* | 2.22 (±0.41) | 2.20 (±0.53) | 0.946 |
| Dosimetric parameter | |||
| V5, % | 26.8 (±7.7) | 32.2 (±7.1) | 0.044 |
| V10, % | 22.1 (±6.5) | 27.4 (±6.0) | 0.041 |
| V20, % | 17.5 (±5.4) | 22.7 (±5.1) | 0.008 |
| V30, % | 14.2 (±5.1) | 19.3 (±4.6) | 0.006 |
| V40, % | 11.3 (±4.8) | 15.6 (±4.3) | 0.012 |
| V50, % | 8.1 (±4.3) | 11.1 (±3.8) | 0.043 |
| V60, % | 2.2 (±3.0) | 2.7 (±2.1) | 0.627 |
| MLD, Gy | 9.5 (±3.0) | 11.8 (±2.8) | 0.031 |
*Data were taken from 27 patients who underwent respiratory function test. Among 27 patients, 16 patients showed grade ≤1 radiation pneumonitis and 11 patients showed grade ≧2 radiation pneumonitis.
CRP; c-reactive protein, KL-6; sialylated carbohydrate antigen, LDH; lactate dehydrogenase, FEV1.0; forced expiratory volume in one second, MLD; mean lung dose, RP; radiation pneumonitis.
Table 3.
Chi-square test for factors related to grade ≥2 radiation pneumonitis.
| p-value | |
|---|---|
| Sex | 0.317 |
| Diabetes mellitus | 0.127 |
| COPD | 0.345 |
| Hypertension | 0.121 |
| Hyperlipidemia | 0.983 |
| CBDCA + PTX | 0.806 |
COPD; chronic obstructive pulmonary disease, CBDCA; carboplatin, PTX; paclitaxel.
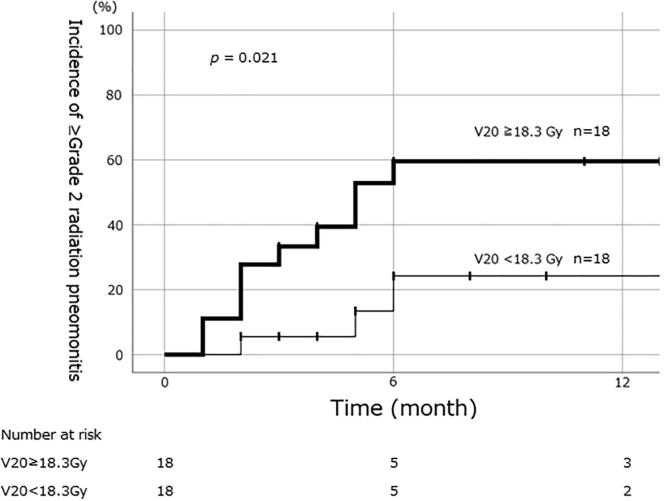
Dosimetric parameters relating to RP are described in Table 2. Mean lung V20 in patients with grade ≥2 RP was significantly higher than that in patients with grade ≤1 RP (22.7% vs 17.5%; P < 0.05). Mean V5, V10, V30, V40 and V50 values were also significantly higher in the grade ≥2 RP group. ROC analysis showed maximum AUC with V30 (AUC = 0.779). Other DVH parameters such as V20 (AUC = 0.756), V40 (AUC = 0.759) and MLD (AUC = 0.742) also showed large AUCs. The 6-month cumulative incidences of RP between the two groups, as determined by cut-off values from ROC analysis, were 60% vs. 24% (V20, ≥18.3 Gy or less) (Fig. 1).
Fig. 1.
Cumulative incidence of grade ≥2 radiation pneumonitis for patients whose lung V20 was ≥18.3 Gy and those whose V20 was <18.3 Gy. The cumulative incidence at 6 months was 60% for the ≥18.3 Gy group and 24% for the <18.3 Gy group.
4. Discussion
The crude incidence of grade ≥3 RP was 8% in this study. Reportedly, among patients who receive CCRT for LA-NSCLC, the range for grade ≥3 RP incidence is 4%–12% [12], [13], [14], which is consistent with our results. Incidence of grade 2 RP was 28% in this study, but varies widely in the literature; Mun et al. reported 16% among patients who received CCRT for LA-NSCLC, and Piotrowski et al reported 47% (29/62) after 3D-CRT for LA-NSCLC [13], [15]. In addition, our own data for the incidence of grade 2 RP after CCRT alone for LA-NSCLC was 25%. At least, incidence of non-severe RP in our study did not deviate from these reports and our own experience. We think that durvalumab does not increase RP incidence, regardless of its severity. In the Pacific trial, incidence of grade 3–4 pneumonitis was 3.4% [8], compared with 5% in the present study. We consider the results of the randomized phase III trial were reproduced in this study with real-world data.
In this study, the lung V5 to V50 and MLD were significantly higher in patients with grade ≥2 RP compared with patients with grade ≤1 RP. Among these DVH parameters, V30 showed the largest AUC; V20, V40 and MLD also showed large AUCs. However, the small differences in AUC among these parameters might be meaningless, because of both the high correlations for these parameters, and the small number of patients in this study. V20 is widely used to predict RP [11], [12]. This study showed a V20 cut-off of 18.3% optimally predicted grade ≥2 RP. Tsujino et al reported a V20 cut-off of 25% optimally to predict grade ≥2 RP. Palma et al also reported that V20 is a predictor of pneumonitis [11]. We think that V20 is a useful predictor of RP after CCRT followed by durvalumab, and the dose-volume relationship between irradiated lung volume and RP does not significantly differ from that of patients treated with CCRT alone.
Relationships between patients’ clinicopathological characteristics and development of RP have been controversial. A review article described 13 studies that showed older age was a significant risk factor for RP, whereas 4 studies found no association between age and RP [16]. Age, sex, Brinkman Index and blood test results were not significant predictors of grade ≥2 RP in the present study, nor did we find any patient characteristics that were significantly associated with RP in this setting. Palma et al reported that specific regimen of chemotherapy such as CBDCA + PTX was significantly associated with RP [11]. In this study, we could not find significant correlation between CBDCA + PTX and RP. It might be due to small number of patients of our study. Further accumulation of patients is required to address this issue.
This study has some limitations—notably, its design as a single-institutional retrospective study with relatively few patients. However, owing to the lack of real-world data about RP after CCRT followed by durvalumab for patients with LA-NSCLC, we believe our findings may be helpful to clinicians. In this study, all patients were treated with elective nodal irradiation (ENI). Irradiated field might be larger with ENI and incidence of RP will be affected by that. However, we still think that “dose-volume relationship” is not affected by that. In this study, all patient were treated with three-dimensional conformal radiotherapy (3D-CRT) technique. It is reported that intensity modulated radiotherapy (IMRT) could reduce the risk of RP [17]. We have to note that incidence and dose-volume relationship might be different between 3D-CRT and IMRT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Cheng T.Y., Cramb S.M., Baade P.D., Youlden D.R., Nwogu C., Reid M.E. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11:1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberg A.J., Brock M.V., Ford J.G., Samet J.M., Spivack S.D. Epidemiology of lung cancer. Chest. 2013;143:e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J., Chirieac L.R. Cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 4.Ahn J.S., Ahn Y.C., Kim J.H. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. https://ascopubs.org/doi/10.1200/JCO.2014.60.0130 [DOI] [PubMed] [Google Scholar]
- 5.Aupérin A., Le Péchoux C., Rolland E., Curran W.J., Furuse K., Fournel P. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. https://ascopubs.org/doi/10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto N., Nakagawa K., Nishimura Y., Tsujino K., Satouchi M., Kudo S. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. https://ascopubs.org/doi/10.1200/JCO.2009.24.5050 [DOI] [PubMed] [Google Scholar]
- 7.Eberhardt W.E., Pöttgen C., Gauler T.C. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE) J Clin Oncol. 2010;33:4194–4201. doi: 10.1200/JCO.2015.62.6812. https://ascopubs.org/doi/10.1200/JCO.2015.62.6812 [DOI] [PubMed] [Google Scholar]
- 8.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. https://www.nejm.org/doi/10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 9.Stewart R., Morrow M., Hammond S.A., Mulgrew K., Marcus D., Poon E. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 10.Postow M.A., Callahan M.K., Wolchok J.D. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. https://ascopubs.org/doi/10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palma D.A., Senan S., Tsujino K., Barriger R.B., Rengan R., Moreno M. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujino K., Hirota S., Endo M., Obayashi K., Kotani Y., Satouchi M. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–115. doi: 10.1016/S0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 13.Liew Mun Sem, Sia Joseph, Starmans MaudH.W., Tafreshi Ali, Harris Sam, Feigen Malcolm. Comparison of toxicity and outcomes of concurrentradiotherapy with carboplatin/paclitaxel or cisplatin/etoposide in stage III non–small cell lung cancer. Cancer Med. 2013;2:916–924. doi: 10.1002/cam4.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steuer C.E., Behera M., Ernani V., Higgins K.A., Saba N.F., Shin D.M. Comparison of concurrent use of thoracic radiation with either carboplatin-paclitaxel or cisplatin-etoposide for patients with stage III non-small-cell lung cancer: a systematic review. JAMA Oncol. 2017;3:1120–1129. doi: 10.1001/jamaoncol.2016.4280. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski T., Matecka-Nowak M., Milecki P. Prediction of radiation pneumonitis: dose-volume histogram analysis in 62 patients with non-small cell lung cancer after three-dimensional conformal radiotherapy. Neoplasma. 2005;52:56–62. [PubMed] [Google Scholar]
- 16.Kong Feng-Ming (Spring), Wang Shulian. Non-dosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015;25:100–109. doi: 10.1016/j.semradonc.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun S.G., Hu C., Choy H., Komaki R.U., Timmerman R.D., Schild S.E. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. https://ascopubs.org/doi/10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]