Abstract
Background
β-Amyloid (Aβ) induces oxidative stress and inflammation of microglial cells, thus leading to Alzheimer’s disease. Methyl jasmonate (MeJA) is reported to have anti-inflammatory and anti-oxidant effects. However, the potential roles of MeJA in Aβ-induced cell activities and the underlying mechanism are unclear.
Methods
Microglial cell line BV-2 was stimulated by 20 μM Aβ and/or 20 μM MeJA and then divided into four groups (control, Aβ, MeJA, and Aβ+MeJA). Cell viability was detected by MTT assay. MDA, SOD activity, and ROS were detected by fluorescence spectrophotometry and immunofluorescence assay. Nrf2 and HO-1 were detected by qRT-PCR and Western blot. Furthermore, inflammatory cytokines (p-NFκB, TLR4, TNF-α, IL-1β, and IL-6) and apoptosis factors (Bcl-2, Bax, and cl-casp-3) were detected by Western blot. TUNEL assay was applied to investigate apoptosis rate. Moreover, the mechanism of how MeJA played anti-oxidative stress and anti-inflammatory roles was investigated by silencing of Nrf2 via siRNA.
Results
The result of MTT assay showed that MeJA improved the decreased viability of BV-2 cells induced by Aβ. The detection of MDA, SOD activity, and ROS showed the oxidative stress levels were decreased in Aβ+MeJA group compared with Aβ group. Nrf2, HO-1, and SOD were significantly up-regulated in Aβ+MeJA group compared with Aβ group (p<0.01). In contrast, inflammatory cytokines were significantly down-regulated in Aβ+MeJA group compared with Aβ group (p<0.05). Similarly, the expressions of apoptosis cytokines and TUNEL assay suggested a decreased apoptosis rate in Aβ+MeJA group compared to Aβ group (p<0.01). Finally, results of Nrf2 knockdown experiment showed down-regulations of anti-oxidative stress factors (Nrf2, HO-1 and SOD), up-regulations of inflammatory cytokines, and increased ratio of Bax to Bcl in Aβ+MeJA+si-Nrf2 group compared with Aβ+MeJA group (p<0.01).
Conclusion
MeJA could relieve Aβ-induced oxidative stress and inflammatory response in microglial cells by activating Nrf2/HO-1 pathway.
Keywords: methyl jasmonate, Nrf2-dependent HO-1 pathway, β-amyloid, oxidative stress, inflammatory cytokines
Introduction
β-Amyloid protein (Aβ) is formed by hydrolysis of amyloid precursor protein (APP). The accumulation of Aβ can significantly increase the level of reactive oxygen species (ROS) and decrease the level of superoxide dismutase (SOD) leading to oxidative stress.1 Oxidative stress is an early and prominent feature of Alzheimer’s disease (AD). It has been reported that oxidative stress plays an important role in the pathogenesis and progression of AD.2,3 Besides that, Aβ can bind to CD36, Toll-like receptor 4 (TLR4) and TLR6 on the surface of microglial cells, which causes production and accumulation of pro-inflammatory cytokines in microglial cells, subsequently contributing to AD.4 Aβ-induced oxidative stress can cause accumulation of phosphorylated NF-κB (p-NFκB) in cell nucleus, followed by up-regulation of TNF-α and overproduction of inflammatory cytokines such as IL-6 and IL-1β.5,6 Previous studies have proved that AD was associated with local inflammation, and the inflammatory response in the brain caused by Aβ was a characteristic of AD as well.7–9 Furthermore, oxidative stress is considered to be closely related to inflammatory response and apoptotic signaling, and the molecular pathways which strengthen their interactions can aggravate immune disorders and accelerate the progression of AD.1,5–7,10,11
Jasmonate is a kind of phytoestrogen and its methyl form (MeJA) is the main bioactive constituent. McKenzie et al have demonstrated that Methyl jasmonate at the appropriate concentration can regulate the inflammatory response of BV-2 cells by reducing the overproduction of ROS and enhancing the phagocytic activity of cells.12 Meanwhile, jasmonate has anti-inflammatory and antioxidant effects by reducing the phosphorylation level of NF-κB.13 Other phytoestrogens including farrerol and resveratrol are reported to have abilities to reduce oxidant stress and inflammation by regulating several molecular signaling pathways such as Nrf2/HO-1, NF-κB, and MAPK.14–17 Primarily, nuclear factor-erythroid 2-related factor 2 (Nrf2), an antioxidant transcription factor, induces the expression of SOD and HO-1 to maintain the intracellular redox equilibrium.18–21 Besides, Nrf2 is reported to inhibit lipopolysaccharides (LPS)-induced expression of pro-inflammatory cytokines through Nrf2/HO-1 signaling pathway.16,23 Deficiency of Nrf2 causes decreased defense capability of neurocytes against oxidative stress injury.24 Therefore, the number of studies related to Nrf2-targeted therapeutic methods against diseases associated with oxidative stress and inflammation are rapidly increasing.14,17,21,23,24 However, it remains unclear whether MeJA plays anti-inflammatory and antioxidant roles in BV-2 cells through Nrf2/HO-1 signaling pathway.
Hence, we simulated the pathophysiological processes of microglial cells BV-2 and investigated the Aβ-induced inflammatory response in vitro, while the anti-oxidative stress and anti-inflammatory effects of MeJA were studied as well. Meanwhile, the expressions of oxidative stress proteins, inflammatory cytokines and apoptosis factors were studied after silencing Nrf2 gene to explore the underlying action mechanism of MeJA on Aβ-induced BV-2 cells.
Materials and Methods
Cell Culture and Treatment
Mouse microglial cell line BV-2 (immortalized microglia that were retrovirus-transfected with oncogene v-raf/v-myc25) was purchased from Chinese Academy of Medical Sciences (CAMS) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, ThermoFisher, USA) supplemented with 10% fetal bovine serum (FBS) and 0.1% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2. Cells were grown in adherent conditions. When the confluence reached 80%, the cells were subcultured.
The cells were cultured with different treatments. Briefly, 1 mM stock solutions of Aβ (Aβ1-42, ≥95% purity, HPLC, Sigma-Aldrich, USA) and MeJA (≥95% purity, HPLC, Sigma-Aldrich, USA) were dissolved in ddH2O. Firstly, BV-2 cells were seeded into 96-well plates (ThermoFisher, USA) with a density of 1×104 cells/well. 200 μL serum-free DMEM was added into each well and the cells were incubated at 37°C in an incubator with 5% CO2 for 24 hr. Then the supernatant of media was discarded and 1×PBS was applied to wash the cells. Next, the new media with different concentrations of Aβ (0, 2, 5, 10, 20, 30, 40 μM) or MeJA (0, 1, 10, 20, 30, 40, 50 μM) were added and the cells were incubated for another 48 hr.
MTT Assay
The viability of BV-2 cells was detected by MTT assay based on previous descriptions.14,16 First, the effects of different concentrations of Aβ and MeJA on cell viability were tested. 50 μL of 5 mg/mL MTT solution was added into each well and the cells were incubated at 37°C in the dark for 4 hr. The cells mentioned previously had already been incubated with Aβ/MeJA media for 48 hr (in the description of cell culture and treatment). After removing the media from the incubator, 200 μL of DMSO was added into each well to dissolve formazan in cells. The absorbance was measured at OD570 in microplate reader (ThermoFisher Multiskan FC, USA). Second, based on previously mentioned MTT results, four groups were set up (normal DMEM for control group, DMEM containing 20 μM Aβ for Aβ group, DMEM containing 20 μM MeJA for MeJA group, DMEM containing both 20 μM Aβ and 20 μM MeJA for Aβ+MeJA group) after the first 24 hr, then incubation was continued for another time period (2, 6, 10, 12, 24, 48 hr). Then, the remaining steps of MTT were repeated. Parallel experiments were performed in triplicate for each group.
Immunofluorescence Assay
The ROS level of BV-2 cells (1×106/well) was detected by ROS assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Briefly, BV-2 cells were cultured in DMEM containing 20 μM Aβ and/or 20 μMMeJA for 48 hr. Then, the medium was replaced by serum-free DMEM containing 10 μM DCFH-DA probe and after that, the BV-2 cells were cultured for another 30 min in darkness. The cells were washed with 1×PBS and stained with 1 μg/mL DAPI (Sigma-Aldrich, USA) for 2 min. Then, the cells were photographed by LCX100 Imaging System (Olympus, Japan) with wavelength of 488 nm. Parallel experiments were performed in triplicate for each group.
Fluorescence Spectrophotometry
According to the information in cell culture and treatment section, four groups of BV-2 cells (control, Aβ, MeJA, and Aβ+MeJA) were setup. The cells were collected and homogenized when the monolayer was formed. Lipid Peroxidation MDA Assay Kit (Sigma-Aldrich, USA) was used to detect MDA according to the manufacturer’s instructions. Briefly, the related reagents were added into the homogenates in order, followed by incubation at 95°C for 40 min. The mixture was cooled on ice and centrifuged at 4000 rpm for 10 min. 200 μL of supernatant was transferred to 96-well plates and the absorbance was detected at 535 nm. Total Superoxide Dismutase Assay Kit (Beyotime Biotechnology, China) was used to determine the SOD activity according to the manufacturer’s instructions. Briefly, test reagents were added to the homogenate and then incubated at 37°C for 20 min. After centrifugation at 4000 rpm for 10 min, the supernatant was transferred into a 96-well plate and the absorbance was detected at 450 nm by LCX100 Imaging System (Olympus, Japan).
Quantitative RT-PCR
Total RNAs were extracted from BV-2 cells by RNeasy Mini Kit (Qiagen, Germany), and reverse transcribed into cDNA by cDNA Reverse Transcriptase Kit (Takara, Japan). The qRT-PCR analysis was performed using ABI 7500 system (Applied Biosystems, USA) with SYBR Green PCR Master Mix (Takara, Japan). The primers were synthetized from Sangon Biotech (Shanghai, China) and shown as follows: Nrf2 forward: 5′ -TCTCCTCGCTGGAAAAAGAA-3′; Nrf2 reverse: 5′-AATGTGCTGGCTGTGCTTTA-3′; HO-1 forward: 5′-CCTCACTGGCAGGAAATCATC-3′; HO-1 reverse: 5′-CCTCGTGGAGACGCTTTACATA-3′; β-actin forward: 5′-GCATTGTAACCAACTGGGAC-3′; β-actin reverse: 5′-TGTTGGCATAGAGGTCTTT-3′. The 2-ΔΔCt method was used to perform the relative quantification.
Western Blot
Total proteins were extracted from BV-2 cells by RIPA lysis buffer and quantified by BCA Protein Assay kit (ThermoFisher, USA). 20 μg of each sample was separated by 12% SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membranes were then blocked with 5% (w/v) fat-free milk for 1 hr, followed by incubation overnight at 4°C with primary antibodies including anti-Nrf2 (65 kDa, rabbit, 1:1000), anti-HO-1 (30 kDa, rabbit, 1:500), anti-SOD (25 kDa, rabbit, 1:2000), anti-TLR4 (95 kDa, rabbit, 1:150), anti-IL-6 (24 kDa, rabbit, 1:1000), anti-IL-1β (31 kDa, rabbit, 1:1000), anti-TNF-α (17 kDa, rabbit, 1:1000), anti-p-NFκB (69 kDa, rabbit, 1:500), anti-Bax (22 kDa, rabbit, 1:1000), anti-Bcl-2 (26 kDa, rabbit, 1:1000), anti-cl-casp-3 (17 kDa, rabbit, 1:1000), which were all purchased from ThermoFisher (USA). Next, the primary antibodies were washed away by 1×PBS). The membranes were then incubated with HRP-labeled anti-rabbit secondary antibodies (1:10,000) for 1 hr at room temperature. After another washing step by 1×PBS, the membranes were rinsed in the Western Lighting Plus-ECL solutions (Perkin Elmer, USA), and finally the immunoreactive bands were detected using CL-XPosure™ Film (ThermoFisher, USA).
TUNEL Detection
Cell apoptosis was evaluated using TUNEL Cell Death Detection Kit (Roche, Switzerland) according to the manufacturer’s instructions. Briefly, four groups of BV-2 cells (control, Aβ, MeJA, and Aβ+MeJA) were setup according to the description in cell culture and treatment section, when the cell confluence reached 70%−80%, the cells were fixed with 70% ethanol and washed with 1×PBS and then incubated with 50 μL of TUNEL assay solution for 1 hr at 37°C. The cells were then washed with 1×PBS. After that, the fluorescein isothiocyanate (FITC) was added (for apoptotic nuclei dUTP labeling) and the cells were incubated for 1 hr in darkness at 37°C. Subsequently, DAPI solution (Sigma-Aldrich, USA) was added (for nuclear staining) to the cells and co-incubated for 10 min in darkness. At last, the fluorescence was detected with the wavelength of 488 nm.
Silencing of Nrf2 by siRNA
The siRNA of Nrf2 and control siRNA were purchased from Santa Cruze, and the siRNA transfection kit (ThermoFisher, USA) was used for transfection. Briefly, the BV-2 cells were seeded into 24-well plates (1×104/well) and cultured in antibiotic-free DMEM. When the confluence reached 60%, the cells were transfected with siRNA of Nrf2 or control siRNA by oligomer-Lipofectamine™ 2000 (ThermoFisher, USA). The cells were cultured in DMEM containing 20 μM Aβ and 20 μM MeJA for 48 hr. Cells were then divided into control group (without transfection and with normal DMEM), Aβ+MeJA+si-Nrf2 group and Aβ+MeJA+si-con group. The transfection efficiency of siRNA of Nrf2 was tested by qRT-PCR and Western blot.
Statistical Analysis
All experiments were repeated at least three times and the data were presented as mean ± SD. Image J was used for obtaining the fluorescence intensity of ROS images, as well as the gray value of Western blot bands. In addition, statistical analysis was conducted using GraphPad Prism version 6.0 (GraphPad Prism Software Inc., San Diego, CA, USA). One-way ANOVA with post hoc Tukey’s test were used to analyze the experimental data. p<0.05 was considered as statistically significant.
Results
MeJA Attenuated Aβ-Induced Toxicity on the Microglial Cells
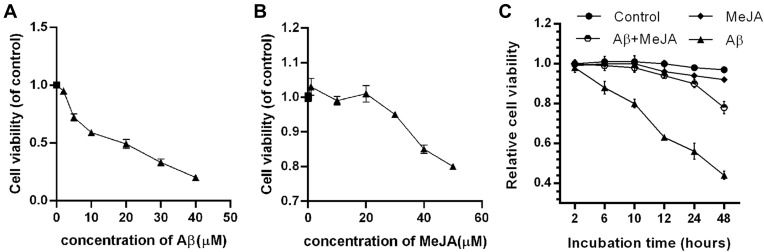
To evaluate the effects of MeJA on Aβ-treated microglial cells, we first tested the cell viability of BV-2 cells in the presence of Aβ or MeJA. The MTT result showed that Aβ inhibited the cell viability in a dose-dependent manner and the viability decreased to about 50% when BV-2 cells were exposed to 20 μM of Aβ (Figure 1A). In addition, MeJA did not significantly affect the cell viability below 20 μM (Figure 1B). Based on these results, we chose 20 μM as the appropriate concentration for the usage of both Aβ and MeJA, and set up four groups (control, Aβ, MeJA, and Aβ+MeJA groups). The result of MTT assay (Figure 1C) showed that there was no significant difference in the cell viability between MeJA group and control group, while the cell viability was decreased in Aβ group and Aβ+MeJA group. It is worth noting that the cell viability of Aβ+MeJA group was much higher than that of Aβ group at each time point. The results indicated that MeJA attenuated Aβ-induced toxicity on the viability of BV-2 cells.
Figure 1.
Relative viability of BV-2 cells under different treatments. BV-2 cells were treated with gradient concentration of Aβ (A) and MeJA (B) for 48 hr. (C) BV-2 cells were treated with 20 μM of MeJA and/or 20 μM Aβ or not.
MeJA Relieved Aβ-Induced Oxidative Stress of Microglial Cells
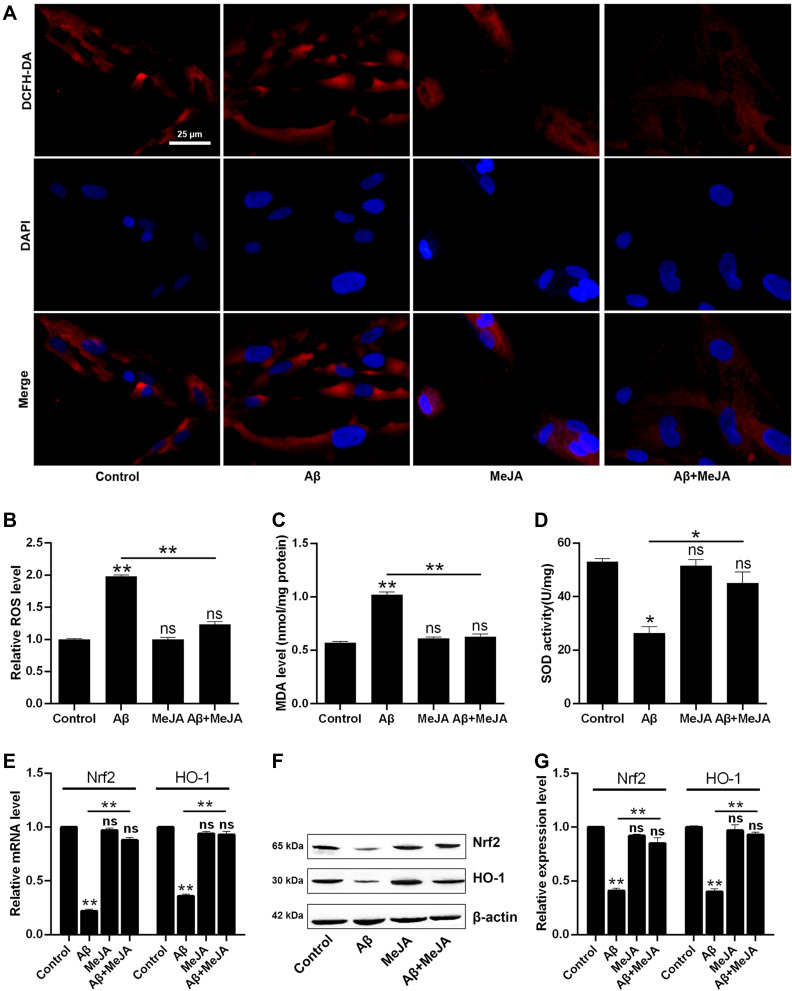
Next, we tested the level of oxidative stress of these four groups by measuring oxidative stress indicators including ROS, MDA, and SOD. The levels of ROS and MDA were the highest in Aβ group, intermediate in Aβ+MeJA group, and the lowest in MeJA and control groups (Figure 2A–C), which indicated that Aβ stimulated the generation of ROS and MDA, while MeJA suppressed this oxidative stress induced by Aβ. Then, we detected the SOD activity among these four groups (Figure 2D) by fluorescence spectrophotometry and the result showed that SOD activity in Aβ group was significantly lower than control group (p<0.05), while the SOD activity in Aβ+MeJA group was higher than Aβ group (p<0.05) and lower than control group. The result indicated that Aβ induced the inactivation of SOD, while MeJA relieved the influence caused by Aβ. Hence, MeJA inhibited Aβ-induced REDOX imbalance in BV-2 cells.
Figure 2.
Relative production and activity of intracellular oxidation index in BV-2 cells under different treatments. The comparison of immunofluorescence images with the scale bar of 25 µm (A) and relative fluorescence intensity (B) of ROS measurement. The MDA relative level (C) and SOD activity (D) were measured by fluorescence spectrophotometry. qRT-PCR (E) and Western blot (F and G) were applied to analyze the mRNA and protein expressions of Nrf2 and HO-1. *p< 0.05; **p< 0.01.
Abbreviation: ns, no significant difference.
Furthermore, to further test the antioxidant activity of MeJA, we detected the expressions of Nrf2 and HO-1 by qRT-PCR (Figure 2E) and Western blot (Figure 2F and G). The result of qRT-PCR showed that the mRNA levels of Nrf2 and HO-1 were significantly decreased in Aβ group compared with those in control group (p< 0.01), while those in Aβ+MeJA group were higher than Aβ group (p< 0.01) but still lower than control group. The result of Western blot was consistent with that of qRT-PCR. These findings indicated that MeJA prevented Aβ-induced down-regulation of Nrf2 and HO-1, and subsequently relieved the oxidative stress in BV-2 cells.
MeJA Reduced Aβ-Induced Expression of Inflammatory Cytokines
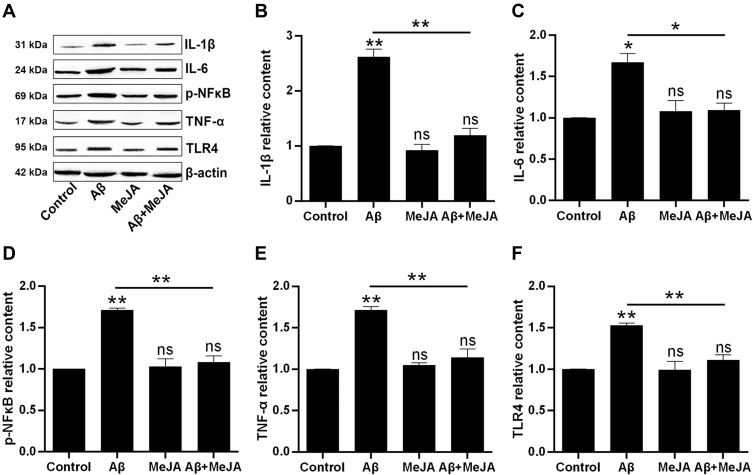
In general, oxidative stress accompanies inflammatory reaction. Thus, we investigated the expression of inflammatory cytokines via Western blot. The result (Figure 3) showed that the expression levels of IL-1β, IL-6, TNF-α, TLR4 and p-NF-κB in Aβ group were significantly up-regulated compared with control group (p<0.05), and those expression levels in Aβ+MeJA group were close to control group, while the expressions of these cytokines were significantly down-regulated in Aβ+MeJA group compared with Aβ group (p<0.05). The result suggested that MeJA inhibited the expression of inflammatory cytokines in BV-2cells induced by Aβ.
Figure 3.
The production of inflammatory cytokines in BV-2 cells under different treatments. The expressions were evaluated by Western blot (A) and the relative contents of the IL-1β (B), IL-6 (C), p-NF-κB (D), TNF-α (E) and TLR4 (F) were organized into histograms. *p<0.05; **p<0.01.
Abbreviation: ns, no significant difference.
MeJA Inhibited Aβ-Induced Apoptosis of Microglial Cells
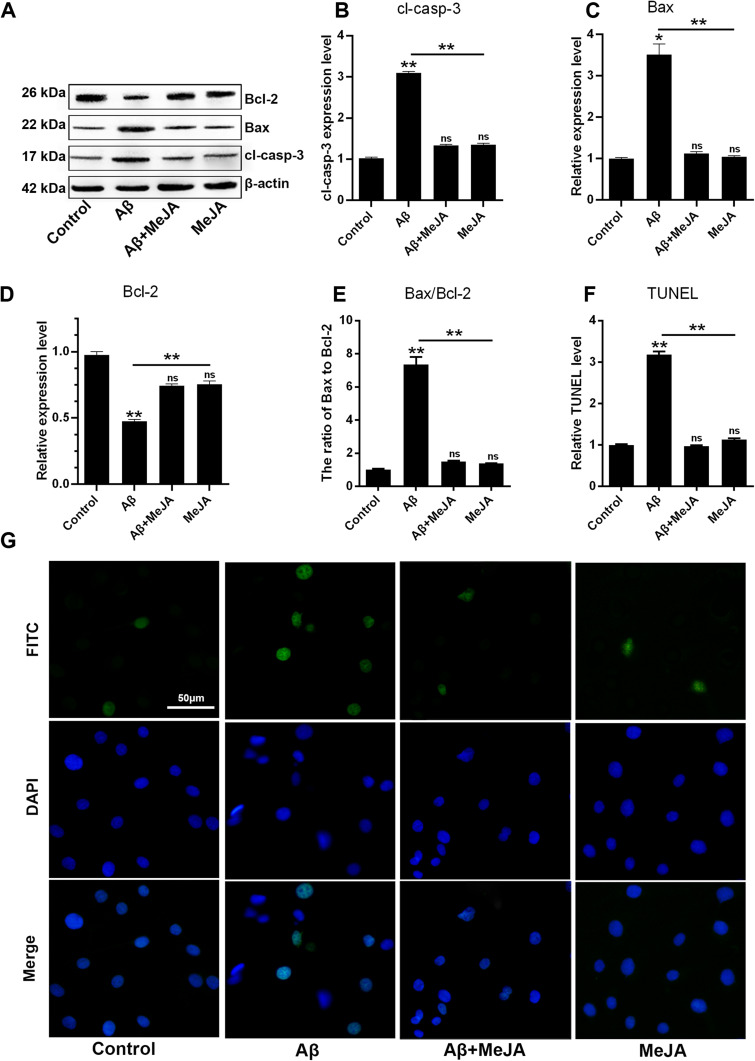
Excessive oxidative stress and inflammatory response can affect cell metabolism and lead to cell apoptosis. Besides, the ratio of Bax to Bcl-2 has been reported to up-regulate the expression of cl-casp-3 thus leading to cell apoptosis.26 In this study, the levels of apoptosis-related markers including Bax, cl-casp-3 and Bcl-2 in four groups were determined through Western blot (Figure 4A–D). In Aβ group, the expressions of Bax and cl-casp-3 were up-regulated, while the expression of Bcl-2 was down-regulated, compared with that in control group (p<0.01), which suggested that Aβ accelerated BV-2 cells’ apoptosis via regulating the expression levels of apoptosis-related markers. Meanwhile, the marker expressions in Aβ+MeJA group showed no significant difference compared with control group. In detail, both Bax/Bcl-2 value (Figure 4E) and cl-casp-3 expression level in Aβ+MeJA group were much lower than those of Aβ group, which suggested that MeJA had inhibitive effects on Aβ-induced apoptosis. Besides, the result of TUNEL assay of BV-2 cells in Aβ group showed a higher number of apoptotic cells than control, Aβ+MeJA and MeJA groups (Figure 4F and G). These findings suggested that Aβ stimulated BV-2 cell apoptosis and MeJA inhibited Aβ-induced apoptosis.
Figure 4.
Apoptosis in BV-2 cells under different treatments. The expressions of Bcl-2, Bax, cl-casp-3 and β-actin were evaluated by Western blot (A) and the relative levels of cl-casp-3 (B), Bax (C), Bcl-2 (D) and the ratio of Bax to Bcl-2 (E) were organized into histograms. TUNEL assay for apoptosis with the scale bar of 50 µm, the images (G) and relative TUNEL level were organized (F). *p<0.05; **p< 0.01.
Abbreviation: ns, no significant difference.
MeJA Protected Microglial Cells Against Aβ-Induced Oxidative Stress, Inflammation and Apoptosis via Nrf2/HO-1 Pathway
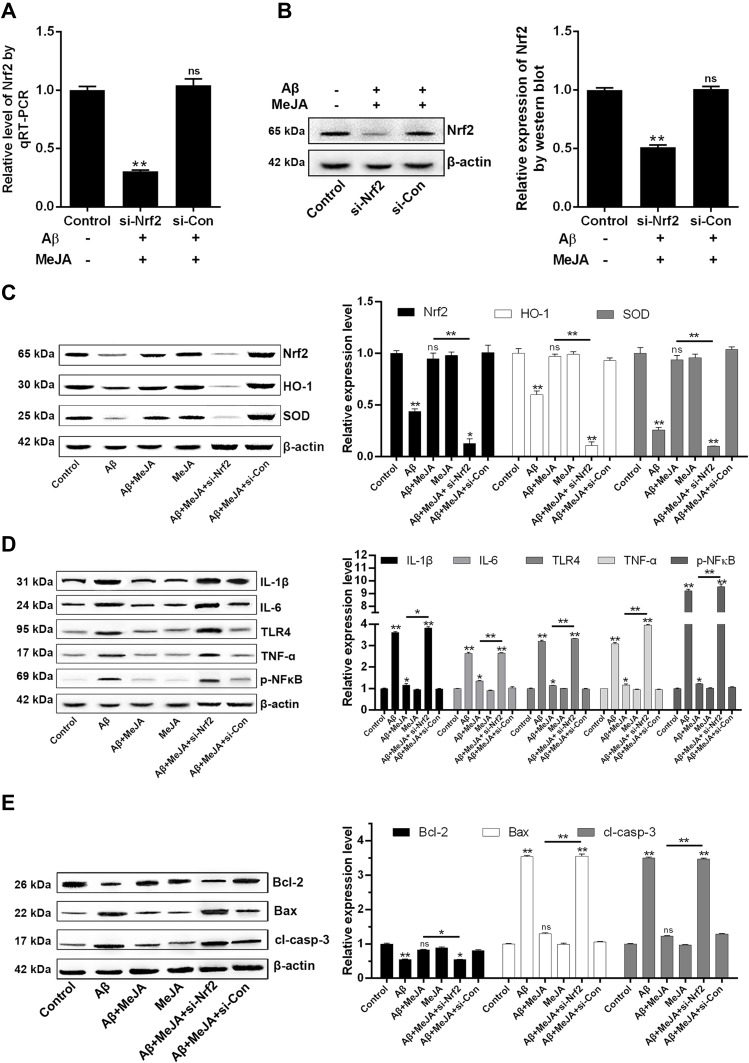
To investigate whether MeJA protects BV-2 cells against oxidative stress and inflammation and apoptosis via Nrf2/HO-1 pathway, we knocked down Nrf2 in BV-2 cells by siRNA and performed a series of evaluations. First, the transfection efficiency was determined by qRT-PCR and Western blot (Figure 5A and B). The results showed that in Aβ+MeJA+si-Nrf2 group, the level of Nrf2 reduced about 70% compared with control group (p<0.01), and there was no significant difference between Aβ+MeJA+si-con group and control group, which indicated the RNA interference was successful. Subsequently, the expressions of the antioxidant-related factors, inflammatory cytokines and apoptosis factors were detected by Western blot to explore the critical effect of Nrf2-dependent HO-1 pathway on Aβ-induced BV-2 cells.
Figure 5.
The expressions of downstream proteins under different treatments. The transfection efficiency of siRNA was detected by qRT-PCR (A) and Western blot (B). The expressions were evaluated by Western blot and the relative contents of antioxidant-related factors (Nrf2, HO-1 and SOD) (C), inflammatory cytokines (IL-1β, IL-6, p-NFκB, TNF-α, TLR4) (D) and apoptosis-related factors (Bcl-2, Bax, cl-casp-3) (E) were organized into histograms. *p<0.05; **p<0.01.
Abbreviation: ns, no significant difference.
The results of Western blot (Figure 5C) showed that the expressions of antioxidant-related factors (Nrf2, HO-1 and SOD) in Aβ+MeJA+si-Nrf2 group were significantly down-regulated compared with control group (p<0.01). There was no significant difference between Aβ+MeJA group and control group, whereas the expressions of antioxidant-related factors were significantly up-regulated compared with Aβ+MeJA+si-Nrf2 group. These findings indicated that MeJA protected BV-2 cells against Aβ-induced oxidative stress via regulating the expression of Nrf2. Furthermore, significant up-regulation (p<0.01) of inflammatory cytokines (IL-1β, IL-6, TNF-α, TLR4 and p-NF-κB) was observed in Aβ+MeJA+si-Nrf2 group and Aβ group compared with those in control group (Figure 5D), while there was little difference between Aβ+MeJA group and control group. Meanwhile, the expressions of inflammatory cytokines in Aβ+MeJA+si-Nrf2 group were significantly up-regulated compared with the wild-type Aβ+MeJA group (p<0.01). These findings suggested that MeJA protected BV-2 cells against Aβ-induced inflammation via Nrf2/HO-1 pathway. Moreover, the expressions of apoptosis markers Bax and cl-casp-3 in Aβ+MeJA+si-Nrf2 group were much higher than control group (p<0.01), whereas there was no significant difference between Aβ+MeJA group and control group (Figure 5E). Conversely, the level of Bcl-2 in Aβ+MeJA+si-Nrf2 group was significantly down-regulated compared with control group, and similar levels of Bcl-2 were found in Aβ+MeJA group and control group. The Bax to Bcl-2 ratio in Aβ+MeJA si-Nrf2 group was about twice that in wild-type Aβ+MeJA group. These findings indicated that MeJA protected BV-2 cells against Aβ-induced apoptosis via Nrf2/HO-1 pathway.
Discussion
In the present study, we demonstrated that MeJA was crucial for Aβ-induced toxicity in BV-2 cells, inflammatory response and apoptosis. MeJA up-regulated the expressions of Nrf2 and HO-1, which inhibited the production of ROS and MDA. Meanwhile, our study also verified that the activated Nrf2/HO-1 pathway down-regulated the inflammatory cytokines (TLR4, p-NFκB, TNF-α, IL-1β and IL-6) and apoptosis factors (Bax and cl-casp-3). Thus, MeJA attenuated Aβ-induced toxicity on the viability of BV-2 cells, and relieved Aβ-induced oxidative stress, inflammation and apoptosis.
Aβ, a key factor leading to AD, incurs excessive ROS production and LPS-stimulated peroxidation.16,27 Christen has demonstrated that Aβ produces free radicals associated with inflammation and induces the development of age-related pathologies including AD.28 McDonald et al have proposed that local inflammatory response of microglia may be the cause of the neuropathological process in the brain of AD patients.29 The ongoing inflammatory processes have been proven in AD patients and NF-kB plays an important role in neurodegenerative disorders.29 ROS generation, inflammatory responses, and apoptosis have been reported to contribute to the process of neurodegenerative diseases.4,30,31
Oxidative stress and inflammation are two main pathogenesis factors of central nervous system diseases, which lead to injury and apoptosis of nerve cells, especially microglia.12,32,33 TLR4, one of the most important types of TLR families, is a transmembrane glycoprotein that plays a key role in the innate immune system and it is related to the secretion of multiple inflammatory cytokines.34,35 Briefly, LPS attaches to the TLR4-MD2 complex and triggers the phosphorylation of NFκB, and p-NFκB leads to the up-regulation of pro-inflammatory cytokines. In the present study, Aβ-induced BV-2 cells showed up-regulation of ROS, MDA, and pro-inflammatory cytokines, which are consistent with the previous studies.14,15,18
Nrf2 is a critical regulator of pro-inflammatory cytokines and intracellular REDOX equilibrium and it is involved in innate immune system.23,36 As a signal transduction medium, MeJA protects the body against oxidative stress by inducing Nrf2 to activate the transcriptional activities of downstream antioxidant genes.37 Besides, MeJA activates the cellular defense mechanism by inducing the expression and activation of related anti-pathogenic factors, which inhibits inflammation.38 Consequently, Solomon et al confirmed the inhibitory effects of MeJA on pro-inflammatory cytokines and Aβ in vivo (mice).39
Blasko et al have reported high level of Aβ in the brain tissue of AD patients and overexpression of pro-inflammatory cytokines.40 MeJA can selectively kill cancer cells without affecting normal cells and it has been proven to have anti-inflammatory and anti-forgetting effects.41 However, the anti-oxidative stress, anti-inflammatory and anti-apoptosis effects of MeJA on BV-2 cells through mediating Nrf2 expression should be verified. For this reason, this research was designed to analyze the expression levels of antioxidant-related factors in BV-2 cells treated with MeJA and/or Aβ, as well as in Nrf2-knockdown and wild groups. The findings of our research verified the results of previous reports, which showed that MeJA down-regulated immunocytokines including TLR4, p-NFκB, TNF-α, IL-1β, IL-6, Bax and cl-casp-3 while it up-regulated Nrf2, HO-1, SOD and Bcl-2 induced by Aβ.6,11,42 Thus, a potential cellular mechanism between MeJA and Nrf2/HO-1 signaling pathway was found in this study (Figure 6).
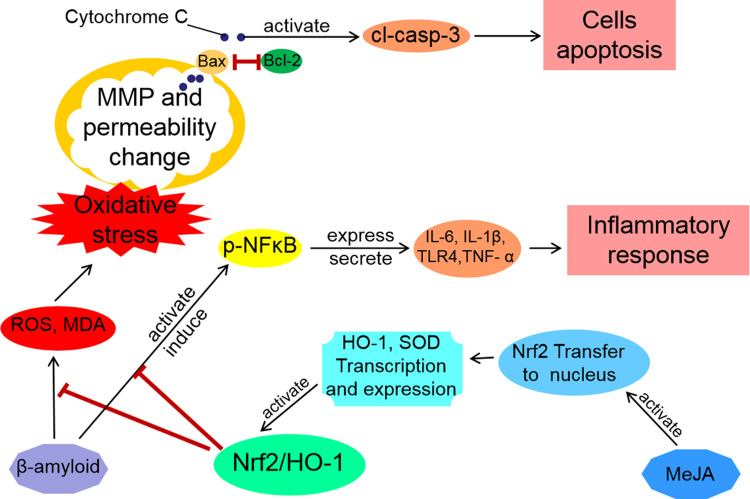
Figure 6.
Schematic representation of cellular mechanism through which MeJA protected BV-2 cells via Nrf2/HO-1 pathway. Two action mechanisms of MeJA on Aβ-treated BV-2 cells were illustrated as antioxidant (inhibition of apoptosis) and anti-inflammatory (maintenance of cell activity) mechanisms.
In conclusion, MeJA protected microglial cells against Aβ-induced oxidative stress and inflammation via Nrf2/HO-1 pathway. The present results might explain the underlying molecular mechanism of how MeJA attenuated Aβ-induced toxicity on the viability of BV-2 cells. In fact, environmental conditions and receptor-mediated effects in different tissues have significant effects on the immune activation of microglia, so we need more repetitive experiments in vivo. Besides that, the safety of using MeJA in vivo should be further tested in the animal model and more data related to the drug resistance are also needed.
Abbreviations
AD, Alzheimer’s disease; APP, amyloid precursor protein; Aβ, β-amyloid protein; Bax, Bcl-2-associated x; Bcl-2, B-cell lymphoma-2; HO-1, Heme oxygenase-1; IL-1β, interleukin 1β; IL-6, interleukin 6; LPS, lipopolysaccharides; MDA, malondialdehyde; MeJA, methyl jasmonate; MMP, mitochondrial membrane potential; Nrf2, nuclear factor-erythroid 2-related factor 2; p-NFkB, phosphor-nuclear factor-kappa B; ROS, reactive oxygen species; SOD, superoxide dismutase; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor α.
Author Contributions
Hua Li and Limei Lv performed the majority of the experiments and wrote the manuscript; Chunyan Wu helped us to perform the experiments; Jisheng Qi participated in manuscript writing; Baolin Shi designed and modified the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang Z, Xiong L, Wang G, Wan W, Zhong C, Zu H. Insulin-like growth factor-1 protects SH-SY5Y cells against beta-amyloid-induced apoptosis via the PI3K/Akt-Nrf2 pathway. Exp Gerontol. 2017;87(Pt A):23–32. doi: 10.1016/j.exger.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 2.Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer’s A beta(1–42) and A beta(25–35). J Am Chem Soc. 2001;123(24):5625–5631. doi: 10.1021/ja010452r [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Hassanein EHM, Shalkami AS, Khalaf MM, Mohamed WR, Hemeida RAM. The impact of Keap1/Nrf2, P38MAPK/NF-kappaB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed Pharmacother. 2019;109:47–56. doi: 10.1016/j.biopha.2018.10.088 [DOI] [PubMed] [Google Scholar]
- 7.Yan Q, Zhang J, Liu H, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23(20):7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s Disease. Front Integr Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagyinszky E, Giau VV, Shim K, Suk K, An SSA, Kim S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J Neurol Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 10.Lin HJ, Tseng CP, Lin CF, et al. A Chinese herbal decoction, modified Yi Guan Jian, induces apoptosis in hepatic stellate cells through an ROS-mediated mitochondrial/caspase pathway. Evid Based Complement Alternat Med. 2011;2011:459531. doi: 10.1155/2011/459531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-kappaB, Muc5ac, TNF-alpha and IL-1beta. Chem Biol Interact. 2015;237:151–165. doi: 10.1016/j.cbi.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 12.McKenzie JA, Klegeris A. Modulation of microglial functions by methyl jasmonate. Neural Regen Res. 2018;13(7):1290–1293. doi: 10.4103/1673-5374.235078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sa-Nakanishi AB, Soni-Neto J, Moreira LS, et al. Anti-inflammatory and antioxidant actions of methyl jasmonate are associated with metabolic modifications in the liver of arthritic rats. Oxid Med Cell Longev. 2018;2018:2056250. doi: 10.1155/2018/2056250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui B, Zhang S, Wang Y, Guo Y. Farrerol attenuates beta-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed Pharmacother. 2019;109:112–119. doi: 10.1016/j.biopha.2018.10.053 [DOI] [PubMed] [Google Scholar]
- 15.Hui Y, Chengyong T, Cheng L, Haixia H, Yuanda Z, Weihua Y. Resveratrol attenuates the cytotoxicity induced by amyloid-beta1-42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res. 2018;43(2):297–305. doi: 10.1007/s11064-017-2421-7 [DOI] [PubMed] [Google Scholar]
- 16.Guo C, Yang L, Wan CX, et al. Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways. Phytomedicine. 2016;23(13):1629–1637. doi: 10.1016/j.phymed.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 17.Ci X, Lv H, Wang L, et al. The antioxidative potential of farrerol occurs via the activation of Nrf2 mediated HO-1 signaling in RAW 264.7 cells. Chem Biol Interact. 2015;239:192–199. doi: 10.1016/j.cbi.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 18.Kwon SH, Ma SX, Hwang JY, Lee SY, Jang CG. Involvement of the Nrf2/HO-1 signaling pathway in sulfuretin-induced protection against amyloid beta25-35 neurotoxicity. Neuroscience. 2015;304:14–28. doi: 10.1016/j.neuroscience.2015.07.030 [DOI] [PubMed] [Google Scholar]
- 19.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi: 10.3390/molecules15107266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah ZA, Li RC, Thimmulappa RK, et al. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147(1):53–59. doi: 10.1016/j.neuroscience.2007.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen-Roetling J, Regan RF. Targeting the Nrf2-heme oxygenase-1 axis after intracerebral hemorrhage. Curr Pharm Des. 2017;23(15):2226–2237. doi: 10.2174/1381612822666161027150616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harder B, Jiang T, Wu T, et al. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem Soc Trans. 2015;43(4):680–686. doi: 10.1042/BST20150020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. doi: 10.1038/ki.2012.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–237. doi: 10.1016/0165-5728(90)90073-V [DOI] [PubMed] [Google Scholar]
- 26.Pawlowski J, Kraft AS. Bax-induced apoptotic cell death. Proc Natl Acad Sci U S A. 2000;97(2):529–531. doi: 10.1073/pnas.97.2.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao C, Gao F, Ou L, et al. Tetrahydroxy stilbene glycoside (TSG) antagonizes Aβ-induced hippocampal neuron injury by suppressing mitochondrial dysfunction via Nrf2-dependent HO-1 pathway. Biomed Pharmacother. 2017;96:222–228. doi: 10.1016/j.biopha.2017.09.134 [DOI] [PubMed] [Google Scholar]
- 28.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71(2):621S–629S. doi: 10.1093/ajcn/71.2.621s [DOI] [PubMed] [Google Scholar]
- 29.McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17(7):2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotman CW, Anderson AJ. A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol Neurobiol. 1995;10(1):19–45. doi: 10.1007/BF02740836 [DOI] [PubMed] [Google Scholar]
- 31.Roth KA. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol. 2001;60(9):829–838. doi: 10.1093/jnen/60.9.829 [DOI] [PubMed] [Google Scholar]
- 32.Sivandzade F, Bhalerao A, Cucullo L. Cerebrovascular and neurological disorders: protective role of NRF2. Int J Mol Sci. 2019;20(14):3433. doi: 10.3390/ijms20143433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova AT, Kostov RV, Kazantsev AG. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018;285(19):3576–3590. doi: 10.1111/febs.14379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidarzadeh M, Roodbari F, Hassanpour M, Ahmadi M, Saberianpour S, Rahbarghazi R. Toll-like receptor bioactivity in endothelial progenitor cells. Cell Tissue Res. 2020;379(2):223–230. doi: 10.1007/s00441-019-03119-2 [DOI] [PubMed] [Google Scholar]
- 35.Yousefi M, Mamipour M, Sokullu SE, Ghaderi S, Amini H, Rahbarghazi R. Toll-like receptors in the functional orientation of cardiac progenitor cells. J Cell Physiol. 2019;234(11):19451–19463. doi: 10.1002/jcp.28738 [DOI] [PubMed] [Google Scholar]
- 36.Thimmulappa RK, Lee H, Rangasamy T, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116(4):984–995. doi: 10.1172/JCI25790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem. 2017;292(41):16817–16824. doi: 10.1074/jbc.R117.800169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gundlach H, Muller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon U, Taghogho EA. Methyl jasmonate attenuates memory dysfunction and decreases brain levels of biomarkers of neuroinflammation induced by lipopolysaccharide in mice. Brain Res Bull. 2017;131:133–141. doi: 10.1016/j.brainresbull.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 40.Blasko I, Apochal A, Boeck G, Hartmann T, Grubeck-Loebenstein B, Ransmayr G. Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiol Dis. 2001;8(6):1094–1101. doi: 10.1006/nbdi.2001.0451 [DOI] [PubMed] [Google Scholar]
- 41.Cesari IM, Carvalho E, Figueiredo Rodrigues M, Mendonca Bdos S, Amoedo ND, Rumjanek FD. Methyl jasmonate: putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int J Cell Biol. 2014;2014:572097. doi: 10.1155/2014/572097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287(13):9873–9886. doi: 10.1074/jbc.M111.312694 [DOI] [PMC free article] [PubMed] [Google Scholar]