Abstract
Biofilm formation and hyphal growth are considered to be the most serious virulence factors of Candida species in blood causing candidemia infections, which are difficult to treat due to the spread of resistant Candida isolates to most antifungal drugs. Therefore, in this study, we investigated the effect of different types and concentrations of selected macroalgal extracts from Cladostephus spongiosus (Phaeophyta), Laurencia papillosa (Rhodophyta), and Codium arabicum (Chlorophyta) in inhibiting those virulence factors of the isolated Candida. Acetone extract of C. spongiosus (AECS) showed a stronger anticandidal activity against the selected strains than ethanol extract. Candida krusei was the highest biofilm producer among the selected isolates. AECS showed an inhibition of C. krusei biofilm formation as well as a reduction in the viability of preformed biofilms. Also, AECS reduced various sugars in the candidal exo-polysaccaride layer (EPS). Scanning electron microscopy (SEM) and light microscopic images revealed an absence of hyphae and an alteration in the morphology of biofilm cells when treated with AECS. Moreover, AECS downregulated the expression of hyphal specific genes, hyphal wall protein 1 (HWP1), Agglutinin-like protein 1 (ALS1) and fourth secreted aspartyl proteinase (SAP4), which confirmed the inhibitory effect of AECS on hyphal growth and biofilm formation. Gas chromatography-mass spectrophotometer (GC-MS) analysis of AECS showed three major compounds, which were non-existent in the ethanol extract, and might be responsible for the anticandidal activity; these revealed compounds were 4-hydroxy-4-methyl-2-pentanone, n-hexadecenoic acid, and phenol, 2-methoxy-4-(2-propenyl). These active compounds of AECS may be promising for future pharmaceutical applications in the treatment of candidemia.
Subject terms: Reverse transcription polymerase chain reaction, Antifungal agents
Introduction
Candida spp. are one of the most common causes of blood stream infections (candidemia), which were found within hospital patients worldwide1. In Egypt alone, the frequency of Candida spp. detected in blood samples ranged between 40 to 45% of populations within hospitals2. These species produced biofilms that led to high mortality rates3. Candida spp. possess a number of virulence factors, which enable the organism to cause hematogenous disseminated infections in susceptible hosts4. The virulence factors of Candida spp. include initial adhesion followed by biofilm production, and morphological transition of yeast cells to their hyphal form5,6. The effectiveness of available antifungals in treating candidemia are in decline due to the development of resistant Candida biofilms and their toxicity7–9. Moreover, azoles that had good broad-spectrum antimicrobial efficacy in candidemia, have many side effects with the prolonged use10,11. Thus, there is an urgent need to develop new antifungal compounds for countering Candida virulence and pathogenesis.
Inhibition of biofilm production and yeast-hyphal transition are predicted to be effective strategies in the treatment of Candida infections when screening for new antifungal agents12. Mainly, agents that inhibit biofilm formation and hyphal growth without affecting the viability of planktonic cells, might be useful antibiofilm agents. Past screens have successfully identified compounds from some plant extracts that exhibit antifungal and antibiofilm activities against C. albicans such as purpurin, chyrsophanol and rhein13–16.
Marine macroalgae are widely employed in folk medicine17,18. As well, marine macroalgae have been shown to produce metabolic compounds with antimicrobial19, antifungal20, anti-inflammatory21, antiviral22, antioxidant23 and anticancer activities24. Bioactive molecules of marine algal origin have high potential to inhibit the growth of many bacterial organisms and to further suppress their biofilm metabolic activities25,26. Also, El-Sheekh27 demonstrated that the extracts of two brown seaweeds, Sargassum vulgaris and Sargassum wightii, exhibited antimicrobial activities. To the best of our knowledge, there are no studies in which marine algal extracts were investigated as alternatives of anticandidal and antibiofilm agents against candidemia. Therefore, this work aims to evaluate the anticandidal and antibiofilm activities of some seaweed extracts with a preliminary identification of the potential inhibitory compounds to find alternative drugs and a promising source of pharmaceutical agents.
Results
Algal extracts showed antifungal activity against the selected Candida species
Among different species of algae collected from the Red sea along the coastal region of Hurghada in Egypt, three species, namely, Cladostephus spongiosus, Laurencia papillosa, and Codium arabicum, were evaluated for their potential anticandidal activities. Table 1 revealed that C. spongiosus and L. papillosa extracts prepared with acetone and ethanol had active compounds that could inhibit growth of the four pathogenic Candida selected strains. While, the methanol extract of these algal extracts did not record an anticandidal activity against all the tested Candida. Acetone extract of C. arabicum showed the lowest anticandidal activity against the selected strains. However, the ethanolic and methanolic fractions of C. arabicum did not show any noticeable activity against all organisms. Further from the results obtained, it was observed that all the algal extracts prepared with acetone and ethanol could record higher inhibitory activities against the tested Candida compared to fluconazole, which did not show inhibitory activity at the same concentration of extracts (10 µg/ml). Among the algal extracts tested for inhibitory activities, acetone extract of C. spongiosus (AECS) showed relatively higher inhibitory activities (20.5, 18.0, 16.7 and 14.7 mm) against the selected Candida species (C. krusei, C. glabrata, C. parapsilosis, and C. albicans, respectively).
Table 1.
Algal extracts and susceptibility of Candida species.
| Algal extract (10 µg/ml) | Solvent | Diameter of inhibition zone (mm) | |||
|---|---|---|---|---|---|
| C. krusei | C. glabrata | C. parapsilosis | C. albicans | ||
| Cladostephus spongiosus | Acetone | 20.50 ± 0.50 | 18.00 ± 0.00 | 16.7 ± 0.29 | 14.67 ± 0.58 |
| Ethanol | 11.67 ± 0.58 | 10.67 ± 0.58 | 5.50 ± 0.50 | 4.50 ± 0.50 | |
| Methanol | 0.00 | 0.00 | 0.00 | 0.00 | |
| F | 1631.286 | 2212.00 | 1824.250 | 871.000 | |
| P-value | *0.000 | *0.000 | *0.000 | *0.000 | |
| Laurencia papillosa | Acetone | 9.67 ± 0.58 | 9.17 ± 0.29 | 9.03 ± 0.06 | 7.33 ± 0.58 |
| Ethanol | 7.5 ± 0.50 | 5.17 ± 0.29 | 5.00 ± 0.00 | 3.50 ± 0.50 | |
| Methanol | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| F | 397.0000 | 1140.500 | 55291.00 | 207.571 | |
| P-value | *0.000 | *0.000 | *0.000 | *0.000 | |
| Codium arabicum | Acetone | 5.33 ± 0.58 | 2.97 ± 0.06 | 3.33 ± 0.29 | 3.33 ± 0.58 |
| Ethanol | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Methanol | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| F | 256.000 | 7921.000 | 400.000 | 100.000 | |
| P-value | *0.000 | *0.000 | *0.000 | *0.000 | |
| Fluconazole (10 µg/ml) | 1.97 ± 0.05 | 0.00 ± 0.00 | 1.00 ± 0.29 | 0.00 ± 0.00 | |
Values are the mean of three replicates ± SD; *significant at P < 0.05
The MIC and MFC values with fungicidal ratios of AECS and fluconazole were shown in Table 2. Both MIC and MFC of AECS gave the lowest value of 80 and 320 µg/ml with a fungicidal ratio of 1:4 in C. krusei compared to fluconazole which gave a fungicidal effect at very high concentration of 2000 µg/ml. C. krusei was the most susceptible strain to AECS treatment and interestingly, C. krusei was found to be the most prolific biofilm producing Candida strain (Fig. S2). Herein, we focus on the antifungal activity of AECS on C. krusei biofilm production and hyphal growth.
Table 2.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of AECS and fluconazole with the corresponding fungicidal ratio.
| Fluconazole | AECS | Isolates | ||||
|---|---|---|---|---|---|---|
| Fungicidal ratio | MFC (µg/ml) | MIC (µg/ml) | Fungicidal ratio | MFC (µg/ml) | MIC (µg/ml) | |
| 1:5 | 2000 | 400 | 1:4 | 320 | 80 | C. krusei |
| 1:6 | 2100 | 350 | 1:4 | 360 | 90 | C. glabrata |
| 1:4 | 1200 | 300 | 1:4 | 400 | 100 | C. parapsilosis |
| 1:5 | 1750 | 350 | 1:5 | 450 | 90 | C. albicans |
AECS inhibits biofilms formation and eradicates the performed biofilm
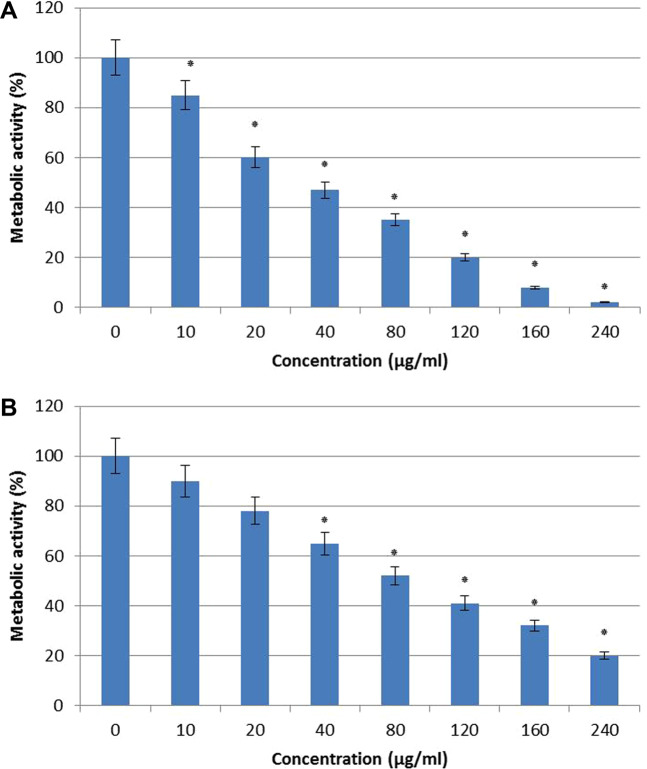
Activity of AECS on C. krusei biofilm formation was quantified and viability was expressed in terms of metabolic activity percentage. The Biofilm inhibitory concentration (BIC) of AECS against C. krusei was 120 µg/ml (Fig. 1A). The BIC80 (biofilm inhibiting concentration) was defined as the lowest concentration of AECS that inhibits 80% metabolic activity of biofilm formation as compared to control. Also, BEC80 for C. krusei was 2-fold higher (240 μg/ml) compared to (BIC80 = 120 μg/ml) (Fig. 1B), as BEC80 (biofilm-eradicating concentration) was defined as the lowest concentration of AECS that eradicates 80% of performed biofilm compared to control.
Figure 1.
Effect of AECS on C. krusei biofilm formation (A) and preformed biofilms (B) BIC80 and BEC80 of AECS against biofilm formation and preformed biofilms = 120 and 240 µg/ml respectively. Results represent the average of three independent experiments ±SD. *p < 0.05 when compared with control.
SEM visualization of C. krusei biofilms
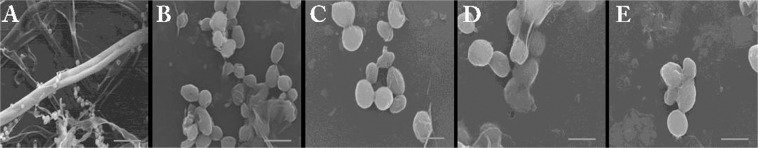
SEM observations provided useful information on the different cellular morphologies present in the biofilm structure. The effect of AECS on C. krusei biofilm and its cellular morphology was monitored by SEM (Fig. 2). SEM images of control plates showed the presence of dense complex structure of biofilm having hyphae and yeast cells (Fig. 2A). In the presence of 80 μg/ml AECS, formation of biofilms was reduced with complete hyphal disappearance, and consisted mostly of yeast cells (Fig. 2B). At BIC80 of AECS (120 μg/ml), biofilm cells were found to have perforated outer membrane with distorted shape (Fig. 2C). Few yeast cells with wrinkled surface can be seen at 160 μg/ml and 240 μg/ml of AECS concentration respectively (Fig. 2D,E). Further increase in AECS concentration (120 μg/ml) led to a complete inhibition of biofilms.
Figure 2.
Scanning electron microscope images for the effect of AECS on C. krusei biofilm formation at 1000× magnification. 0 μg/ml (A), 80 μg/ml (B), 120 μg/ml (C), 160 μg/ml (D) and 240 μg/ml (E) of AECS. Scale bar represents 20 μm.
AECS inhibits the EPS production and hyphal growth of C. krusei
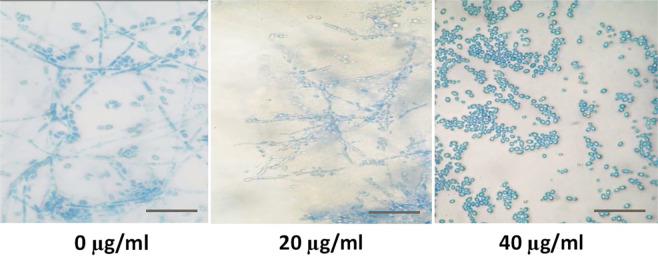
The major virulence factors of Candida species include yeast-to-hyphal transition, and EPS production. EPS ensures the mechanical stability and the physical architecture of the formed biofilms. As a result, we used different concentrations of AECS (20, 40, and 80 µg/ml) to study their effects on the EPS production of C. krusei. Our results showed that the different tested concentrations of AECS reduced the amount of sugar content in treated C. krusei compared to the control as in Fig. 3 that showed the ability of AECS to decrease sugar content formed by C. krusei biofilms. Furthermore, our results showed that AECS inhibits the hyphal growth of C. krusei in a dose dependent manner (Fig. 4). Microscopically, massive C. krusei hyphae were observed in control plates. In the meantime, hyphal growth was moderate at 20 μg/ml, and absent at 40 μg/ml of extract, indicating a directly proportional relation between concentration of AECS and inhibition of hyphal growth.
Figure 3.

Effect of AECS on the EPS layer of C. krusei biofilms. AECS showed a concentration dependent reduction of sugars when compared to that of the control. *p < 0.05.
Figure 4.
Microscopic visualization for the effect of AECS on C. krusei hyphal growth at 40x magnification. Scale bar represents 5 μm.
AECS downregulates C. krusei hyphal specific genes
To determine possible molecular mechanism of AECS inhibition of C. krusei hyphal growth, we tested the expression level of hyphal growth associated genes such as HWP1, ALS1, and SAP4 genes. Expression of these genes in AECS treated cells was significantly reduced by 5-fold, 2.5-fold, and 3.3-fold, respectively, when compared to the control (Fig. 5).
Figure 5.
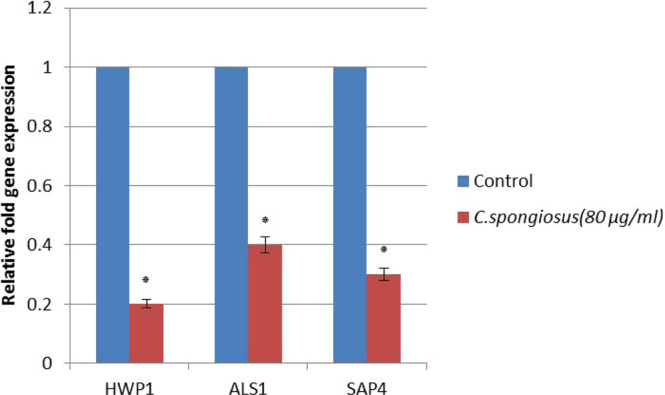
Effect of AECS on the expression of C. krusei hypha specific genes. *p < 0.05.
Chemical analysis of the different C. spongiosus extracts using GC-MS
As a next step, it was necessary to check the chemical composition of the different C. spongiosus extracts using GC–MS. The chemical constituents, molecular weight and peak area of each component were listed in Tables (3, 4). Our results indicated that the major compounds in AECS were 4-hydroxy-4-methyl-2-pentanone (50.47%), n-hexadecanoic acid (6.46%) and Phenol, 2-methoxy-4-(2-propenyl) (9.21%). While, the three major compounds in the ethanol extract of C. spongiosus were 9,12,15-octadecatrienoic acid ethyl ester (22%), Hexadecanoic acid ethyl ester (16.4%), and 2-Hexadecen-1-ol,3,7,11,15-tetramethyl (15.25%). Preliminary screening suggested that these different major compounds in AECS might be the active compounds that cause C. krusei biofilm inhibition and hyphal growth. Meanwhile AECS is a mixture of several compounds, each component might contribute to the biofilm inhibition than if they acted alone. So, further study will be done for the isolation and the purification of active compounds with a comprehensive toxicological analysis to determine its safety as it is beyond the scope of this paper.
Table 3.
Chemical constituents of C. spongiosus acetone extract.
| S.No | RT | Compound name | PA(%) | Mf | MW |
|---|---|---|---|---|---|
| 1 | 8.83 | 4-hydroxy-4-methyl-2-pentanone | 50.47992 | C6H12O2 | 116 |
| 2 | 25.01 | Phenol,2-methoxy-4-(2-propenyl) | 9.213392 | C10H12O2 | 164 |
| 3 | 27.85 | 4,7-Octadecadiynoic acid,methyl ester | 0.467299 | C19H30O2 | 290 |
| 4 | 29.07 | Phenol,2-methoxy-4-(2-propenyl)-acetate | 1.582964 | C12H14O3 | 206 |
| 5 | 32.54 | Oleic Acid | 0.941824 | C18H34O2 | 282 |
| 6 | 32.74 | cis-11-Eicosenoic acid | 5.487069 | C20H38O2 | 310 |
| 7 | 35.24 | Tetradecanoic acid | 2.036633 | C14H28O2 | 228 |
| 8 | 36.22 | 2-Pentadecanone6,10,14-trimethyl | 1.12189 | C18H36O | 268 |
| 9 | 37.55 | Stearic acid,3-(octadecyloxy)propyl ester | 0.304654 | C39H78O3 | 594 |
| 10 | 37.87 | 9-Hexadecenoic acid | 0.111443 | C16H30O2 | 254 |
| 11 | 39.66 | n-Hexadecanoic acid | 16.46548 | C16H32O2 | 256 |
| 12 | 42.94 | Oleic acid, eicosyl ester | 2.982072 | C38H74O2 | 562 |
| 13 | 46.83 | Octadecanoic acid,2-hydroxy-1,3propanediylester | 1.726582 | C39H76O5 | 624 |
| 14 | 49.33 | Hexa-t-butylselenatrisiletane | 2.944481 | C24H54SeSi3 | 506 |
| 15 | 51.60 | Cyclodecasiloxane,eicosamethyl | 0.423269 | C20H60O10Si10 | 740 |
| 16 | 53.57 | Decanedioic acid, diisooctyl ester | 2.894215 | C26H50O4 | 426 |
Note: RT-Retention time; MF-Molecular formula; MW-Molecular Weight; PA-Peak area *
Table 4.
Chemical constituents of C. spongiosus ethanol extract.
| S.No | RT | Compound name | PA(%) | Mf | MW |
|---|---|---|---|---|---|
| 1 | 6.03 | Oxime-, methoxy-phenyl | 2.265608 | C8H9NO2 | 151 |
| 2 | 9.35 | Octadecanal, 2-bromo- | 0.800369 | C18H35BrO | 346 |
| 3 | 10.89 | Propanedioic acid, [2-[(4-methylphenyl) sulfonyl]ethylidene]-, dimethyl ester | 5.877589 | C14H16O6S | 312 |
| 4 | 14.54 | 9-Octadecenoic yl)methyl ester acid(2-phenyl-1,3-dioxolan-4- | 0.308225 | C28H44O4 | 444 |
| 5 | 15.52 | Phenol2,4-bis(1,1-dimethylethyl | 0.985806 | C14H22O | 206 |
| 6 | 16.83 | Octasiloxane hexadecamethyl | 0.195271 | C16H50O7Si8 | 578 |
| 7 | 18.66 | Cis-13-Eicosenoic acid | 3.9762 | C20H38O2 | 310 |
| 8 | 21.88 | 2-Hexadecen-1-ol,3,7,11,15-tetramethyl | 15.25358 | C20H40O | 296 |
| 9 | 22.38 | Isopropyl linoleate | 2.058717 | C21H38O2 | 322 |
| 10 | 23.24 | Docosanoic acid, methyl ester | 1.347766 | C23H46O2 | 354 |
| 11 | 24.32 | Eicosapentaenoic acid | 1.367173 | C20H30O2 | 302 |
| 12 | 24.51 | Hexadecadienoic acid, methyl ester | 1.257589 | C17H30O2 | 266 |
| 13 | 24.65 | 6,9,12-Octadecatrienoic acid,, methyl ester | 3.249543 | C19H32O2 | 292 |
| 14 | 25.16 | Hexadecanoic acid, ethyl ester | 16.43712 | C18H36O2 | 284 |
| 15 | 25.27 | Hexadecanoic acid | 7.717059 | C16H32O2 | 256 |
| 16 | 28.41 | Linoleic acid ethyl ester | 7.557009 | C20H36O2 | 308 |
| 17 | 28.57 | 9,12,15-Octadecatrienoic acid, ethyl ester, | 22.00678 | C20H34O2 | 306 |
| 18 | 35.65 | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 3.768015 | C24H38O4 | 390 |
| 19 | 39.43 | 17-Pentatriacontene | 3.441183 | C35H70 | 490 |
Note: RT-Retention time; MF-Molecular formula; MW-Molecular Weight; PA-Peak area *
Discussion
Secondary metabolites of marine algae of potential interest have been extensively documented25. According to several reports, antimicrobial activity depends on algal species, extraction method, type of solvent and the resistance of the tested organism26. In the present study, acetone was the most effective solvent for the extraction of the bioactive compounds followed by ethanol. Furthermore, C. spongiosus was the most effective marine algae against the selected Candida species. These results are in agreement with many earlier reports17,18. Our data elucidated that AECS showed MIC and MFC at 80 μg /ml and 320 μg /ml against C. krusei. These results are consistent with the previous findings of Mickymaray and Allturaiki28 who reported that U. prolifera demonstrated an MIC and MFC at 500 and 1000 μg/ml against A. niger.
Bioactive molecules of marine algal origin have high potentiality to subjugate the growth of many infectious organisms and to suppress their biofilm metabolic activity26. Biofilm formation and hyphal morphogenesis are considered the most important virulence factors of Candida species29. The present study showed that secondary metabolites of AECS have the potential to attenuate these virulence factors.
AECS reduced the metabolic activity of the matured C. krusei biofilms in vitro and acts as a dominant antibiofilm agent that prevents biofilm formation and removes the existing biofilm. These results agreed with Dulger30 who reported that AECS has antibacterial and antibiofilm activities. SEM images of the C. krusei biofilm demonstrated the presence of dense hyphae in absence of the extract. However, it showed deformed and swollen cells at BIC80. These morphological alterations of the cells resulting in cell death as reported previously for sophorolipid treatment against C. albicans31. Moreover, cells deformation and distortion of cell membrane have been reported as the mechanisms of antimicrobial activity for many biosurfactants32.
AECS suppressed the expression of hyphal genes illustrating the molecular mechanism of AECS in inhibition the hyphal growth. This result is in accordance with Haque31 who reported the inhibition of C. albicans hyphal growth by sophorolipid using the same genes. To the best of our knowledge, there was not any scientific reports revealing the role of AECS against biofilm formation and hyphal growth of Candida sp.
As a next step, it was necessary to check the chemical composition of bioactive secondary metabolites in the different solvent extracts. The differences in the anticandidal effects of the algal extracts may be attributable to differences in the active compounds that present in the algae after their extraction with different solvents. The GC-MS analysis indicated that the chemical composition of the most promising AECS had 3 major peaks in comparison with the ethanolic extract; 4-hydroxy-4-methyl-2-pentanone was the major component, which showed the highest peak area percentage compared with the other components. On the other hand, this compound was not observed in the ethanolic extracts of C. spongiosus. Additionally, this compound had previously detected by GC-MS in acetone extract of the red algae Peterocladia Capillaceae and Laurencia pinnatifida showing a potent antimicrobial activity33. As well, this compound was detected as a volatile oil fraction from Phaeophyceae and Rhodophyceae that had an antimicrobial activity34. The second major component in AECS was n-hexadecanoic acid, which also was detected using GC-MS from Rhodophyceae35, and was reported to have an anticandidal activity36. In addition, n-hexadecanoic acid was found as a major component in the acetone extract of Sargassum hystrix with a strong antimicrobial activity33. Moreover, the third major component observed in AECS was Phenol, 2-methoxy-4-(2-propenyl), which previously identified by GC-MS analysis in methanol extract of Ulva lactuca with reported high antimicrobial and antioxidant activities37. Collectively, these results suggest that AECS is a mixture of several compounds, and each component might contribute to the biofilm inhibition than if they acted alone. Therefore, the current study suggested that the AECS is a potential source of natural anticandidal agents. It possessed certain metabolites with potent anticandidal properties that may be used for the treatment of blood candidemia infections as it can inhibit the candidal growth by suppressing biofilm formation, hyphal growth and its adhesion genes. Further study is required to characterize the antibiofilm activity of AECS in vivo by studying the antagonistic effect of its purified components against C. krusei biofilm and its safety.
Materials and methods
Organisms and growth conditions
Four Candida spp. strains (C. krusei, C. glabrata, C. parapsilosis, and C. albicans) were kindly provided by Dr. Mona Osama (Clinical Microbiology Unit, Tanta University Hospital, Faculty of Medicine, Tanta, Egypt). The selected strains were isolated from blood samples collected from the intensive care unit (ICU) and dialysis units in the Tanta University hospital in July 2016. All patients provided written informed consent and the study protocol was approved by the review board of Tanta University Hospitals for the collection of swabs from the Laboratories of Clinical Microbiology Unit at Tanta University Hospital, Faculty of Medicine, Tanta, Egypt. The clinicians followed the guidelines of the Declaration of Helsinki. One strain per patient was studied. Strains were stored at −70 °C. Phenotypic identification was confirmed with the API Candida system (bioMérieux Vitek, Hazelwood, MO, USA) following the manual instructions according to standard method of Buchaille38. The specific number code for each species is shown in the supplementary data (Fig. S1). A frozen glycerol stock of each strain was cultured on sabouraud dextrose broth (SDB; Ifco Laboratories, Detroit, MI, USA) and incubated at 37 °C for 24 h.
Algal collection
Three seaweeds species, Cladostephus spongiosus (Phaeophyta), Laurencia papillosa (Rhodophyta) and Codium arabicum (Chlorophyta), were collected from Hurghada coastal along the Red Sea (27°15′28″ N; 33°48′42″ E), Egypt, and identified according to Aleem39, Abbott and Hollenberg40 and Taylor41. Collected algal samples were preserved in polythene bags and transferred to the laboratory under cooled conditions to keep temperatures at 4–8 °C.
Extraction of algal bioactive compounds using organic solvents
About 2 kg of the three isolated algal species were harvested, separately rinsed with sterile-filtered seawater and shade-dried, cut into small pieces, and powdered in a mixer grinder. Then, 5 g of powdered sample of each algal species was extracted separately and soaked with 40 ml of different solvents (acetone, ethanol and methanol) for 48 h. The obtained extracts were filtrated and concentrated in a rotatory evaporator at 40 °C. The residual solvent was removed with a vacuum pump. Then, the weighted crude extracts were well preserved in airtight containers and kept at −20 °C for further analysis42,43.
Anticandidal activity of selected algal extracts
An agar well diffusion method as detailed in El-Zawawy and Hafez44 was conducted to determine the most effective algal extract against the four selected strains. Briefly, sabouraud dextrose agar (SDA) plates were inoculated with 100 μl of each Candida strain (1 × 106 cells/ml) with wells of size 8 mm filled with 10 µg/ml of each algal extract dissolved in different solvents (acetone, ethanol and methanol). Each solvent (100 µg/ml) was added as a control, which did not show any antifungal activities (data not shown). Fluconazole (10 µg/ml) (Diflucan, Pizer) was used as a positive control. Then, these plates were incubated at 37 °C for 48 h. After the incubation period, the results were observed and the diameter of the inhibition zone around each well was measured to determine the most effective extract. All tests were performed in triplicate.
Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
Minimal inhibitory concentration (MIC) of the most effective extract and fluconazole against the four selected strains was performed using 96-well microtiter plates. Selected strains were added in SDB supplemented with varying concentrations of acetone extract of C. spongiosus (AECS) and fluconazole, then incubated at 37 °C for 48 h. After incubation, the fungal growth was assayed at 600 nm using a Biotek plate reader. The MIC was recorded as the lowest concentration that produced complete suppression of visible growth45.
The MFC of AECS and fluconazole was determined according to Borman45. Briefly, (10 µg/ml) from MIC to last concentration wells of AECS and fluconazole were transferred separately to SDA plates, which were then incubated at 37 °C for 48 h. The MFC was recorded as the lowest drug concentration at which fungal growth was completely inhibited after 48 h of incubation.
Determining the cell viability of preformed biofilms in Candida strains
The ability to obtain quantitatively the metabolic activity of cells in preformed biofilms of the four Candida strains were tested by a reduction assay46 using colorimetric XTT [2,3-bis (2-methoxy-4-nitro-5sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt]. A cell suspension of each Candida strain was prepared in SDB at a density of 1 × 106 cells/ml, after that 100 μl were added to each well in microtiter plates. The plates were incubated at 37 °C for 48 h. At the end of incubation, medium was aspirated from the wells and nonadherent cells were removed by washing the biofilms 3-times with a sterile phosphate buffered saline (PBS). Residual PBS of the wells was removed. To each well of prewashed biofilms, 900 μl of fresh broth, 90 μl of XTT salt solution (0.5 mg/ml) and 10 μl menadione solution (1 mM) were added and incubated at dark at 37 °C for 5 h. During incubation, biofilm metabolism reduces XTT tetrazolium salt to XTT formazan. Then, the absorbance was measured spectrophotometrically at 490 nm to obtain the strain which is the higher biofilm producer.
Effect of AECS on biofilm formation and preformed biofilm of the higher biofilm producer strain
The inhibitory activity of AECS on biofilm formation was assessed in vitro according to Ramage47. A cell suspension of the selected strain was prepared in SDB (1 × 106 cells/ml) and added to microtiter plates (100 μl per well) with 100 μl of different concentrations of AECS (10, 20, 40, 80, 120, 180, 240 µg/ml). Similarly, 100 μl of SDB with 100 μl of acetone without algal extract were added into wells as a control. Microtiter plates were incubated at 37 °C for 48 h.
Preformed biofilms were prepared as described previously in microtiter plates, then different concentrations of AECS (100 μl) were added into the wells of prewashed biofilms. For the control, 100 μl of SD broth medium with 100 μl of acetone without AECS. Microtiter plates were then incubated, and biofilm metabolic activity was determined as mentioned above by colorimetric XTT assay46.
Biofilm imaging using scanning electron microscopy (SEM)
Untreated and treated biofilms with AECS of selected strains were washed with PBS and air-dried in desiccators48. Samples were coated with gold/palladium (40%/60%) and observed in a scanning electron microscope (JEOL, JSM–5200 LV, Tokyo, Japan) at Tanta University, Tanta, Egypt.
Quantification of exopolysaccharides (EPS) of AECS treated biofilm
This assay was used to estimate the amount of exopolysaccharides in AEC treated preformed biofilm compared to untreated biofilm as a control. Preformed biofilms were prepared as described previously. The non-adherent cells were discarded and 500 µl of 0.9% NaCl was added to the wells of the plate and washed thoroughly. Then, cell suspensions in 0.9% NaCl were transferred to sterile test tubes with an equal volume of 5% phenol. Then, 5% v/v of concentrated sulfuric acid containing 0.2% hydrazine sulfate was added and incubated in dark for 1 h and the absorbance was measured at 490 nm according to Nithya49.
Effect of AECS on candidal hyphal growth
Hyphal growth assay was performed in 10 ml of modified sabouraud glucose broth (MSGB) (Sigma) supplemented with 10% fetal bovine serum (FBS, Invitrogen). A cell suspension of the selected strain (1 × 106 cells/ml) was incubated with different concentrations of AECS (0, 20, 40, 80 μg/ml) at 37 °C with agitation (200 rpm) for 5 h. Aliquots of samples were stained using Lactophenol cotton blue and allowed to dry for 5 min, then visualized under a light microscope using an 40x objective lens and photographed (Nikon Eclipse Ti 100, Japan)50.
Real time PCR (qRT-PCR) expression analysis of candidal hyphal specific genes
Effect of AECS on the expression of hyphal specific genes, hyphal wall protein 1 (HWP1), Agglutinin-like protein 1 (ALS1) and fourth secreted aspartyl proteinase (SAP4), was evaluated by qRT-PCR. Hot phenol/chloroform extraction method51 was used in extraction of total RNA from AECS treated (80 μg/ml) and untreated (0 μg/ml) hyphal growth of selected strain. Quantitative RT-PCR amplification mixtures (25 ml) contained 10 ng template cDNA, Light Cycler Hybridization Probes Master Mix kit (Roche diagnostics, Tenay, Turkey), and SYBR Green I master mix buffer with fluorescein. Light Cycler (Roche diagnostics, Tenay, Turkey) and Light Cycler 3.5 software were used52,53.
Gas chromatography-mass spectrometer (GC-MS) analysis
Different extracts from C. spongiosus (acetone and ethanol) were investigated for their phytoconstituents using GC-MS (Trace GC Ultra, USA), at the National Research Centre (NRC), El Dokky, Giza Governorate. The identification of unknown compounds was based on comparing their retention time relative to those of the known compounds by matching spectral peaks available with Wiley 9 Mass Spectral Library54.
Statistical analysis
All data were expressed as mean ± standard deviation of three replicates and submitted to variance analysis using SPSS-20.
Supplementary information
Acknowledgements
The authors thank Dr. Ahmed Abd Elkhalek (Lecturer of genetics, Mubarak city for scientific research, Alexandria) for his kind assistance and monitoring the data of Real time PCR experiment and Dr. Chad Brabham for his input on the drafting of this manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not –for-profit sectors.
Author contributions
Nessma A. El Zawawy: Conceptualization, Methodology, Writing-Original Draft preparation, Writing- Reviewing and Editing. Rania A. El- Shenody: Methodology, Writing-Original Draft preparation. Sameh Ali: Software, Writing- Reviewing and Editing. Mohamed El-Shetehy: Methodology, Writing- Reviewing and Editing.
Data availability
The datasets used and analyzed during this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66000-1.
References
- 1.Kreger-Van Rij, N.J. The Yeasts: a taxonomic study. 3rd Edition, Elsevier Science Publishers B.V., Amsterdam, The Netherlands (1984).
- 2.Kumamoto CA. Candida biofilms. Curr. Opin. Microbiol. 2002;608:611–5. doi: 10.1016/s1369-5274(02)00371-5. [DOI] [PubMed] [Google Scholar]
- 3.Hegazi M, Abdelkader A, Zaki M, El-Deek B. Characteristics and risk factors of candidemia in pediatric intensive care unit of a tertiary care children’s hospital in Egypt. J. Infect. Dev. Ctries. 2014;624:634–8. doi: 10.3855/jidc.4186. [DOI] [PubMed] [Google Scholar]
- 4.Christensen GD, Simpson WA, Bisno AL, Beachy EH. Adherence of biofilm producing strains of Staphylococci epidermidis to smooth surfaces. Infect. Immun. 1982;318:326–7. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattb M, Culioli G, Piovetti L, Chitour S, Valls R. Comparison of various extraction methods for identification and determination of volatile metabolites from brown alga Dictyopteris membranacea. J. Chromatogr. 2007;1143:1147–7. doi: 10.1016/j.chroma.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 6.Carradori S, Chimenti P, Fazzari M, Granese A, Angiolella L. Antimicrobial activity, synergism and inhibition of germ tube formation by Crocus sativus-derived compounds against Candida spp. J. Enzyme Inhib. Med. Chem. 2016;189:193–31. doi: 10.1080/14756366.2016.1180596. [DOI] [PubMed] [Google Scholar]
- 7.Simões LC, Simões M, Vieira MJ. Biofilm interactions between distinct bacterial genera isolated from drinking water. App. Environ. Microbiol. 2007;6192:6200–73. doi: 10.1128/AEM.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;1325:1337–8. doi: 10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandai D, Tabana YM, Ouweini AE, Ayodeji IO. Resistance of Candida albicans biofilms to drugs and the host immune system. Jundishapur J. Microbiol. 2016;373:385–9. doi: 10.5812/jjm.37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel R. Prophylactic fluconazole in liver transplant recipients: A randomized, double-blind, placebo-controlled trial. Int. Med. 2000;729:737–131. doi: 10.7326/0003-4819-131-10-199911160-00003. [DOI] [PubMed] [Google Scholar]
- 11.Addy M, Moran J. Mechanisms of stain formation on teeth, in particular associated with metal ions and antiseptics. Adv. Dent. Res. 1995;450:456–9. [Google Scholar]
- 12.Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov. 2009;214:222–14. doi: 10.1016/j.drudis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Xiang W, Song QS, Zhang HJ, Guo SP. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia. 2008;501:504–79. doi: 10.1016/j.fitote.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Kang K, Fong WP, Tsang PW. Novel antifungal activity of purpurin against Candida species in vitro. Med. Mycol. 2010;904:911–48. doi: 10.3109/13693781003739351. [DOI] [PubMed] [Google Scholar]
- 15.Marioni J, da Silva MA, Cabrera JL, Montoya SC, Paraje MG. The anthraquinones rubiadin and its 1-methyl ether isolated from Heterophyllaea pustulata reduces Candida tropicalis biofilms formation. Phytomedicine. 2016;1321:1328–23. doi: 10.1016/j.phymed.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Janeczko M, Maslyk M, Kubinski K, Golczyk H. Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development, and biofilm formation of Candida albicans. Yeast. 2017;253:265–34. doi: 10.1002/yea.3230. [DOI] [PubMed] [Google Scholar]
- 17.Ismail A, BelHadj Salah K, Ahmed M, Mastouri M, Bouraoui M. Antibacterial antifungal activities of brown algae Zonariatournefortii (J.V. Lamouroux) Allelopath. J. 2014;143:154–34. [Google Scholar]
- 18.Elnabris KJ, Elmanama AA, Chihadeh WN. Antibacterial activity of four marine seaweeds collected from the coast of Gaza Strip, Palestine. Mesopot. J. Mar. Sci. 2013;81:92–28. [Google Scholar]
- 19.Zbakh H, Chiheb H, Bouziane H, Sánchez VM, Riadi H. Antibacterial activity of benthic marine algae extracts from the Mediterranean coast of Morocco. J. Microb. Biotech. Food Sci. 2012;219:228–1. [Google Scholar]
- 20.Bhadury P, Wright CP. Exploitation of marine algae: biogenic compounds for potential antifouling application. Planta. 2004;561:578–219. doi: 10.1007/s00425-004-1307-5. [DOI] [PubMed] [Google Scholar]
- 21.Jaswir I, Monsur HA. Anti-inflammatory compounds of macro algae origin: a review. J. Med. Plants Res. 2011;7146:7154–5. [Google Scholar]
- 22.Devi GK, Manivannan K, Thirumaran G, Rajathi FA, Anatharaman P. In vitro antioxidant activities of selected seaweeds from Southeast coast of India. Asi. Pac. J. Trop. Med. 2011;4:205–211. doi: 10.1016/S1995-7645(11)60070-9. [DOI] [PubMed] [Google Scholar]
- 23.Devi GK, Manivannan K, Thirumaran G, Rajathi FAA, Anantharaman P. In vitro antioxidant activities of selected seaweeds from Southeast coast of India. Asian Pac. J. Trop. Med. 2011;205:211–6. doi: 10.1016/S1995-7645(11)60070-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Thomas NV, Li X. Anticancer compounds from marine macroalgae and their application as medicinal foods. Adv. Food Nutr. Res. 2011;213:224–64. doi: 10.1016/B978-0-12-387669-0.00016-8. [DOI] [PubMed] [Google Scholar]
- 25.Tsang PW, Bandara HM, Fong WP. Purpurin suppresses Candida albicans biofilmformation and hyphal development. PLoS ONE. 2012;7:e50866. doi: 10.1371/journal.pone.0050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Sheekh MM, El-Shafay SM, El-Ballat EM. Production and characterization of antifungal active substance from some marine and freshwater algae. Int. J. Enviro. Sci. Engine. 2015;6:85–92. [Google Scholar]
- 27.Nieminen MT, et al. A novel antifungal is active against Candida albicans biofilms and inhibits muta-genic acetaldehyde production in vitro. PLOS ONE. 2014;9:e97864. doi: 10.1371/journal.pone.0097864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mickymaray S, Alturaiki W. Antifungal Efficacy of marine macroalgae against fungal isolates from bronchial asthmatic cases. Molecules. 2018;23:1–14. doi: 10.3390/molecules23113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K. Mucins suppress virulence traits of Candida albicans. MBio. 2014;11:901–911. doi: 10.1128/mBio.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dulger B, Acioglu N, Rdugan H, Aysel V. Antimicrobial Activity of Some Brown Algae from Turkey. Asi. J. Chem. 2009;21(4113):4117. [Google Scholar]
- 31.Haque F, Alfatah M, Ganesan K, Bhattacharyya M. Inhibitory Effect of Sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci. Rep. 2016;31(6):23575. doi: 10.1038/srep23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudiña EJ, Rangarajan V, Sen R, Rodrigues LR. Potential therapeutic applications of biosurfactants. Trends in pharmacological sciences. 2013;667:675–34. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 33.El Shafay SM, Ali SS, El-Sheekh MM. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aqua. Res. 2016;65:74–42. [Google Scholar]
- 34.De Rosa S, et al. Chemical composition of Corallina mediterraneaa reschoug and Corallina granifera Ell. Et Soland. Z. Naturfarsch. C. J. Biosci. 2003;58:325–332. doi: 10.1515/znc-2003-5-606. [DOI] [PubMed] [Google Scholar]
- 35.Hattb M, Culioli G, Piovetti L, Chitour S, Valls R. Comparison of various extraction methods for identification and determination of volatile metabolites from brown alga Dictyopteris membranacea. J. Chromatogr. A. 2007;2(1143(1-2)):1–7. doi: 10.1016/j.chroma.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 36.Manilal A, et al. Evaluating the in vitro antagonism of secondary metabolites fractionated from the brown algae, Sargassum swartzii against human Candida spp. Transl. Biomed. 2016;7(1):51. [Google Scholar]
- 37.Sujina MG, Soumya JD, Manjusha WA. Assessing the anticancer, anti-inflammatory, antimicrobial and antioxidant potential of bioactive compounds present in marine algae Ulva Lacutuca. World. J. Pharm. Res. 2016;5:1482–1500. [Google Scholar]
- 38.Buchaille L, Freydiere A, Gill Y. 1999.Evaluation of six commercial systems for identification of medically important yeasts. Eur. J. Clin. Microbiol. Infect. Dis. 1998;17:479–488. doi: 10.1007/BF01691130. [DOI] [PubMed] [Google Scholar]
- 39.Aleem AA. Contributions to the study of the marine algae of the Red Sea III- Marine algae from Obhor, in the vicinity of Jeddah, Saudi Arabia. Bull. Fac. Sci. KAU Jeddah. 1978;99:118–2. [Google Scholar]
- 40.Abbott, L.A. & Hollenberg, L.G. Marine algae of California Stanford University Press, Stanford, California, USA (1976).
- 41.Taylor, W.S. Marine algae of the easterntropical and subtrobical coasts of Americas. ANN. Arbor the university of Michigan press (1985).
- 42.Patra J, Rath S, Jen K, Rathod V, Thatoi H. Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: a study on inhibition of Glutathione-S transferase activity. Turk. J. Biol. 2008;119:125–37. [Google Scholar]
- 43.Mohanta TK, Patra JK, Rath SK, Pal DK, Thatoi HN. Evaluation of antimicrobial activity & phytochemical screening of oil & nuts of Semi carpusan acardium L. F. Sci. Res. Essays. 2007;2:486–490. [Google Scholar]
- 44.El Zawawy NA, Hafez EE. Efficacy of Pluchea dioscoridis leaf extract against pathogenic Candida albicans. J. Infect. Dev. Ctries. 2011;11:334–3428. doi: 10.3855/jidc.8447. [DOI] [PubMed] [Google Scholar]
- 45.Borman AM, et al. MIC Distributions and Evaluation of Fungicidal Activity for Amphotericin B, Itraconazole, Voriconazole, Posaconazole and Caspofungin and 20 Species of Pathogenic Filamentous Fungi Determined Using the CLSI Broth Microdilution Method. J. Fungi. 2017;27:2252–2262. doi: 10.3390/jof3020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nett JE, Cain MT, Crawford K, Andes DR. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J. Clin. Microbiol. 2011;49:1426–1433. doi: 10.1128/JCM.02273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramage G, VandeWalle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2011;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum sensing molecule. Appl. Environ. Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nithya C, Devi MG, KaruthaPandian S. A novel compound from the marine bacterium Bacillus pumilus S6-15 inhibits biofilm formation in Gram positive and Gram-negative species. Biofouling. 2011;27(519):528. doi: 10.1080/08927014.2011.586127. [DOI] [PubMed] [Google Scholar]
- 50.Shafreen RM, Muthamil S, Pandian SK. Inhibition of Candida albicans virulence factors by novel levofloxacin derivatives. Appl. Microbiol. Biotechnol. 2014;98:6775–6785. doi: 10.1007/s00253-014-5719-2. [DOI] [PubMed] [Google Scholar]
- 51.Mannan AU, Sharma S, Ganesan K, Total RNA. isolation from recalcitrant yeast cells. Anal. Biochem. 2009;77(79):389. doi: 10.1016/j.ab.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Carlous H, Dyck K, Dijck PV. Candida albicans and Staphylococcus Species: A Threatening Twosome. Front. Microbiol. 2019;10:2162. doi: 10.3389/fmicb.2019.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen R. Quantification on the Light Cycler. In Meuer, S., Wittwer, C.& Nakagawara, K. (eds), Rapid Cycle Real-time PCR, Methods and Applications. Springer Press, Heidelberg. 21:34 (2001).
- 54.Annegowda HV, et al. TLC–bioautography-guided isolation, HPTLC and GC–MS assisted analysis of bioactive of Piper betle leaf extract obtained from various extraction techniques: in vitro evaluation of phenolic content, antioxidant and antimicrobial activities. Food Anal. Methods. 2013;6:715–726. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during this study are available from the corresponding author upon request.