Abstract
Background
Seaweeds are an important source of bioactive compounds which are applied in various aspects of medicinal investigations. The present study was conducted to investigate cytoxicity (in-vitro and in-vivo) and wound healing activity of different seaweed species in Sri Lanka.
Methods
Twenty-three seaweed samples, belonging to Phaeophyta (Brown), Chlorophyta (Green) and Rhodophyta (Red) were used for the experiments. Samples were collected from the inter-tidal and the sub-tidal habitats around Sri Lankan coast (Southern, Northern and North-western). Aqueous seaweed extracts were tested for cytotoxic and wound healing activity; in-vitro and in-vivo. To determine toxicity of aqueous seaweed extracts, brine shrimp lethality assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay on mouse fibroblasts (L929) cell line were performed. Cell migration induction of seaweed extracts was assessed by scratch wound healing assay using L929 cell line. Based on the our previous experiments S.ilicifolium (SW23) was selected for the in vivo study to confirm our hypothesis. Albino mice (BALB/c) were divided into three groups (12 in each) and a circular area (44.07 ± 02.51 mm2) of full skin was excised to create a wound in mice group II and III. Group III received aqueous extract of Sargasum illicifolium (400 mg/kg BW/day for 12 days, orally), Group II received distilled water for 12 days whereas Group I was used as the control group and it was tested without forming wounds and without providing any treatment. Further, the expression level of Tumor Necrosis Factor (TNF-α) and Transforming Growth Factor-β (TGF-β) via RT-PCR were measured every three days until the end of the experiment.
Results
Phytochemical tests showed positive results to flavonoids in all the selected green seaweeds and alkaloids were observed in red seaweeds. In the toxicity assay, red seaweed, Acanthophora spicifera (SW17) was found to be highly effective on nauplii of brine shrimp (LC50 = 0.072 μg/μl). LC50 value of green seaweed species, Caulerpa racemosa (SW02 and SW08) and Caulerpa sertularioides (SW10) was not found within the tested concentration series. The highest cytotoxic effect on L929 cell line was exhibited by aqueous extracts of red seaweed; Jania adhaereus with 50.70 ± 7.304% cell viability compared with control group. The highest cell migration activity was observed in L929 cell line group treated with extracts of green seaweed namely; Halimeda opuntin (SW07) and extracts of brown seaweed namely; Stoechospermum polypodioides (SW11). Extracts of S. illicifolium (SW23) exhibited a significantly enhanced wound healing activity in mice group III within three days (P < 0.05) with an open wound area of 17.35 ± 1.94 mm2 compared with control group (26.29 ± 2.42 mm2). TGF-β gene expression peaked on 6th day of post-wound and subsequently decreased on 9th day of post-wound in mice group III. TNF-α expression was suppressed in mice group III whereas it was elevated in group II. TGF-β expression is enhanced in the treatment group compared to the control group.
Conclusions
Aqueous extracts of selected seaweeds are a significant source of potential compounds with wound healing properties, which might be helpful in the healing of various wounds. This also infers that many species of brown and red seaweeds have the potential of wound healing, specifically, Sargasum illicifolium and Jania adhaereus could be a potential candidate for in-vivo studies related to wound healing and cancer therapy in the near future.
Keywords: Seaweeds, Phytochemical, Brine shrimp, Cell viability, L929, Wound healing, Albino mice, Toxicity, m-RNA expression, TGF-β, TNF-α, Immunology, Biochemistry, Molecular biology, Toxicology, Animal behavior, Surgery, Pharmacology, Alternative medicine, Laboratory medicine
Seaweeds, Phytochemical, Brine shrimp, Cell viability, L929, Wound healing, Albino mice, Toxicity, m-RNA expression, TGF-β, TNF-α; Immunology; Biochemistry; Molecular biology; Toxicology; Animal behavior; Surgery; Pharmacology; Alternative medicine; Laboratory medicine
1. Introduction
Seaweeds are considered as an economically valuable natural resource and a rich source of extraordinary therapeutic agents that are important in the pharmaceutical industry (Dhargalkar and Pereira, 2005). However, the available information and scientific data with regards to the medicinal potential of these plants is lacking with. Therefore, many studies have been conducted to evaluate the toxicity of medicinal plants as an initial step before investigating other therapeutic potentials of any natural extract (Badakhshan et al., 2009; Wangchuk, 2018). Phytochemical screening is a process of evaluating phytochemical constituents present in the natural extracts using standard established tests (Audu et al., 2007; Prasad et al., 2008; Santhi, 2011). Currently, several studies have been conducted to investigate the therapeutic value of seaweeds and pointed marine algae as an outstanding source of active biochemical compounds (Torres et al., 2014). Biological resources including seaweed may contain toxic compounds and the evaluation of cytotoxicity is required prior to focusing on any other activity. The cytotoxic effect can be evaluated in-vitro or in-vivo (Carballo et al., 2002). Brine shrimp (Artemia sp.) lethality assay could be used as an indicator for presence of general toxic compounds in an extract. Since this is a low cost and relatively easy assay, cytotoxicity of the aqueous extracts could be screened efficiently (Olowa and Nuneza, 2013; Hamidi, et al., 2014). Based on the ability to inactivate laboratory hatched brine shrimp (Artemia sp.) nauplii by cytotoxic compounds, brine shrimp lethality assay was proposed by Carballo et al. (2002). This method has been introduced as a safe, practical and economically cheap method used to determine bioactive and cytotoxic effect of synthetic compounds (Almeida et al., 2002). MTT assay was conducted to assess the cytotoxicity effect of seaweed extracts against mouse fibroblasts cell line (L929) (Premarathna et al., 2019). The scratch wound healing assay has been broadly adapted and modified to study the effects of a variety of experimental conditions on cell migration and proliferation. In a typical scratch wound healing assay, many factors that alter the motility and/or growth of the cells can lead to increase or decrease in the rate of “healing” the gap (Premarathna et al., 2019).
Wound healing is a specific process leading to the restoration of injured tissues (Boateng et al., 2008). In the past, without any theoretical knowledge, human beings have been using many plant resources based on experiential observations for the treatment of wounds, cuts, and burns (Wang et al., 2011). In general, wound healing is a dynamic process that can be divided into five overlapping phases: hemostasis, inflammation, migration, proliferation and maturation (Velnar et al., 2009). In wound healing, TGF-β1 expression is important for inflammation, angiogenesis, re-epithelialization, and connective tissue regeneration. With the onset on an injury, cytokine expression levels are shown to be increased (Stenzel et al., 2011; Stratman et al., 2009). TGB-β1, produced by macrophages, T cells and platelets is a potent stimulus of fibroblasts and involve in a critical role in the proliferative stage (Gharaee-Kermani and Phan, 2001). It is also interesting to note that once the wound field is sterilized, TGF-b1 may be able to deactivate superoxide production from macrophages in-vitro. This helps to protect the surrounding healthy tissue and prepare the wound for granulation tissue formation (Tsunawaki et al., 1988). In superficial skin wounds, TGF-β stimulates contractile response and control the orientation of collagen fibers (Farahani and Kloth, 2008). The current wound healing drugs are not satisfactory, due to their low availability, high cost and numerous detrimental side effects (allergic reaction, damage skin integrity, etc.) (Kumar et al., 2007). Almost all the wound healing processes are found to be affected by bioactive compounds isolated from natural resources such as tannins, alkaloids, and triterpenoids (Kumar et al., 2007; Nayak and Pereira, 2006). Since seaweeds have shown several biological activities including wound healing, seaweeds have a great potential to be used to extract new therapeutic compounds. The available information on the medicinal potential of Sri Lankan seaweeds is not corroborated with creditable scientific data. For this reason, the present study was carried out to examine the phytochemical constituents, in-vivo and in-vitro cytotoxic effect and wound healing ability aqueous extracts of different seaweed species available in Sri Lanka.
2. Materials and methods
2.1. Seaweeds sample collection
Twenty-three different seaweeds species, belonging to Phaeophyta (Brown), Chlorophyta (Green) and Rhodophyta (Red) samples were collected from inter-tidal and sub-tidal habitat in Southern, Northern and North-western coastal areas in Sri Lanka (see Figure 1).
Figure 1.
Map of seaweed samples collection location. 01-Ahangama (N 05⁰ 58.006′ E 080⁰ 22.482′), 02-Thalpe (N 05⁰ 59.792′ E 080⁰ 16.898′), 03-Chilaw (N 07⁰ 36.220′ E 079⁰ 47.120′), 04-Negambo (N 07⁰ 12.170′ E 079⁰ 48.570′), 05-Kankasanthurai (N 09⁰ 48.592′ E 080⁰ 02.546′), 06-Point Pedro (N 09⁰ 49.501′ E 080⁰ 15.119′).
Firstly, the harvested seaweed samples were washed thoroughly with seawater (at the site), then with tap water (fresh water) to remove contaminants (sand particles, impurities and epiphytes) and finally, cleaned with distilled water. All those cleaned samples were transported to the laboratory at the Department of Veterinary Pathobiology, Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Sri Lanka under sterilized and cold conditions. Collected seaweed species were identified using identification guide (Coppejans et al., 2009). Furthermore, the seaweed species were authenticated using the “Peradeniya Royal Botanical Gardens: National Herbarium in Sri Lanka” and a voucher herbarium of seaweeds (Figure 2) were deposited in the laboratory for future reference. The samples of seaweeds were dried, at 40 °C for four days until a constant weight was achieved. Then the samples were ground (Electrical Herbal Grinder; CS-700, China) to a 0.5 mm particle size powder and stored in the freezer at -20 °C (see Table 1).
Figure 2.
Voucher specimens. SW01-Ulva lactuca, SW02-Caulerpa racemosa, SW03-Gracilaria corticata, SW0-Padina antillarum, SW05-Sargassum illicifolium, SW06-Sargassum polycystem, SW07-Halimeda opuntin, SW08-Caulerpa racemosa, SW09-Turbinaria ornata, SW10-Caulerpa sertularioides, SW11-Stoechospermum polypodioides, SW12-Sargassum illicifolium, SW13-Sargassum illicifolium, SW14-Gracilaria corticata, SW15-Padina antillarum, SW16-Ulva lactuca, SW17-Acanthophora spicifera, SW18-Gelidiopsis variabilis, SW19-Gracilaria corticata, SW20-Chaetomorpha antennina, SW21-Chaetomorpha crassa, SW22-Jania adhaereus, SW23-Sargassum illicifolium.
Table 1.
Name of Seaweed species, collected location and voucher numbers.
| Collect Date | Voucher number | Type | Species Name | Location | GPS Point |
|---|---|---|---|---|---|
| 18/02/2016 | SW 01 | Green | Ulva lactuca | Thalpe | N 05⁰ 59.792′ E 080⁰ 16.898′ |
| 18/02/2016 | SW 02 | Green | Caulerpa racemosa | Ahangama | N 05⁰ 58.006′ E 080⁰ 22.482′ |
| 18/02/2016 | SW 03 | Red | Gracilaria corticata | Ahangama | N 05⁰ 58.006′ E 080⁰ 22.482′ |
| 18/02/2016 | SW 04 | Brown | Padina antillarum | Ahangama | N 05⁰ 58.006′ E 080⁰ 22.482′ |
| 18/02/2016 | SW 05 | Brown | Sargassum ilicifolum. | Thalpe | N 05⁰ 59.792′ E 080⁰ 16.898′ |
| 24/02/2016 | SW 06 | Brown | Sargassum polycystem | Point Pedro | N 09⁰ 49.501′ E 080⁰ 15.119′ |
| 24/02/2016 | SW 07 | Green | Halimeda opuntin | Kankasanthurai | N 09⁰ 48.592′ E 080⁰ 02.546′ |
| 24/02/2016 | SW 08 | Green | Caulerpa racemosa | Point Pedro | N 09⁰ 49.501′ E 080⁰ 15.119′ |
| 24/02/2016 | SW 09 | Brown | Turbinaria ornata | Kankasanthurai | N 09⁰ 48.592′ E 080⁰ 02.546′ |
| 24/02/2016 | SW 10 | Green | Caulerpa sertularioides | Kankasanthurai | N 09⁰ 48.592′ E 080⁰ 02.546′ |
| 24/02/2016 | SW 11 | Brown | Stoechospermum polypodioides | Point Pedro | N 09⁰ 49.401′ E 080⁰ 14.593′ |
| 24/02/2016 | SW 12 | Brown | Sargassum ilicifolum | Kankasanthurai | N 09⁰ 48.592′ E 080⁰ 02.546′ |
| 18/03/2016 | SW 13 | Brown | Sargassum ilicifolum | Negambo | N 07⁰ 12.170′ E 079⁰ 48.570′ |
| 18/03/2016 | SW 14 | Red | Gracilaria corticata | Negambo | N 07⁰ 12.170′ E 079⁰ 48.570′ |
| 18/03/2016 | SW 15 | Brown | Padina antillarum | Negambo | N 07⁰ 12.170′ E 079⁰ 48.570′ |
| 18/03/2016 | SW 16 | Green | Ulva lactuca | Negambo | N 07⁰ 12.170′ E 079⁰ 48.570′ |
| 18/03/2016 | SW 17 | Red | Acanthophora spicifera | Negambo | N 07⁰ 12.170′ E 079⁰ 48.570′ |
| 18/03/2016 | SW 18 | Red | Gelidiopsis variabilis | Chilaw | N 07⁰ 36.220′ E 079⁰ 47.120′ |
| 18/03/2016 | SW 19 | Red | Gracilaria corticata | Chilaw | N 07⁰ 36.220′ E 079⁰ 47.120′ |
| 18/03/2016 | SW 20 | Green | Chaetomorpha antennina | Chilaw | N 07⁰ 36.220′ E 079⁰ 47.120′ |
| 18/03/2016 | SW 21 | Green | Chaetomorpha crassa | Chilaw | N 07⁰ 36.220′ E 079⁰ 47.120′ |
| 18/03/2016 | SW 22 | Red | Jania adhaereus | Chilaw | N 07⁰ 36.220′ E 079⁰ 47.120′ |
| 21/01/2017 | SW 23 | Brown | Sargassum ilicifolum | Ahangama | N 05⁰ 58.006′ E 080⁰ 22.482′ |
2.2. Preparation of extracts and estimation of the concentration of seaweed extract
The powdered samples were weighed and 5 g of each sample was suspended/dissolved separately in 25 ml of distilled water. Then the samples were dissolved via sonication for 1 h using ultrasound sonicator (Branson 2510; Danbury, USA). The temperature was maintained at 40 °C throughout the process. Then, the samples were shaken in a roller overnight at room temperature (DENLEY-SPIRAMIX 5, UK) and the extracts were centrifuged (Beckman Avanti, UK) at 15000 rpm for 10 min at 4 °C and supernatants were taken for further analysis. Following methods (A and B) were used to calculate the concentrations of aqueous seaweed extracts.
-
(A).
All the residuals removed during the process of water extraction was taken and kept in 40 °C dry oven until it gives constant weight. Then the concentration was analyzed using the following equation
-
(B).
5 ml of each seaweed extract was added to petri dishes separately and labeled. The extract was kept in the dryer at 40 °C temperature until the samples gets a constant weight. Finally, dry weights of the samples were recorded and concentrations were calculated as the concentration per 1 ml of the extract.
2.3. Preliminary phytochemical screening
Seaweeds contain many chemical constituents which are therapeutically active or inactive such as carbohydrates, triterpenoids, alkaloids, glycosides, tannins, flavonoids, lipids and other similar secondary metabolites (Tuhin et al., 2017). Based on the literature, aqueous extraction product mainly includes high hydrophilic compounds, metals, ions, and water soluble proteins/enzymes, glycoproteins, amino acids, peptides, nucleotides, sugars, and polysaccharides (Bouchard et al., 2007). Several methods were followed to screen specific compounds in the seaweed extracts of this study.
2.4. Brine shrimp lethality assay (BSLA)
BSLA assay has been noted as a useful tool for the isolation of bioactive compounds from plant extracts (Prakash et al., 2015). The assay was conducted according to Prakash et al. (2016) with some modifications. Filtered, seawater (pH range; 7.85 ± 0.5) was put in a small plastic container (hatching chamber) with a partition for dark (covered) and light areas. Brine Shrimp eggs (0.5 g) were added into the dark side of the chamber. The eggs were hatched for 24–36 h at room temperature with strong aeration under continuous light regime. Then, the phototropic nauplii were collected from the illuminated side of the jar. 10–15 brine shrimp nauplii with 50 μl of seawater were added to into each well of 96 well plate filled with different concentrations (Two-fold dilution series was made separately in 96 well plates) of seaweed extracts. Distilled water was used as the negative control and the absolute ethanol was used as positive control. Well plates were incubated for 24 h and after 24 h incubation number of dead and survived shrimp nauplii were counted under the light microscope and recorded. The brine shrimps that did not show any movement for more than 2 min were recorded as dead nauplii. The percentage mortality (%M) was calculated by dividing the number of dead nauplii by the total number, and then multiplied by 100. These values were used to calculate LC50 values of each aqueous seaweed extract against brine shrimp. To certify that the mortality detected in the bioassay is due to the effect of the bioactive compounds present in seaweed sample and not due to starvation (Ullah et al., 2013), mortality rate of nauplii in both negative and positive control was compared with the mortality rate of seaweed treated nauplii. Cytotoxicity of seaweed extracts was evaluated by comparing the LC50 values.
2.5. In-vitro assay
2.5.1. Cell viability (MTT assay)
The mouse fibroblasts cell line (L929, ATCC, USA) was maintained at Roswell Park Memorial Institute (RPMI-1640) medium (Invitrogen, Gibco, UK), supplemented with 10 ml/L Penicillin-Streptomycin (PSA), 2 g/L sodium bicarbonate (NaHCO₃), 5 ml/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10% fetal bovine serum (FBS, Invitrogen, Gibco, UK). L929 cells were cultured and maintained under an atmosphere of 5% CO2 and 95% air incubator (MCO-20AIC, Japan) at 37 °C. The cells were grown in 96-well flat bottom plates (Corning Glass-work, NY) at a density of approximately 2×105 cells/well and incubated until it reaches 70%–80% confluence level. The medium was removed from the wells and washed with Phosphate buffered saline to remove the dead cells. 50 μl of RPMI medium, separately supplemented with aqueous extracts of all seaweeds samples was introduced per well plated with L929 cells. Ten dilutions of a two-fold serial dilution were used with triplicates and diluent in 8-wells were used for respectively concentration. The treated cells were incubated further for 24 h. In addition, positive and negative control tests were also prepared using absolute ethanol and distilled water, separately. After 24 h, the treated mixtures were removed and washed with PBS to remove any residual traces of chemicals. 10 μl of MTT (5 mg/ml in PBS) regent was added to each well and further incubated for 4 h at 37 °C. Finally, the formazan crystals were dissolved in 100 μl Dimethyl Sulfoxide (DMSO) and the absorbance values was measured on ELISA reader (Muitiskan Ex, German) at 570 nm (Pieters et al., 1988). All experiments were carried out in triplicates and the same experiment was conducted three times and the mean values of all experiments were analysed.
2.5.2. Scratch wound healing assay
The scratch assay was performed to study cell migration and proliferation ability with respect to the treatments. Mouse fibroblast (L929) cells were grown in RPMI-1640 supplemented with 10% FBS. 2×105 cells/well were seeded into 24-well cell culture plates and incubated for 24 h, till the wells reach 70–80% confluence as a monolayer. Without changing the medium, cellular monolayer scratched with 200 μl pipette tip and an artificial linear wound was introduced. The medium was removed in the wells and gently washed with Phosphate buffered saline to remove dead/unattached cells. Replenished the wells with 150 μl fresh medium supplemented with 150 μl the of the aqueous extract of seaweeds (The best concentration recorded from above cell viability assay was taken for this experiment). The wound size was observed after 24 h and 48 h incubation. Scratch wound closures were observed using an inverted microscope and digital photograph were taken by a digital camera (Nikon Coolpix 4500; Nikon, Japan). Photographs were analyzed by Carl Zeiss Microscopy GmbH software followed by monitoring the width of the scratch area at 0h, 12h and 24 h time intervals to quantify cell migration ability.
2.6. In-vivo assay
2.6.1. Experiment I - wound healing
2.6.1.1. Dose selection
A pilot dose-response study was conducted to decide the toxic effects of aqueous extracts of selected seaweed species on mice. Doses of aqueous extracts of seaweeds 100 mg/kg BW, 200 mg/kg BW and 400 mg/kg BW were orally administered to mice once daily for 14 days. Animals were observed individually after oral treatment at least once after the first 45 min, periodically after the first 24 h and 48 h for their behavioral changes and stimulations were monitored for 14 days. In the acute toxicity studies, if any behavioral change or mortality and histopathology investigation were detected, the dose administered was assigned as a toxic dose. Therefore, no signs of behavioral changes or mortality were observed by 100, 200 and 400 mg/kg BW doses administered to mice during this initial dose screening in-vivo experiment toxicity assay. Therefore, the maximum dose of 400 mg/kg BW of mice was chosen to administer to mice during the in-vivo experiments of this study.
2.6.1.2. Experimental animals
Healthy albino mice (BALB/c), total of 36 with average body weight (BW) of 34.79 ± 1.03 g and age of 8 weeks were selected. All the mice were marked and acclimatized for seven days before the experiment. Mice were sheltered separately in individual ventilated cages with the laboratory conditions of temperature regulated at 28 ± 2 °C, humidity (65 ± 5%), with a 12 h light/12 h dark cycle and fed with standard pellet diet and water ad libitum. All the mice were acclimatized for 7 days prior to the study. The study was approved by the Animal Ethics Committee of the Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Sri Lanka (Ref No. ERC/FVMA/UOP/2013/10), which is on par with the international standards of ethics on animal experimentations.
2.6.1.3. Excision wound model
A full-thickness excision wound was made on general anesthesia by Ketamine (120 mg/kg) (Uhlig et al., 2015), the shaved dorso-lateral skin with 8 mm Piercing sterile biopsy punch. The shaved skin area was cleaned with 70% ethanol and wounds were created by excising a circular 07.48 ± 0.05 mm diameter portion of the wound area 44.02 ± 0.70 mm2. Immediately after wounding, all animals were kept in separate cages with tissue towel beds and fly proof environment by covering of mosquito net. Cage beds were cleaned twice a day with antiseptics (1% Savlon).
2.6.1.4. Treatment of animal
The mice were divided into three different groups (n = 12): Group III; wounds were created and received S. illicifolium extracts (400 mg/kg BW/day for 12 days, orally); Group II; wounds were created and received distilled water for 12 days, orally; Group I; normal mice kept intact without wound and any treatment.
2.6.1.5. Measurement of wound contraction and area
The progressive healing changes in all the treatment and control group were measured with a vernier caliper and digital photographs of the wound areas were taken on every three days starting from the first day of wound treatment until the end of the experimental period. The number of days taken for healing of the wound was estimated (Chatterjee and Chakravorty, 1993) and reduction in the wound area was expressed as percentage of the original wound size (Nayak et al., 2009). Changes in wound areas were analyzed and used as an indication of the percentage of wound contraction. It was calculated by using the following formula:
| Percentage of wound healing = (Healed area/Total wound area) × 100 |
(Healed area = Total wound area − Present wound area).
2.6.1.6. Histopathology
A specimen sample of tissue from the healed wound was isolated from treatment and control group of mice and fixed in 10% neutral buffered formalin and dehydrated with a sequence of ethanol-xylene series of solutions, processed and blocked with paraffin at 40-6 °C and sectioned at 3–6 μm in thickness. Paraffin was removed by dehydration and again rehydrated sections were stained with hematoxylin and eosin stain (H & E) for histopathological observations. The wounded tissue sections were assessed for extent of wound contraction, angiogenesis, collagen formation, granulation tissue and epithelization. The progress of epithelization was recorded from the border of normal skin toward the wound center. The microscopic slides were photographed and examined under magnification (40X and 100X).
2.6.2. Experiment II - cytokine expression
2.6.2.1. RNA isolation and amplification
Total RNAs were isolated from the treatment and control wound skin samples using PureLink RNA mini kits (USA) according to the manufacturer's instructions. RNA was amplified with random primers as following modifications to inhibit the degradation of RNA by abundant skin RNAases. RNA isolation with potential interference of protein were removed by adding the homogenate in lysis buffer 1 ml with 2-mercaptoethanol 10 μl at room temperature for 3 min and then samples were homogenized using QIAshredder Mini Spin Columns (QIAGEN, USA). Supernatant was recovered and RNA was isolated through a RNeasy mini column with one volumes of chilled ethanol (70%) into a RNase-DNase free tube.
2.6.2.2. cDNA preparation and PCR amplification
The first strand cDNA synthesis was accomplished in 1μl M-MLV Reverse transcriptase (Dongsheng biotech, China) and RNase Inhibitor (Dongshengbiotech, China). Total RNAs of wounded treatment and control mice tissue samples were reverse-transcribed at 70 °C for 5 min in a 20 μl reaction mixture containing mouse Moloney leukemia virus reverse transcriptase (Dongshengbiotech, China) with Random Primers (Dongsheng biotech, China). cDNA was amplified together with specific primers for Tumor Necrosis Factor (TNF-α), Transforming Growth Factor β1 (TGF- β1) and β-actin as control with the primers and conditions mentioned in Table 2.
Table 2.
Sequences of the primers used for RT-PCR, Transcript Sequence annealing temperature (⁰C), Cycle and Product size (bp).
| Transcript | Sequence | Annealing temperature (⁰C) | Cycle | Produc size (bp) |
|---|---|---|---|---|
| TNF-α | (F) 5-CAGCCTCTTCTCATTCCTGCTTGTG-3 | 60 | 35 | 511 |
| (R) 5-CTGGAAGACTCCTCCCAGGTATAT-3 | ||||
| TGF-β1 | (F) 5-CGGGGCGACCTGGGCACCATCCATGAC-3 | 60 | 35 | 405 |
| (R) 5-CTGCTCCACCTTGGGCTTGCGACCCAC-3 | ||||
| beta-actin | (F) 5-TTCTACAATGAGCTGCGTGTGGC-3 | 60 | 35 | 456 |
| (R) 5-CTCATAGCTCTTCTCCAGGGAGGA-3 |
(F) Forward primer, (R) reverse primer.
Polymerase chain reactions (PCR) were performed in 25 μl of the total volume containing 5×PCR buffer, 25 mM MgCl2, 2 mM deoxynucleotide triphosphate (dNTP), 10 μM forward and reverse primers, and Taq DNA polymerase (0.25μl). PCR conditions comprised of an initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for 45 s, extension at 72 °C for 1 min and the final extension at 72 °C for 7 min. The PCR products were run on a 2% agarose gel stained with ethidium bromide and subsequently specific bands purified using a gel extraction kit according to the manufacturer's instruction (DongshengBiotech, China). The intensities of specific bands were photographed with a charge-coupled device imaging system (Gel Doc.). The specific bands of Purified PCR products were quantified using NanoDrop Spectrophotometers (2000, Termo Scientific, USA). RT-PCR was performed at least three times for each sample and specific PCR products quantify were determined as described above.
2.6.3. Experiment III - toxicity study
After treatment of three mice from each group were sacrificed on the 12th, 15th, 19th and 26th days of the experimental period. Blood samples were collected to heparin tubes and the serum was separated by centrifugation at 2500 rpm at 4 °C for 10 min for the assessment of biochemical parameters. The Serum enzyme levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine levels (Kihdze et al., 2016) using commercially available human diagnostic test kits (Human Wiesbaden, Germany) according to the manufacturer's instructions. The Erba Mannheim spectrophotometer (Model:chem-7, Germany) was used for the serum enzyme measurement. Vital organs of mice (liver, kidney, heart, spleen, and lung) were isolated and weighed. Furthermore, specimens from the organs were collected, examined for any gross changes and preserved for histopathological and toxicological investigations. Behavioral changes, food consumption, and water intake were recorded every three days until the end of the experiment. Body weights of mice have recorded before the starting the experiment and at the end of the experimental period.
2.7. Statistical analysis
Prism software package (Graph Pad, Version 4.03; San Diego, CA, USA) for Windows and Excel software were used for all statistical analysis. Lethality concentrations LC50 were assessed. ANOVA test was done to find out the significant difference between test extractions and control. Multiple comparisons between the significant levels of interactions of the variables were done by Tukey's method. Values were expressed as the Mean ± SEM and P < 0.05 considered as the presence of statistically significant difference.
3. Results
3.1. Extraction matter concentration analysis
Concentration values obtained by the both the methods were more or less the same (see Table 3).
Table 3.
Concentration (μg/μl) of aqueous seaweed extracts.
| Sample Number | Species Name | Concentration (μg/μl) |
|---|---|---|
| SW 01 | Ulva lactuca | 39.08 ± 0.34 |
| SW 02 | Caulerpa racemosa | 43.84 ± 0.21 |
| SW 03 | Gracilaria corticata | 25.80 ± 0.59 |
| SW 04 | Padina antillarum | 27.20 ± 0.64 |
| SW 05 | Sargassum ilicifolum. | 32.08 ± 0.11 |
| SW 06 | Sargassum polycystem | 12.80 ± 0.12 |
| SW 07 | Halimeda opuntin | 07.64 ± 0.09 |
| SW 08 | Caulerpa racemosa | 17.32 ± 0.63 |
| SW 09 | Turbinaria ornata | 23.36 ± 0.38 |
| SW 10 | Caulerpa sertularioides | 17.00 ± 0.22 |
| SW 11 | Stoechospermum polypodioides | 46.84 ± 0.69 |
| SW 12 | Sargassum ilicifolum. | 16.96 ± 0.55 |
| SW 13 | Sargassum ilicifolum. | 60.01 ± 0.35 |
| SW 14 | Gracilaria corticata | 41.12 ± 0.41 |
| SW 15 | Padina antillarum | 36.48 ± 0.63 |
| SW 16 | Ulva lactuca | 95.44 ± 0.33 |
| SW 17 | Acanthophora spicifera | 00.17 ± 0.03 |
| SW 18 | Gelidiopsis variabilis | 14.88 ± 0.46 |
| SW 19 | Gracilaria corticata | 22.84 ± 0.28 |
| SW 20 | Chaetomorpha antennina | 34.04 ± 0.31 |
| SW 21 | Chaetomorpha crassa | 39.08 ± 0.24 |
| SW 22 | Jania adhaereus | 84.20 ± 0.16 |
| SW 23 | Sargassum ilicifolum | 31.16 ± 0.12 |
3.2. Phytochemical screening of seaweeds
The seaweeds were technically certified through the identification of the phytochemicals responsible for their use in therapeutic values of health care. The results of qualitative chemical analysis of the aqueous extract of seaweeds are charted in Table 4.
Table 4.
The results of phytochemical screening of aqueous seaweed extracts.
| Sample Number | Species Name | Saponins | Tannins | Flavonoids | Alkaloids | Triterpenes | References |
|---|---|---|---|---|---|---|---|
| SW 01 | Ulva lactuca | ++ | + | + | - | - | Paiva et al. (2017) |
| SW 02 | Caulerpa racemosa | + | +++ | ++ | - | ++ | Lee et al. (2013) |
| SW 03 | Gracilaria corticata | ++++ | + | +++ | +++ | ++ | De Almeida et al. (2011) |
| SW 04 | Padina antillarum | + | - | - | - | - | Arunkumar et al. (2010); Ponnanikajamideen et al. (2014) |
| SW 05 | Sargassum illicifolium | ++ | + | + | + | - | Permeh et al. (2012); Basha and Muthukumar, 2014 |
| SW 06 | Sargassum polycystem | + | + | ++ | + | - | Permeh et al. (2012); Mehdinezhad et al. (2016) |
| SW 07 | Halimeda opuntin | - | - | + | - | +++ | Liu et al. (2011); Al-Wathnani et al. (2012) |
| SW 08 | Caulerpa racemosa | - | + | + | + | +++ | Lee et al. (2013) |
| SW 09 | Turbinaria ornata | + | + | + | - | - | Neelamathi and Kannan (2016); Ermakova et al. (2016) |
| SW 10 | Caulerpa sertularioides | - | - | + | - | + | Viswanathan and Nallamuthu (2013); Lee et al. (2013) |
| SW 11 | Stoechospermum polypodioides | + | + | + | + | ++ | Anbu et al. (2017) |
| SW 12 | Sargassum illicifolium | ++ | + | + | + | - | Permeh et al. (2012); Basha and Muthukumar, 2014 |
| SW 13 | Sargassum illicifolium | + | + | + | + | - | Permeh et al., 2012; Basha and Muthukumar, 2014 |
| SW 14 | Gracilaria corticata | + | + | ++ | +++ | + | De Almeida et al. (2011) |
| SW 15 | Padina antillarum | + | + | - | - | - | Arunkumar et al. (2010); Ponnanikajamideen et al. (2014) |
| SW 16 | Ulva lactuca | ++ | + | + | - | - | Paiva et al. (2017) |
| SW 17 | Acanthophora spicifera | + | + | ++ | - | + | Domettila et al. (2013) |
| SW 18 | Gelidiopsis variabilis | - | + | + | ++ | - | Lee et al. (2013); Raposo et al. (2013) |
| SW 19 | Gracilaria corticata | + | + | ++ | ++ | + | De Almeida et al. (2011) |
| SW 20 | Chaetomorpha antennina | ++ | + | + | - | - | Pierre et al. (2011); Domettila et al. (2013) |
| SW 21 | Chaetomorpha crassa | + | + | + | - | - | Pierre et al. (2011); Kumbhar et al. (2014) |
| SW 22 | Jania adhaereus | + | + | + | +++ | + | Karabay-Yavasoglu et al. (2007); Mohy El-Din and El-Ahwany (2016) |
| SW 23 | Sargassum illicifolium | ++ | + | + | + | - | Permeh et al., 2012; Basha and Muthukumar, 2014 |
+ Present; - Absent.
3.3. Brine shrimp assay
The crude extracts of Sargassum polycystum (SW06; 12.80 μg/μl), Turbinaria ornate (SW09; 23.36 μg/μl), Sargassum illicifolium (SW13; 16.96 μg/μl), Padina antillarum (SW15; 36.48 μg/μl), Gelidiopsis variabilis (SW18; 14.88 μg/μl), Gracilaria corticata (SW19; 22.84 μg/μl), Chaetomorpha antennina (SW20; 34.04 μg/μl), Chaetomorpha crassa (SW21; 39.08 μg/μl) and Sargasum illicifolium (SW23; 31.16 μg/μl) showed 100% mortality of brine shrimp nauplii followed by 24 h incubation. Moreover, the crude extracts of Gracilaria corticata (SW14; 41.12 μg/μl), Acanthophora spicifera (SW17; 0.20 μg/μl) and Jania adhaerens (SW22; 84.2 μg/μl) exhibited a remarkable toxic effect of brine shrimp nauplii after 2 h of incubation period (see Table 5).
Table 5.
LC50 values in μg/μl of aqueous extracts of different seaweed samples after incubation for 24 h. 50% lethal concentration for brine shrimp nauplii was considered as LC50 value.
| Voucher number and species | LC50 value (μg/μl) | Voucher number and species | LC50 value (μg/μl) |
|---|---|---|---|
| SW01;Ulva lactuca | 49.71 | SW13;Sargassum illicifolium | 7.63 |
| SW02;Caulerpa racemosa | - | SW14;Gracilaria corticata | 4.93 |
| SW03;Gracilaria corticata | 12.93 | SW15;Padina antillarum | 16.05 |
| SW04;Padina antillarum | - | SW16;Ulva lactuca | - |
| SW05;Sargassum illicifolium | 18.43 | SW17;Acanthophora spicifera | 0.03 |
| SW06;Sargassum polycystem | 5.76 | SW18;Gelidiopsis variabilis | 9.74 |
| SW07;Halimeda opuntin | 37.6 | SW19;Gracilaria corticata | 1.91 |
| SW08;Caulerpa racemosa | - | SW20;Chaetomorpha antennina | 16.7 |
| SW09;Turbinaria ornata | 3.89 | SW21;Chaetomorpha crassa | 21 |
| SW10;Caulerpa sertularioides | - | SW22;Jania adhaereus | 10.06 |
| SW11;Stoechospermum polypodioides | - | SW23;Sargasum illicifolium | 9.93 |
| SW12;Sargassum illicifolium | 5.30 |
All tested seaweed species have shown concentration dependent mortality of brine shrimp nauplii after 24 h incubation. Collectively all red seaweed species showed significantly low LC50 values compared with all other tested brown and green seaweed samples. Ulva lactuca collected from Negombo (North-western coastal area) showed the highest LC50 value out of all green seaweed samples.
3.4. In-vitro assay
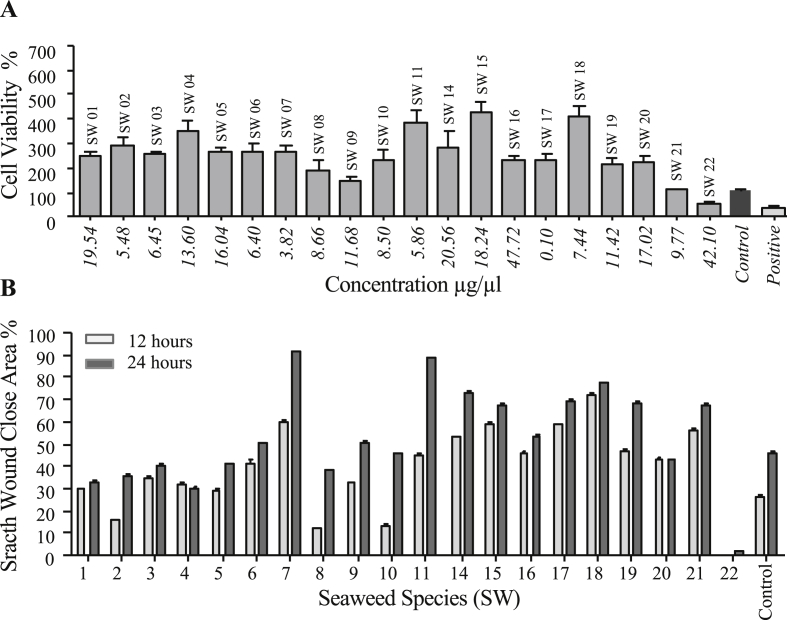
Most of the tested aqueous seaweed extracts did not appear significant cytotoxic effect on L929 cells. Based on the results, cell proliferation and migration activity were observed in the wells with L929 cell line treated with most of the aqueous extracts of brown seaweeds. Furthermore, almost all tested aqueous extracts of Green, Brown and Red seaweed samples, except Red algae Jania adhaereus (SW22), did not show a significant cytotoxic effect on L929 cells (see Figure 3 & Figure 4).
Figure 3.
L929 cell viability and proliferation percentage against aqueous extracts of seaweed samples after 24 h incubation. A; Cell viability is given as a percentage with compared to the control test. B; L929 cell proliferation observed as a percentage of the scratch wound close area compared to the control test. SW01-Ulva lactuca; 19.54 μg/μl, SW02-Caulerpa racemose; 5.48 μg/μl, SW03-Gracilaria corticata; 6.45 μg/μl, SW04-Padina antillarum; 13.60 μg/μl, SW05-Sargassum illicifolium; 16.04 μg/μl, SW06-Sargassum polycystem; 6.40 μg/μl, SW07-Halimeda opuntin; 3.82 μg/μl, SW08-Caulerpa racemose; 8.66 μg/μl, SW09-Turbinaria ornate; 11.68 μg/μl, SW10-Caulerpa sertularioides; 8.50 μg/μl, SW11-Stoechospermum polypodioides; 5.86 μg/μl, SW14-Gracilaria corticata; 20.56 μg/μl, SW15-Padina antillarum; 18.24 μg/μl, SW16-Ulva lactuca; 47.72 μg/μl, SW17-Acanthophora spicifera; 0.10 μg/μl, SW18-Gelidiopsis variabilis; 7.44 μg/μl, SW19-Gracilaria corticata; 11.42 μg/μl, SW20-Chaetomorpha antennina; 17.02 μg/μl, SW21-Chaetomorpha crassa; 9.77 μg/μl, SW22-Jania adhaereus; 42.10 μg/μl, All values are expressed as mean±SEM (n=08). One-way analysis of variance (ANOVA) Tukey’s comparisons test. ∗indicates comparison with the Control, ∗p<0.05.
Figure 4.
The migration and proliferation ability on L929 cells against the seaweed extracts. Microscopic inspection (40×magnifications) at 12 h and 24 h of scratch wound healing. SW01-Ulva faciata, SW02-Caulerpa racemosa, SW03-Gracilaria corticata, SW04-Padina antillarum, SW05-Sargassum illicifolium, SW06-Sargassum polycystem, SW07-Halimeda opuntin, SW08-Caulerpa racemosa, SW09-Turbinaria ornata, SW10-Caulerpa sertularioides, SW11-Stoechospermum polypodioides, SW14-Gracilaria corticata, SW15-Padina antillarum, SW16-Ulva lactuca, SW17-Acanthophora spicifera, SW18-Gelidiopsis variabilis, SW19-Gracilaria corticata, SW20-Chaetomorpha antennina, SW21-Chaetomorpha crassa,SW22-Jania adhaereus. Cell proliferation is given as a percentage compared to the control test. All values are expressed as mean ± SEM (n = 08). One-way analysis of variance (ANOVA) Tukey's comparisons test. ∗indicates significantly difference with Control, ∗p < 0.05.
3.5. In-vivo assay
3.5.1. Wound healing
The progressive healing changes in wounds of mice from each group during the experiment are presented below.
The rates of wound contraction were expressed as a percentage (%) of healed wound area (Table 6).
Table 6.
Wound healing area % (mm2) of each test group over a period of 12 days.
| Day | Control | Treatment |
|---|---|---|
| 01 | 43.75 ± 02.21 | 44.70 ± 00.43 |
| 03 | 26.29 ± 02.42 | 17.35 ± 01.94a∗ |
| 06 | 15.12 ± 02.48 | 07.71 ± 01.15a∗ |
| 09 | 02.92 ± 00.76 | 00.06 ± 00.02a∗ |
| 12 | 00.00 ± 00.00 | 00.00 ± 00.00 |
Data are expressed as values: mean ± SE (n = 12) and analyzed by two-way analysis of variance. ∗p < 0.05 when compared to control group animals. a = when compared with control group, (∗) indicates statistically significant difference from respective group using ANOVA, followed by Tukey's comparisons test (p > 0.05). (†) indicates statistically no significant difference from respective group using ANOVA, followed by Tukey's comparisons test (p > 0.05). Control group received an equal volume of distilled water, orally; untreated wounds and Treatment group received S. illicifolium extracts (400 mg/kg BW/day for 14 days, orally).
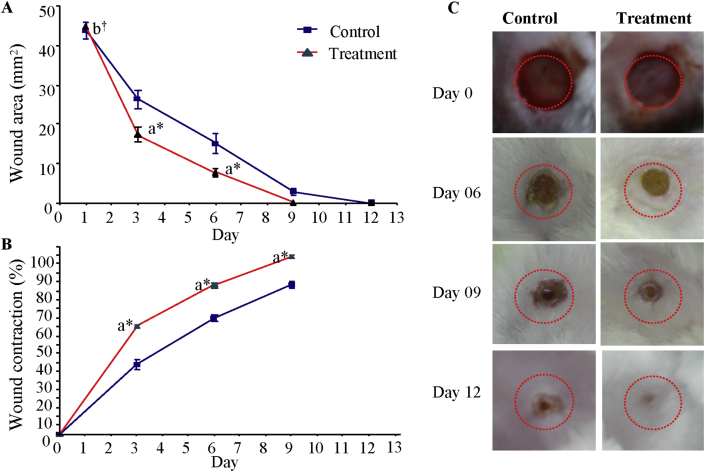
There was a significant difference (p < 0.05) in wound closure between the control and treatment group on the 3rd and 6th and 9th day. S. illicifolium extracts exhibited a significantly enhanced wound healing activity in mice group III within three days (p < 0.05) with open wound area of 17.35 ± 1.94 mm2 compared with control group 26.29 ± 2.42 mm2. On day 6th, the treatment group and control group had an open wound area of 7.70 ± 1.15 mm2 and 15.12 ± 2.48 mm2, respectively. On day 9th, treatment group and the control group had an open wound area of 0.058 ± 0.022 mm2 and 2.92 ± 0.76 mm2, respectively. On the 12th day the wound area of treatment group mice was completely healed and epithelialized (see Figure 5).
Figure 5.
Changes in wound area at each time point to the original wound area of mice in control and treatment group over a period of 12 days. A; Wound healing area (mm2) of test groups, B; Wound healing percentages (%) of test and control groups, C; Digital photographs of mice showing various stages of wound healing. Day 0 picture was taken immediately after injury. Values of diameter of wounds are expressed as mean ± SEM (n = 12). a = when compared with control group, (∗) indicates statistically significant difference from respective group using ANOVA, followed by Tukey's comparisons test (p > 0.05). (†) indicates statistically no significant difference from respective group using ANOVA, followed by Tukey's comparisons test (p > 0.05). Control group received an equal volume of distilled water, orally; untreated wounds and Treatment group received S. illicifolium extracts (400 mg/kg BW/day for 12 days, orally).
The extracts of S. illicifolium showed the highest level of wound healing activity within three days (61.20%) compared with the control group (39.91%).
3.5.2. Hitopathological changes
In the treatment group, 82.75% of the original wound area healed within 6 days after injury, with angiogenesis and collagen accumulation. The renewed epidermis had enclosed the wound area completely at the 9th day of S. illicifolium treatment group. Primitive degree of granular tissue formation was reported in the control group, but there were no differentiated epidermal layers compered to treatment group.
Images were taken with a microscope facilitated with a camera (×40 and ×100 magnifications) (Carl Zeiss Microscopy, Germany). Histopathological findings showed that the aqueous extract of S. illicifolium has enhanced the tissue granulation and re-epithelization significantly compared with the control group. S. illicifolium extracts treatment resulted decrease in inflammation, increase in the rate of tissue proliferation as well as remodeling, and reepithelization (Figure 6).
Figure 6.
Photomicrographs epidermis sections of wound tissues; hematoxylin and eosin stain staining (H & E; 40 and 100). A; Histopathological skin sections determine the changes in wound healing events B; Granulation tissue of the wounded skin. Arrows pointing events during wound healing; s, scab; re, re- epithelialization; IC, inflammatory cells; nv, neovascularization, GT: granulation tissue, mnc: mononuclear; F: fibroblasts; CF: collagen fiber, NE: new epithelium. Control (C): received an equal volume of distilled water, orally; untreated wounds, Treatment (T): received S. illicifolium extracts (400 mg/kg BW/day for 12 days, orally).
3.5.3. Cytokine expression
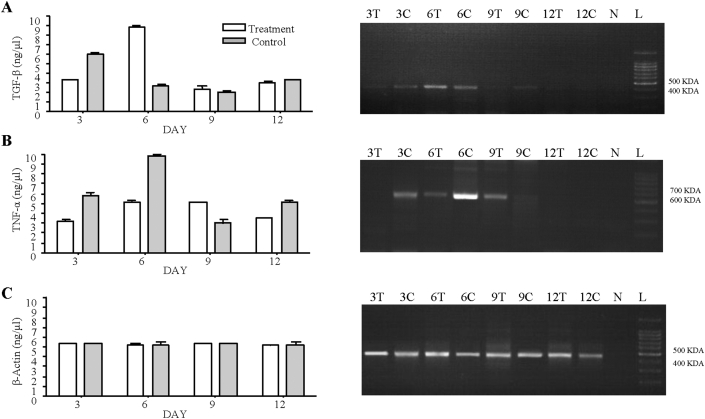
RT-PCR analysis showed the high level of TGF-β expression in S. illicifolium treated wound skin at 6 days. The lowest level of TNF-α expression shown in S. illicifolium treated wound skin when compared with control group during the healing process. Equal level of β-Actin expression were seen in S. illicifolium treated wound skin and control. β-Actin expression did not indicate any significant difference in treatment and control wounded skin. TGF-β and TNF-α expression were significantly different during the healing process of wound tissue between treatment and control groups (see Figure 7).
Figure 7.
RT-PCR analysis for mRNA expression of mice wound tissue samples over a period of 12th days. A; TGF-β expression during the wound healing of treatment and control (Ratios of TGF-β on 3,6,9 and 12 days post injury treatment and control). B; TNF-α expression during the wound healing of treatment and control (Ratios of TNF-α on 3,6,9 and 12 days post injury treatment I and control). C; β-Actin expression during the wound healing of treatment and control (Ratios of β-Actin on 3,6,9 and 12 days post injury treatment I and control). Control: Wound were created and received an equal volume of distilled water, orally, Treatment: Wound were created and received S. illicifolium extracts (400 mg/kg BW/day for 14 days, orally). Values are expressed as mean ± SEM (n = 4 animals); Data is compared against control group. One way analysis of variance (ANOVA) Tukey-Kramer multiple comparisons test ∗p < 0.05. 3T–3 Day Treatment, 3C- 3 Day Control, 6T - 6 Day Treatment, 6C - 6 Day Control, 9T - 9 Day Treatment, 9C - 9 Day Control, 12T - 12 Day Treatment, 12C - 12 Day Control, N – Negative Control, L - Ladder. Full, non-adjusted images of RT-PCR blots include in Supplementary Figure 1.
3.5.4. Toxicity study
3.5.4.1. Body weight and behaviour changes
Although the mice in treatment group showed the highest body weight compared to the control groups there was no significant difference between the treatment group and the control groups. The animals were physically active and were consuming food and water in a regular way.
3.5.4.2. Biochemical parameters
Toxicity studies of the seaweeds extracts showed no signs and symptoms such as restlessness, respiratory distress, diarrhea, convulsions, and coma and it was found safe at 400 mg/kg dosage. Studied biochemical parameters for the toxicity assessment; serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and serum creatinine levels of mice in each group are given below (see Figure 8).
Figure 8.
Biochemical values of mice of each test group over a period of the 26th day. A; Serum aspartate aminotransferase (AST) levels in mice, B; Alanine aminotransferase (ALT) levels in mice, C; Serum creatinine levels in mice. Data are expressed as mean ± SEM (n = 12 mice); Data is compared against control group. a = when compared with control group, b = when compared with treatment II Group, (∗) indicates statistically significant difference from respective group using ANOVA, followed by Tukey's comparisons test (p > 0.05). (†) indicates statistically no significant difference from respective group using ANOVA, followed by Tukey's comparisons test (p > 0.05). Control group received an equal volume of distilled water, orally; untreated wounds and Treatment group received S. illicifolium extracts (400 mg/kg BW/day for 12 days, orally).
The biochemical parameters that were analyzed in this experiment groups were compared between experimental groups, such as the Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and serum creatinine, levels not exhibit significant different from their normal ranges (AST/SGOT: 54–98 IU/L, ALT/SGPT: 17–77 IU/L, Serum Creatinine; 0.2–0.9 mg/dl for laboratory mice, research animal resources, University of Minnesota) in all three groups.
These results indicated the fact that mice vital organs have not any considerable damage due to the feeding of experimental seaweeds extracts.
3.5.4.3. Histopathological sections of vital organ
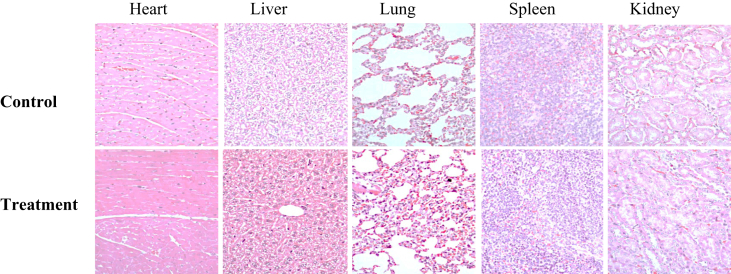
Histopathological sections of vital organs of mice of each treatment group for day 12th, day 15th, day 19th, and day 26th days exhibited normal histology compared with control groups. No considerable morphological changes were observed in sections of vital organs. Normal morphology of vital organs provided strong evidence for safety and non-toxic effects of ingestion of S. illicifolium. These results indicated the fact that vital organs have not any considerable damage due to S. illicifolium extract administrated orally as the time period (see Figure 9).
Figure 9.
Histopathological sections of heart, liver, lungs, spleen, and kidney in mice treated with seaweed extracts and the control. Vital organs of mice stained with hematoxylin and eosin stain (H & E; 40×). Control (C): received an equal volume of distilled water, orally, Treatment (T): received S. illicifolium extracts (400 mg/kg BW/day for 12 days, orally).
4. Discussion
Among the natural marine resources, seaweeds are considered as a rich source of natural bioactive compounds (Vo et al., 2011). It is reported that more than 2400 marine natural compounds had been isolated from seaweeds present in subtropical and tropical worldwide algal beds (Manilal et al., 2009a, Manilal et al., 2009b, Manilal et al., 2009c). For a long time, human beings have used marine algae as food supplements and medicines because seaweeds are a great source of pharmacologically active metabolites with antineoplastic, antimicrobial, antiviral (Faulkner, 2000; Tziveleka et al., 2003) and antitumor activities (Mayer and Gustafson, 2003). Also, those active compounds have shown potential immune stimulant activity as well (König and Wright, 1996). In Sri Lanka, there are sandy and rocky coastal regions where algal species are distributed copiously (Premarathna et al., 2020).
In the present study aqueous extracts of twenty-three different seaweed species collected from different coastal sites in Sri Lanka showed promising wound healing activity in-vitro and in-vivo. Phytochemical compounds present in selected samples were also investigated and the seaweeds samples contained natural phytochemicals such as saponins, flavonoids, tannins, alkaloids and triterpenes. The result of qualitative chemical analysis of the aqueous extract of seaweeds and references reported in literature are tabulated in Table 4. Moreover, during the phtochemical analysis, saponins, flavonoids, tannins, alkaloids and triterpenes were reported in the most of the aqueous extracts of red and brown seaweed samples compared with green seaweed samples. In this context, saponins, flavonoids, tannis, akaloid and terpenoids were mainly recorded in aqueous extracts of Gracilaria corticata. Flavonoids, tannins, alkaloids and triterpenes were absent in aqueous extracts of Padina antillarum and triterpene was totally absent in aqueous extracts of Sargassum species and Ulva lactuca but present in the aqueous extracts of Halimeda opuntin, Caulerpa racemosa and Caulerpa sertularioide. Flavonoids were observed in aqueous extracts of all the seaweeds except aqueous extract of Padina antillarum. Recent reports of antiviral, anti-fungal, antioxidant, anti-inflammatory, antiallergenic, anti-thrombic, anti-carcinogenic, hepatoprotective and cytotoxic activities of flavonoids have been generated an interest on studies regarding flavonoid containing plants (Veitch, 2007; Jiang et al., 2008) and these flavonoids are considered as a natural drug which possess numerous promising biological and pharmacological importance. Aqueous extracts of Gracilaria corticata reported the highest amount of alkaloids compared with other seaweed extracts and alkaloids were absent in Ulva fasciata. Many reports revealed the presence of alkaloids in seaweeds and some of seaweed species have been investigated for their biological activities as well (Adikalaraj et al., 2011). Alkaloids are commonly found to have antimicrobial properties (Wangchuk et al., 2016) against both gram-positive and gram-negative bacteria (Lavanya and Veerappan, 2011). During this study saponins were detected in all the aqueous extracts of red seaweeds and green seaweed; Ulva lactuca. Saponins had showed numerous biological properties including antimicrobial, anti-inflammatory, anti-feedent and haemolytic effects (Xu et al., 2000). The highest amount of tannins was recorded in the aqueous extracts of Caulerpa racemosa out of other green seaweed extracts. The results of the phytochemical investigation of seaweeds revealed the presence of various secondary metabolites in a varied degree. Recently, many studies have been conducted on the phytochemistry of seaweeds worldwide (Selvin and Lipton, 2004; Fayaz et al., 2005, Somepalli et al., 2007; Adikalaraj et al., 2011). It suggests that seaweeds have a great medicinal value and have been extensively used in the pharmaceutical industry. Based on the literature, the environmental conditions and geographical distribution like factors including light, water temperature, salinity, nutrients, minerals availability may effect on the chemical composition of marine algal and seagrass species (Ranahewa et al., 2018).
The brine shrimp lethality assay is very important as a preliminary toxicity screening of any biological compound or product since it is a comparatively easy and cheap method which provides the basic information useful to extend further cytotoxicity tests; in-vitro or in-vivo though a few of the studies have been carried out to check the cytotoxicity of seaweeds using brine shrimp test (Zhang et al., 2013). Toxicity index values (LC50) of extracts are used to find out whether the extracts are toxic or non-toxic. If LC50 value of an extract is greater than 1 mg/ml that extract is considered as a non-toxic extract (Meyer et al., 1982 and Clarkson et al., 2004). LC50 values of the most of samples were greater than 1 mg/ml and considered as non-toxic whereas SW17-Acanthophora spicifera (LC50 = 0.033 μg/μl) was the only species that exhibited toxic results in brine shrimp lethality assay. Since LC50 value of SW17 was in between 0-100 μg/ml that species is considered as a highly toxic species which is compatible with studies of Clarkson et al. (2004). It was observed that even the lowest tested concentration of Acanthophora spicifera, (0.20 μg/μl) was detrimental to brine shrimp nauplii. This inhibitory effect of the extract might be due to any toxic compound present in the active fraction that possesses ovicidal and larvicidal properties. According to the Hamidi, et al., 2014 and Wijesekara et al. (2011), cytotoxic potency of a natural extract is considered as a clue for the presence of antitumor compounds. The cytotoxic property of plant materials are due to the presence of antitumor compounds (Ara et al., 1999) and many of the secondary metabolites produced by the marine red algae are well known for their cytotoxic property (Harada and Kamei, 1997). Further, the crude extracts or any tested concentrations of green seaweed species Caulerpa racemosa (SW02, 43.84 μg/μl), Halimeda opuntia (SW07, 7.64 μg/μl), Caulerpa racemosa (SW08, 17.32 μg/μl), Caulerpa sertularioides (SW10, 17.00 μg/μl) and Ulva lactuca (SW16, 95.44 μg/μl) did not exhibit any abnormal behavior or mortality during the study period. The present study indicated that the brine shrimp lethality assay is a reliable method for the assessment of toxic effect of seaweeds and lends support for the further studies. The aqueous extracts red and brown seaweed showed brine shrimp larvicidal activity and the degree of lethality is directly proportional to the concentration of the extracts. Based on the results, the brine shrimp lethality of the seaweed extracts were found to be concentration dependent. The observed lethality of the seaweed extracts on brine shrimps indicated the presence of potent cytotoxic components of seaweeds.
The MTT assay was carried out using living, metabolically active cells to assess cell viability and proliferation followed by treating the cells with different concentrations of testing extracts. The absorbance value from the MTT assay is directly proportional to the viable cell count of the respective well (Mosmann, 1983). In this study, cytotoxic effect of the aqueous seaweed extracts have been compared with the negative control and other seaweed extract treated cells. The highest cytotoxicity against L929 cell line was recorded by Jania adhaereus (red algae) extract (42.10 μg/μl concentration) and it accounts for 50.70 ± 7.304% cell growth inhibition. Cell viability and proliferation activity have increased with the increase of the concentration of many seaweeds extracts. In this context, a promising cell proliferation was recorded in the wells treated with Padina antillarum (SW15, 431.5 ± 41.13%). The morphology of the treated cells does not show any variation when compared with the control cells. Moreover, seaweed extracts of Stoechospermum polypodioides (SW15; 5.86 μg/μl) and Gelidiopsis variabilis (SW18, 7.44 μg/μl) have also shown a potential cell proliferation activity on L929 cell line. Therefore, phytochemical compounds reported in afore mentioned seaweed extracts could be responsible for the cell proliferation and wound healing activity exhibited during this study. This study provides only basic data regarding induction of epithelial cell proliferation and further studies are necessary for isolation and identification of biological active substances of these extracts responsible for wound healing activity. When comparing the cell proliferation or/and migration effect of the aqueous extract of Halimeda opuntin and Stoechospermum polypodioides with all other seaweed extracts on L929 cell line, Halimeda opuntin be considered as a potential source of bioactive compounds which are having wound healing properties. According to previous study results, even the lowest concentration of Sargassum species has showed greater effect on cell proliferation and migration compared to the control group (Premarathna et al.,2019). Thus in-vitro assays indicate that the aqueous extracts of most of the selected seaweeds possess significant wound healing properties which might be helpful in healing of various wounds in animals. This also infers that all the seaweeds are comprised of potent wound healing agents, specifically seaweed could be a potential candidate for wound healing therapy in near future. All the tested seaweed species except J. adhaereus did not show any significant cytotoxic effect compared to control group. Therefore, this result suggests that the bioactive compounds of these seaweed species can be isolated and can be used for further medicinal investigations including wound healing activity.
The wound healing process of repairing the skin injury is a dynamic, complex, and well-organized process (Reinke and Sorg, 2012). Assessment of cell migration activity of epithelial cells towards the wounded area is necessary during the investigation of wound healing potential of any biological resource. The in vitro scratch assay is considered one of the earliest developed, an easy and comparatively low-cost methods used to assess directional cell migration (Liang et al., 2007). It has been widely used for gene knockdown or chemical exposure. In general, cell migration activity of cells treated with S. illicifolium extracts (0.97 μg/μl, 97.83 ± 0.05%) (Premarathna et al., 2019) and Halimeda opuntin extracts (SW11; 3.82 μg/μl, 91.63 ± 0.28) showed highest cell migration ability compared with other seaweeds extracts and control groups. Aqueous extracts of J. adhaereus (SW22, 42.10 μg/μl, 00.60 ± 0.07%) (Mean ± SEM, n = 08) (P > 0.05) showed significant cytotoxic effect compared with other seaweed extracts and control.
Mammalian wound healing activity is a kind of complex process mainly occurs due to cell migration and proliferation and it aligns with various systematized cellular activities. The wound repair in multicellular organisms is a process by which a damaged skin or tissue is restored to its normal state by forming several layers to cover the wound. Wound healing mainly depends on the repairing ability of the wounded tissue, the extent of damage and the general health state of the wounded tissue. The normal healing cascade begins with an orderly process of hemostasis and fibrin deposition, which leads to an inflammatory cell cascade, characterized by neutrophils macrophages and lymphocytes within the tissue (Mast et al., 1969). At the end of healing, the number of inflammatory cell decreases and the fibroblast cell increases. The fibroblast cell in connective tissue which produces collagen and other fibres are needed to repair the tissue injury (Ross, 1969). In the tissue repair process, inflammatory cells promote the migration and proliferation of endothelial cells leading to neovascularization of connective tissue cells which synthesize extracellular matrices including collagen resulting in re-epithelialization of wounded tissue (Shimizu et al., 2000). In the present study, it is revealed that in the excision wound model, mice fed with aqueous extracts of S. illicifolium (SW23; 400 mg/kg) showed a significant increase in wound healing process than that of the control. This might be due to the activity; one or more bioactive compounds present in the tested seaweeds extracts on the wound healing process and it may have enhanced the activity at certain phase in the wound healing process. Histopathological findings revealed that the healing process was significantly fast in the mice group that treated orally with S. illicifolium extract (SW23; 400 mg/kg). The histopathological sections proved that the content of the granulation tissue, the rate of wound contraction and epithelialization in the tissue sections taken from the animal group treated orally with S. illicifolium extracts was significantly increased compared to the control group.
Enhanced healing activity may also be attributed to increased synthesis of collagen accumulates and angiogenesis (Gao et al., 2010; Gerard et al., 2010). Angiogenesis helps to improve granulation of tissues by providing good blood supply to the injury site, thus provides adequate amount of required nutrients and oxygen for the healing process (Al-Bayaty et al., 2012). Wound healing involves regeneration of specialized cells by the proliferation of surviving cells and connective tissue response characterized by the formation of granulation tissue (Whaley and Burt, 1996). Thus, the effect of S. illicifolium on wound contraction and epithelialization reported during this study suggests that aqueous extracts of S. illicifolium may contain bioactive compound which has the ability to enhance epithelial cell migration, proliferation and the action of myofibroblasts. In this context, the decrease in the diameter of the whole wound was determined as the wound contraction, for the easiness of differentiation of wound contraction from re-epithelialization process. Further, brown seaweed possesses alginates, polysaccharides, laminarin, fucoidan, cellulose, mannitol, galactans and fucans, (Lordan et al., 2011). Fucoidans has been reported an effect on the inflammatory activity and antioxidant properties (Wang et al., 2019). Nevertheless, the non-halogenated and halogenated terpenoids in the seaweeds (Kasanah et al., 2015) might also be directly involve to wound healing process. Therefore, the factors mentioned above could be responsible for the wound healing activity exhibited by the aqueous extracts of S. illicifolium during this study.
In-vivo toxicity findings of this study showed that there is no toxic effect from the S. illicifolium aqueous extracts (400 mg/kg) on mice (Figure 8). Changes in organ weight are considered as a sensitive indicator. Therefore, the comparison of organ weight of treatment group with the control group was carried out to estimate the toxic effects in toxicological studies (Michael et al., 2007; Sellers et al., 2007). There was no significant difference of mean weight of all the vital organs between the treatment group and control groups. Moreover, biochemical methods such as serum ALT/AST analysis are also used to estimate the toxic effect of any chemical or a treatment. In general, levels of serum enzymes; ALT and AST tend to increase significantly, If the liver cells got impaired due to influence of the introduction of infectious agents, chemicals or any other causative agents (Contreras-Zentella and Hernández-Muñoz, 2016). Based on that, the serum ALT and ASL levels of mice of treatment group and control groups were investigated and such condition was not observed in in any group of mice. In toxicological studies, the liver and kidneys are considered as vital organs thus are widely used to test possible toxic effects due to ingested toxins in experimental trials (Alferah, 2012).
The expression of TGF-β is very important as inflammatory mediators in scar less wound repair. Overexpression of TGF-β in mice dermal wound healing would create an accelerated scarless wound healing. Also, TNF-α expression mediated signals have a role in promoting leukocyte infiltration at the wound site and negatively affect on wound healing, probably by reducing angiogenesis and collagen accumulation (Mori et al., 2002). In the current experiment, wound healing activity was evaluated in the injured tissue specimens collected from control and treatment based on TGF-β and TNF-α expression. However, an acceleration of wound healing was observed and similarly, a significant upregulation of TGF-β expression levels at three to six days after injury was also recorded in the treatment group compared with the control group. The level of TGF-β expression returns near baseline level after reepithelialization because TGF-β plays a significant role during the process of reepithelialization which begins around day 6 in wounded mice. The higher expression level of TNF-α is related to the inhibition of wound reepithelialization. Further, low levels of TNF-α promote wound healing by indirectly stimulating inflammation and accelerate the wound healing process. The results of the current study showed low expression levels of TNF-α at 6 days after injury in the treatment group compared to the control groups. However, TNF-α expression was significantly increased after 6th day of injury in the wound tissues of mice in control group due to prolonged period of time indicated wound healing delay. The expression of TGF-β and suppression of TNF-α at 6 days appears to coincide with the proliferative phase of wound repair in injured treatment mice wounds. It has been proposed that the cellular infiltrate present on the treatment mice group at day 6 is likely a combination of inflammatory cells and fibroblasts. Specifically, more collagen have been increasingly observed in wound tissue at early 6 days after injury in wound site of treated mice compared with control mice. S. illicifolium treated mice has been shown considerably higher epithelial gaps when compared to the control animals at 6th days after injury and the epithelial thickness was significantly decreased at these time points.
The TGF-β consist in the granulation tissue was expected to be essential for effectual healing because TGF-β stimulates angiogenesis, fibroblast proliferation, myofibroblast differentiation and matrix deposition (Desmoulière et al., 1993; Roberts and Sporn, 1996; Roberts et al., 1986). However, wounds of the treatment and control group were completely reepithelialized at 12th day of post-wounding. Comparatively, excision wounds healed due to granulation tissue formation and reepithelialization were distinguishable in the wound of mice in treatment and control groups. This finding is supported by a various types of studies in animal models that confirmed a convenient effect of exogenous TGF-β for wound restoration; the rate of healing and strength of the wound healed (Alferah, 2012). These data suggest the role of TGF-β compensated during healing engaged by formation of granulation tissue. These results represent the significance of the excision wounds for the investigation of growth factors in wound healing. Therefore, TGF-β is involved in the turnover of the wound healing process directly, it would be necessary for future experiments to examine how seaweeds extracts influence on TGF-β cytokine expression after injury. Additionally, TNF-α is suppressed during wound healing process thus, it would also be necessary to investigate the role of cytokines in both the expression and suppression through the wound healing.
Therefore, further studies are required to determine and isolate bio-active compound(s); from these seaweeds with therapeutic value. The findings of this study would provide baseline information on the cytotoxicity of the tested seaweed species that could be used in further studies in the investigation of their bioactive compounds and potential therapeutic uses. In additional, some other studies using bio-active compound(s) of S. illicifolium are required to appreciate the complete the wound healing mechanism. Indeed, studies are required to evaluate longer post-injury time points for stabilization of TGF-β and TNF-α mRNA in pre-injury levels. Further research on these species will enable thorough understanding of the therapeutic value through isolation of bioactive compounds.
5. Conclusion
The examined seaweed species could be collected and utilized effectively in product preparation that will be beneficial to the mankind. Phytochemicals as flavonoids and alkaloids were exceptionally present in Gracilaria corticata. This species can be further worked out to extract their active metabolites. Brine shrimp lethality assay was conducted to investigate cytotoxicity of aqueous seaweed extracts. Green seaweed species Caulerpa racemosa and Caulerpa sertularioides and brown seaweed species Padina antillarum did not exhibit mortality within tested concentrations. Crude extracts of brown and red seaweeds species have shown thigh mortality rate compared to green seaweeds. In additional, remarkable cytotoxic effect on L929 cell line was exhibited by one of the red seaweed named; Jania adherens when compared to the control. Extract of Halimeda opuntin and Stoechospermum polypodioides has properties that render it capable of promoting accelerated scratch wound healing activity compared with control and other seaweeds species. The final conclusion of the study was that Sargassum ilicifolum as a candidate for more studies on in-vivo wound healing evaluation would provide great insight for its potential. Histopathological findings also revealed that healing process was significantly fast in the mice group treated with Sargassum ilicifolum aqueous extracts by enhancing epithelialization and stimulating tissue proliferation. Also the results of this current study clearly indicated that the continuous oral administration of Sargassum illicifolium aqueous extracts in experimental mice up to 400 mg/kg dosage do not exert any acute/sub-acute toxic effect. Therefore, this seaweed source has wound healing properties and it does not show any toxic effect on mice. It can be a new marine resource for wound medicine and to demonstrate that marine algae can be a potential candidate sources as wound drugs. However, further investigations should be conducted to elucidate the mechanisms of action of the Sargassum illicifolium extracts, expanding the knowledge of healing process. This also infers the seaweeds are potent anticancer agents, specifically Jania adhaereus could be a potential candidate for cancer therapy in the near future. Further research studies are being carried out on those species of seaweeds from the same habitat in order to provide complete data on the nutritive and medicinal potential of these plants.
Declarations
Author contribution statement
A.D. Premarathna, T.H. Ranahewa, S.K. Wijesekera and R.P.V.J. Rajapakse: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
D.L. Harishchandra and K.J.K. Karunathilake: Performed the experiments.
R.N. Waduge, R.R.M.K.K. Wijesundara, A.P. Jayasooriya and V. Wijewardana: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by National Research Council (NRC) in Sri Lanka, Grant No: NRC 15-028.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank Mr. N.G.J Perera and Mr. K.L.M.S Bandara, staff of Animal House, Faculty of Medicine, University of Peradeniya, Sri Lanka. The authors also wish to thank Ms. H.M.N.D Herath, Department of Pathobiology, Faculty of Medicine, University of Peradeniya, Sri Lanka. Technical support given by Mr. N.A.N.D Perera, Mr. K.B.A.T Bandara, K.A Wijesekera and Mr. D.P.G.S.P Jayasinghe, Department of Veterinary Pathobiology, Faculty of Veterinary Medicine and Animal Science is also appreciated. Mr. I.P.G.H.U Dissanayake, Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, is appreciated. The authors also wish to thank “Parasitology Research Group” members, Department of Veterinary Pathobiology, Faculty of Veterinary Medicine and Animal Science, University of Peradeniya Sri Lanka.
Contributor Information
Amal D. Premarathna, Email: amaldharmapriya@gmail.com.
R.P.V.J. Rajapakse, Email: jayanthar@pdn.ac.lk.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adikalaraj G., Johnson M., Patric Raja D., Janakiraman N. Pharmacognostical and phytochemical evaluation of selected seaweeds of Rhodophyceae. Nat. Prod.: Ind J. 2011;7(6):1–9. [Google Scholar]
- Al-Bayaty F.H., Abdulla M.A., Hassan M.I.A., Ali H.M. Effect of Andrographis paniculata leaf extract on wound healing in rats. Nat. Prod. Res. 2012;26(5):423–429. doi: 10.1080/14786419.2010.496114. [DOI] [PubMed] [Google Scholar]
- Al-Wathnani H., Ara I., Tahmaz R.R., Al-Dayel T.H., Bakir M.A. Bioactivity of natural compounds isolated from cyanobacteria and green algae against human pathogenic bacteria and yeast. J. Med. Plants Res. 2012;6(18):3425–3433. [Google Scholar]
- Alferah M.A. Toxicity induced histological changes in selected organs of male (Wistar) rats by lawsonia inermis leaf extract. Eur. J. Med. Plants. 2012:151–158. [Google Scholar]
- Almeida L., Tovar E., Fonseca J.A., Vasques F. Schedulability analysis of real-time traffic in WorldFIP networks: an integrated approach. IEEE Trans. Ind. Electron. 2002;49(5):1165–1174. [Google Scholar]
- Anbu A., Arun E.S., Gopal V. Phytochemical and pharmacological profile of Brown marine algae stoecheospermum marginatum. J. Acad. Ind. Res. (JAIR) 2017;5(8):120. [Google Scholar]
- Ara J., Sultana V., Ehteshamul-Haque S., Qasim R., Ahmad V.U. Cytotoxic activity of marine macro-algae on Artemia salina (Brine shrimp) Phytother Res.: Int. J. Devoted Pharm. Texicol. Eval. Nat. Prod. Deriv. 1999;13(4):304–307. doi: 10.1002/(SICI)1099-1573(199906)13:4<304::AID-PTR439>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Arunkumar K., Sivakumar S.R., Rengasamy R. Review on bioactive potential in seaweeds (marine macroalgae): a special emphasis on bioactivity of seaweeds against plant pathogens. Asian J. Plant Sci. 2010;9(5):227. [Google Scholar]
- Audu S.A., Mohammed I., Kaita H.A. Phytochemical screening of the leaves of Lophira lanceolata (Ochanaceae) Life Sci. J. 2007;4(4):75–79. [Google Scholar]
- Badakhshan M.P., Sasidharan S., Rameshwar N.J., Ramanathan S. A comparative study: antimicrobial activity of methanol extracts of Lantana camara various parts. Pharmacogn. Res. 2009;1(6):348. [Google Scholar]
- Basha S.F., Muthukumar C. Preliminary phytochemical screening and invitro angiotension activity of bioactive compound-steroid isolated from Sargassum ilicifolium. Int. J. Pharm. Pharm. Sci. 2014;6(2):299–301. [Google Scholar]
- Boateng J.S., Matthews K.H., Stevens H.N., Eccleston G.M. Wound healing dressings and drug delivery systems: a review. J. Pharmaceut. Sci. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- Bouchard A., Hofland G.W., Witkamp G.J. Properties of sugar, polyol, and polysaccharide water− ethanol solutions. J. Chem. Eng. Data. 2007;52(5):1838–1842. [Google Scholar]
- Carballo J.L., Hernández-Inda Z.L., Pérez P., García-Grávalos M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002;2(1):17. doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee T.K., Chakravorty A. Wound healing properties of the new antibiotic (MT81) in mice. Ind Drugs-Bombay. 1993;30:450. 450. [Google Scholar]
- Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Matsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92(2-3):177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Contreras-Zentella M.L., Hernández-Muñoz R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell Long. 2016;2016 doi: 10.1155/2016/3529149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppejans E., Leliaert F., Dargent O., Gunasekara R., De Clerck O. Vol. 6. Belgian Development Cooperation; Brussels: 2009. p. 265. (Sri Lankan Seaweeds: Methodologies and Field Guide to the Dominant Species). [Google Scholar]
- De Almeida C.L.F., Falcão D.S., Lima D.M., Gedson R., Montenegro D.A., Lira N.S., Athayde-Filho D., Petrônio F., Rodrigues L.C., De Souza M.D.F.V., Barbosa-Filho J.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011;12(7):4550–4573. doi: 10.3390/ijms12074550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhargalkar V.K., Pereira N. 2005. Seaweed: Promising Plant of the Millennium. [Google Scholar]
- Domettila C., Joselin J., Jeeva S. Phytochemical analysis on some south Indian seaweeds. J. Chem. Pharmaceut. Res. 2013;5(4):75–278. [Google Scholar]
- Ermakova S.P., Menshova R.V., Anastyuk S.D., Malyarenko O.S., Zakharenko A.M., Thinh P.D., Ly B.M., Zvyagintseva T.N. Structure, chemical and enzymatic modification, and anticancer activity of polysaccharides from the brown alga Turbinaria ornata. J. Appl. Phycol. 2016;28(4):2495–2505. [Google Scholar]
- Farahani R.M., Kloth L.C. The hypothesis of ‘biophysical matrix contraction’: wound contraction revisited. Int. Wound J. 2008;5(3):477–482. doi: 10.1111/j.1742-481X.2007.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2000;17(1):7–55. doi: 10.1039/a809395d. [DOI] [PubMed] [Google Scholar]
- Fayaz M., Namitha K.K., Murthy K.C., Swamy M.M., Sarada R., Khanam S., Subbarao P.V., Ravishankar G.A. Chemical composition, iron bioavailability, and antioxidant activity of Kappaphycus alvarezzi (Doty) J. Agric. Food Chem. 2005;53(3):792–797. doi: 10.1021/jf0493627. [DOI] [PubMed] [Google Scholar]
- Gao F., Liu Y., He Y., Yang C., Wang Y., Shi X., Wei G. Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol. 2010;29(2):107–116. doi: 10.1016/j.matbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Gerard C., Bordeleau L.J., Barralet J., Doillon C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31(5):824–831. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M., Phan S.H. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr. Pharmaceut. Des. 2001;7(11):1083–1103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- Hamidi M.R., Jovanova B., Panovska T.K. Toxicоlogical evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced Pharm. Bull. 2014;60(1):9–18. [Google Scholar]
- Harada H., Kamei Y. Selective cytotoxicity of marine algae extracts to several human leukemic cell lines. Cytotechnology. 1997;25(1–3):213. doi: 10.1023/A:1007987010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Zhan W.Q., Liu X., Jiang S.X. Antioxidant activities of extracts and flavonoid compounds from Oxytropis falcate Bunge. Nat. Prod. Res. 2008;22(18):1650–1656. doi: 10.1080/14786410701875686. [DOI] [PubMed] [Google Scholar]
- Karabay-Yavasoglu N.U., Sukatar A., Ozdemir G., Horzum Z. Antimicrobial activity of volatile components and various extracts of the red alga Jania rubens. Phytother Res.: Int. J. Devoted Pharm. Texicol. Eval. Nat. Prod. Deriv. 2007;21(2):153–156. doi: 10.1002/ptr.2045. [DOI] [PubMed] [Google Scholar]
- Kasanah N., Triyanto T., Seto D.S., Amelia W., Isnansetyo A. Antibacterial compounds from red seaweeds (Rhodophyta) Indonesian J. Chem. 2015;15(2):201–209. [Google Scholar]
- Kihdze T.J., Mayowa A.A., Joseph O., Oc E.J., Ghaife T.G., Bulus A., Van Aerschot A., Laekeman G., Ganafa A.A. Phytochemical and antidiabetic evaluation of the methanolic stem bark extract of Spathodea campanulata (P. Beauv.) bignoniaceae. Phcog. J. 2016;8(3) [Google Scholar]
- König G.M., Wright A.D. Marine natural products research: current directions and future potential. Planta Med. 1996;62(3):193–211. doi: 10.1055/s-2006-957861. [DOI] [PubMed] [Google Scholar]
- Kumar B., Vijayakumar M., Govindarajan R., Pushpangadan P. Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. J. Ethnopharmacol. 2007;114(2):103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kumbhar R.D., Rode S.P., Sabale A.B. Phycochemical screening of seaweeds from Sindhudurg district of Maharashtra. Int. J. Pharmaceut. Sci. Rev. Res. 2014;29:77–81. [Google Scholar]
- Lavanya R., Veerappan N. Antibacterial potential of six seaweeds collected from gulf of mannar of southeast coast of India. Adv. Biol. Res. 2011;5(1):38–44. [Google Scholar]
- Lee J.C., Hou M.F., Huang H.W., Chang F.R., Yeh C.C., Tang J.Y., Chang H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Canc. Cell Int. 2013;13(1):55. doi: 10.1186/1475-2867-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.C., Park A.Y., Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2(2):329. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Liu M., Hansen P.E., Lin X. Bromophenols in marine algae and their bioactivities. Mar. Drugs. 2011;9(7):1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordan S., Ross R.P., Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar. Drugs. 2011;9(6):1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manilal A., Sujith S., Kiran G.S., Selvin J., Shakir C., Gandhimathi R., Panikkar M.V.N. Biopotentials of seaweeds collected from southwest coast of India. J. Mar. Sci. Technol. 2009;17(1):67–73. [Google Scholar]
- Manilal A., Sujith S., Kiran G.S., Selvin J., Shakir C. Cytotoxic potentials of red alga, Laurencia brandenii collected from the Indian coast. Global J. Pharmacol. 2009;3(2):90–94. [Google Scholar]
- Manilal A., Sujith S., Kiran G.S., Selvin J., Shakir C., Gandhimathi R., Lipton A.P. Antimicrobial potential and seasonality of red algae collected from the southwest coast of India tested against shrimp, human and phytopathogens. Ann. Microbiol. 2009;59(2):207–219. [Google Scholar]
- Mast B.A., Cohen I.K., Diegelmann B., Lindblad W.J. Vol. 1992. Saunders Company; Philadelphia: 1969. p. 344. (Wound Healing, Biochemical and Clinical Aspects). [Google Scholar]
- Mayer A.M., Gustafson K.R. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int. J. Canc. 2003;105(3):291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- Mehdinezhad N., Ghannadi A., Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Res. Pharm. Sci. 2016;11(3):243. [PMC free article] [PubMed] [Google Scholar]
- Michael B., Yano B., Sellers R.S., Perry R., Morton D., Roome N., Johnson J.K., Schafer K. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007;35(5):742–750. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- Mohy El-Din S.M., El-Ahwany A.M. Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea) J. Taibah Univ. Sci. 2016;10(4):471–484. [Google Scholar]
- Mori R., Kondo T., Ohshima T., Ishida Y., Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. Faseb. J. 2002;16(9):963–974. doi: 10.1096/fj.01-0776com. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nayak B.S., Pereira L.M.P. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Compl. Alternative Med. 2006;6(1):41. doi: 10.1186/1472-6882-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak B.S., Raju S.S., Eversley M., Ramsubhag A. Evaluation of wound healing activity of Lantana camara L.–a preclinical study. Phytother Res.: Int. J. Devoted Pharm. Texicol. Eval. Nat. Prod. Deriv. 2009;23(2):241–245. doi: 10.1002/ptr.2599. [DOI] [PubMed] [Google Scholar]
- Neelamathi E., Kannan R. Screening and characterization of bioactive compounds of Turbinaria ornate from the gulf of Mannar, India. J. Agri. Environ. Sci. 2016;16(2):243–251. [Google Scholar]
- Olowa L.F., Nuñeza O.M. Brine shrimp lethality assay of the ethanolic extracts of three selected species of medicinal plants from Iligan City, Philippines. Mortality. 2013;1(T2):T3. [Google Scholar]
- Paiva L., Lima E., Neto A.I., Marcone M., Baptista J. Nutritional and functional bioactivity value of selected Azorean macroalgae: Ulva compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J. Food Sci. 2017;82(7):1757–1764. doi: 10.1111/1750-3841.13778. [DOI] [PubMed] [Google Scholar]
- Permeh P., Saeidnia S., Mashinchian-Moradi A., Gohari A.R. Sterols from Sargassum oligocystum, a brown algae from the Persian Gulf, and their bioactivity. Nat. Prod. Res. 2012;26(8):774–777. doi: 10.1080/14786419.2010.548812. [DOI] [PubMed] [Google Scholar]
- Pierre G., Sopena V., Juin C., Mastouri A., Graber M., Maugard T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioproc. Eng. 2011;16(5):937–945. [Google Scholar]
- Pieters R., Huismans D.R., Leyva A., Veerman A.J.P. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Canc. Lett. 1988;41(3):323–332. doi: 10.1016/0304-3835(88)90294-7. [DOI] [PubMed] [Google Scholar]
- Ponnanikajamideen M., Malini M., Malarkodi C., Rajeshkumar S. Bioactivity and phytochemical constituents of marine brown seaweed (Padina tetrastromatica) extract from various organic solvents. Int. J. Pharm. Therapeut. 2014;5(2):108–112. [Google Scholar]
- Prakash S., Ahila N.K., Ramkumar V.S., Ravindran J., Kannapiran E. Antimicrofouling properties of chosen marine plants: an eco-friendly approach to restrain marine microfoulers. Biocatal. Agri. Biotechnol. 2015;4(1):114–121. [Google Scholar]
- Prakash S., Ramasubburayan R., Ramkumar V.S., Kannapiran E., Palavesam A., Immanuel G. In vitro-Scientific evaluation on antimicrobial, antioxidant, cytotoxic properties and phytochemical constituents of traditional coastal medicinal plants. Biomed. Pharmacother. 2016;83:648–657. doi: 10.1016/j.biopha.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Prasad R.N., Viswanathan S., Devi J.R., Nayak V., Swetha V.C., Archana B.R., Parathasarathy N., Rajkumar J. Preliminary phytochemical screening and antimicrobial activity of Samanea saman. J. Med. Plants Res. 2008;2(10):268–270. [Google Scholar]
- Premarathna A.D., Ranahewa T.H., Wijesekera S.K., Wijesundara R.R.M.K.K., Jayasooriya A.P., Wijewardana V., Rajapakse R.P.V.J. Wound healing properties of aqueous extracts of Sargassum illicifolium: an in vitro assay. Wound Med. 2019;24(1):1–7. [Google Scholar]
- Premarathna A.D., Kumara A.M.C.P., Jayasooriya A.P., Jayanetti D.E., Adhikari R.B., Sarvananda L. Distribution and diversity of seaweed species in South coastal waters in Sri Lanka. J. Oceanogr. Mar. Res. 2020;7:196. [Google Scholar]
- Ranahewa T.H., Gunasekara A.J.M., Premarathna A.D., Karunarathna S.C., Jayamanne S.C. A comparative study on the diversity of seagrass species in selected areas of puttalam lagoon in Sri Lanka. J. Oceanogr. Mar. Res. 2018;6(185):2. [Google Scholar]
- Raposo M.F.D.J., De Morais R.M.S.C., de Morais B., Miranda A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs. 2013;11(1):233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke J.M., Sorg H. Wound repair and regeneration. Eur. Surg. Res. 2012;49(1):35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- Roberts A.B., Sporn M.B. Transforming growth factor- In: Clark Raf., editor. The Molecular and Cellular Biology of Wound Repair. second ed. Plenum; New York: 1996. pp. 275–308. [Google Scholar]
- Roberts A.B., Sporn M.B., Assoian R.K., Smith J.M., Roche N.S., Wakefield L.M., Heine U.I., Liotta L.A., Falanga V., Kehrl J.H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. Unit. States Am. 1986;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Wound healing. Sci. Am. 1969;220:40–50. doi: 10.1038/scientificamerican0669-40. [DOI] [PubMed] [Google Scholar]
- Santhi R. Phytochemical screening of Nerium oleander leaves. Int. Res. J. Pharm. 2011;2(1):131–135. [Google Scholar]
- Sellers R.S., Mortan D., Michael B., Roome N., Johnson J.K., Yano B.L., Perry R., Schafer K. Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007;35(5):751–755. doi: 10.1080/01926230701595300. [DOI] [PubMed] [Google Scholar]
- Selvin J., Lipton A.P. Biopotentials of Ulva fasciata and Hypnea musciformis collected from the peninsular coast of India. J. Mar. Sci. Technol. 2004;12(1):1–6. [Google Scholar]
- Shimizu N., Watanabe T., Arakawa T., Fujiwara Y., Higuchi K., Kuroki T. Pentoxifylline accelerates gastric ulcer healing in rats: roles of tumor necrosis factor alpha and neutrophils during the early phase of ulcer healing. Digestion. 2000;61(3):157–164. doi: 10.1159/000007752. [DOI] [PubMed] [Google Scholar]
- Stenzel D., Franco C.A., Estrach S., Mettouchi A., Sauvaget D., Rosewell I., Schertel A., Armer H., Domogatskaya A., Rodin S., Tryggvason K. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 2011;12(11):1135–1143. doi: 10.1038/embor.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman A.N., Malotte K.M., Mahan R.D., Davis M.J., Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood J. Am. Soc. Hematol. 2009;114(24):5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres F.A., Passalacqua T.G., Velásquez A.M., de Souza R.A., Colepicolo P., Graminha M.A. New drugs with antiprotozoal activity from marine algae: a review. Revista Brasileira de Farmacognosia. 2014;24(3):265–276. [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Tuhin R.H., Begum M.M., Rahman M.S., Karim R., Begum T., Ahmed S.U., Mostofa R., Hossain A., Abdel-Daim M., Begum R. Wound healing effect of Euphorbia hirta linn.(Euphorbiaceae) in alloxan induced diabetic rats. BMC Compl. Alternative Med. 2017;17(1):423. doi: 10.1186/s12906-017-1930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziveleka L.A., Vagias C., Roussis V. Natural products with anti-HIV activity from marine organisms. Curr. Top. Med. Chem. 2003;3(13):1512–1535. doi: 10.2174/1568026033451790. [DOI] [PubMed] [Google Scholar]
- Uhlig C., Krause H., Koch T., de Abreu M.G., Spieth P.M. Anesthesia and monitoring in small laboratory mammals used in anesthesiology, respiratory and critical care research: a systematic review on the current reporting in top-10 impact factor ranked journals. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M.O., Haque M., Urmi K.F., Zulfiker A.H.M., Anita E.S., Begum M., Hamid K. Anti–bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pacific J. Trop. Biomed. 2013;3(1):1–7. doi: 10.1016/S2221-1691(13)60015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2007;24(2):417–464. doi: 10.1039/b511238a. [DOI] [PubMed] [Google Scholar]
- Velnar T., Bailey T., Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009;37(5):1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Viswanathan S., Nallamuthu T. Screening of phytochemical and antibacterial activity of three different seaweeds from gulf of mannar, Tamilnadu. Phykos: J. Phycoll Soc. 2013;43(1):32–38. [Google Scholar]
- Vo T.S., Ngo D.H., Van Ta Q., Kim S.K. Marine organisms as a therapeutic source against herpes simplex virus infection. Eur. J. Pharmaceut. Sci. 2011;44(1-2):11–20. doi: 10.1016/j.ejps.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Wang J.P., Ruan J.L., Cai Y.L., Luo Q., Xu H.X., Wu Y.X. In vitro and in vivo evaluation of the wound healing properties of Siegesbeckia pubescens. J. Ethnopharmacol. 2011;134(3):1033–1038. doi: 10.1016/j.jep.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xing M., Cao Q., Ji A., Liang H., Song S. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar. Drugs. 2019;17(3):183. doi: 10.3390/md17030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangchuk P. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J. Biolog. Active Prod. NAt. 2018;8(1):1–20. [Google Scholar]
- Wangchuk P., Sastraruji T., Taweechotipatr M., Keller P.A., Pyne S.G. Anti-inflammatory, anti-bacterial and anti-acetylcholinesterase activities of two isoquinoline alkaloids–Scoulerine and Cheilanthifoline. Nat. Prod. Commun. 2016;11(12) 1934578X1601101207. [PubMed] [Google Scholar]
- Whaley K., Burt A.D. Inflammation, healing and repair. Muir's Textbook Pathol. 1996;13:112–165. [Google Scholar]
- Wijesekara I., Pangestuti R., Kim S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84(1):14–21. [Google Scholar]
- Xu Y.X., Chen H.S., Liang H.Q., Gu Z.B., Liu W.Y., Leung W.N., Li T.J. Three new saponins from Tribulus terrestris. Planta Med. 2000;66(6):545–550. doi: 10.1055/s-2000-8609. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Han J., Mu J., Feng Y., Gu X., Ji Y. Bioactivity and constituents of several common seaweeds. Chin. Sci. Bull. 2013;58(19):2282–2289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.