Abstract
Background
Faecal immunochemical testing (FIT) is suboptimal in detecting advanced neoplasia (AN). To increase the sensitivity and yield of a FIT-based screening programme, FIT could be combined with risk factors for AN. We evaluated the incremental yield of adding a family history questionnaire (FHQ) on colorectal cancer (CRC) and Lynch syndrome-associated tumours to the Dutch FIT-based screening programme.
Methods
Six thousand screen-naive individuals, aged 59–75 years, were invited to complete a FIT (FOB-Gold, cut-off 47 µg Hb/g faeces) and a validated online FHQ. Participants with a positive FIT and/or positive FHQ, confirmed after genetic counselling, were referred for colonoscopy. Yield of detecting AN per 1000 invitees for the combined strategy was compared with the FIT-only strategy.
Results
Of the 5979 invitees, 1952 (32.6%) completed the FIT only, 2379 (39.8%) completed both the FIT and FHQ and 95 (1.6%) completed the FHQ only. Addition of the FHQ to FIT-based screening resulted in one extra case of AN detected after 16 additional colonoscopies, resulting in a yield of 19.6 (95% CI, 16.4–23.5) for the combined strategy versus 19.5 (95% CI, 16.3–23.3) for the FIT-only strategy (p = 1.0).
Conclusions
The addition of an FHQ to one round of FIT screening did not increase the detection of AN compared with FIT only (ClinicalTrials.gov NCT02698462).
Subject terms: Population screening, Colorectal cancer, Genetic testing, Cancer screening, Population screening
Background
Colorectal cancer (CRC) is the third most prevalent cancer in the Netherlands.1 CRC predominantly originates from adenomas.2 As the progression of adenomas to cancer takes 10–15 years, there is a long window of opportunity for intervention.2 Colonoscopy enables detection and treatment of adenomas and early cancer, reducing both CRC-related incidence and mortality.3
Faecal immunochemical testing (FIT) is a screening method that selects individuals at high risk of having advanced adenomas or CRC, the combination referred to as advanced neoplasia (AN). Screening participants with a positive FIT are subsequently invited for colonoscopy. FIT has a low burden, facilitating participation in FIT-based screening. In the Netherlands, the participation rate is around 72%.4 Yet, since the sensitivity of FIT is not perfect, not all individuals with AN are detected in one screening round. Depending on the faecal haemoglobin concentration cut-off level and brand, pooled sensitivity was 79% for CRC and 6–56% for AN.5,6
To increase the detection rate of AN in FIT-based screening, FIT could be combined with risk factors for AN. Studies have reported that a false-negative FIT result was more often observed in participants with a family history of CRC than in those without.7,8 In this context, a positive family history is defined by the diagnosis of a hereditary CRC syndrome, such as Lynch syndrome, and/or the so-called “familial CRC syndrome”, defined by the number and age of relatives with CRC but which does not yet have a known genetic basis. Of all the CRC cases, 15–30% seem to have such a familial risk and 2–5% are related to a hereditary CRC syndrome.9
International guidelines recommend individuals with a positive CRC family history to undergo regular colonoscopy surveillance instead of participating in FIT-based screening programmes.10,11 Colonoscopy surveillance has shown to reduce CRC-related mortality for individuals with Lynch syndrome by 72% and up to 81% for those with a familial CRC syndrome.12,13 However, colonoscopy uptake in individuals with a positive family history for CRC varies widely (12–51%).14 Furthermore, only 12–33% of CRC patients and their relatives who qualify for referral to a clinical geneticist are actually referred.15,16 Contrary to the guideline recommendations, many individuals with a hereditary or familial CRC syndrome do not receive periodic colonoscopy surveillance. Instead, they will be invited for screening whenever an organised population-based screening programme is in place.
In the Australian FIT-based population screening programme, 4% of the participants had a positive family history of CRC or polyps, warranting colonoscopy surveillance.17 Only 50% of those individuals had recently undergone surveillance.17 Another study showed that offering colonoscopy surveillance to participants with a positive FIT or CRC family history increased sensitivity in detecting AN, at the expense of specificity.18 In this study, the definition of a positive CRC family history was limited to first-degree relatives with CRC, regardless of their age.18
The limited sensitivity of FIT and the limited identification of individuals with a positive family history call for the exploration and evaluation of simple online questionnaire to improve the current situation. We designed a study to evaluate the incremental yield of adding a validated online family history questionnaire (FHQ) on CRC and Lynch syndrome-associated tumours to the Dutch organised FIT-based screening programme.19 In this study, participants with a positive FIT and/or a positive FHQ were offered a colonoscopy. We compared the yield of this combined strategy with that of the FIT-only screening strategy. As a secondary aim, we studied the identification of individuals with a hereditary or familial CRC syndrome by the FHQ and the subsequent referral rates for genetic counselling and colonoscopy.
Methods
Study population and study design
We performed a prospective population-based CRC screening trial embedded in the national CRC screening programme. Six thousand screening-naive individuals, aged from 58–59 to 74–75 years, were invited to sample an FIT and to complete an FHQ. Invitees were selected from four areas in the province of North Holland based on their year of birth (1941, 1945, 1953, 1955 and 1957). These areas reflected the nationwide CRC screening programme in 2014 regarding socioeconomic status (SES), ethnicity and participation rates. Within these areas, invitees were selected with an age and sex distribution comparable to that of the national CRC screening programme population in 2016. SES was based on postal code areas. There were no specific exclusion criteria for participation in the study.
Study procedures
Invitation for FIT and FHQ
In line with standard procedure of the national FIT-based screening programme, all invitees received an FIT pre-announcement letter. This letter contained a study invitation letter including a personal login code and information leaflet. Using this personal login code, a validated online FHQ could be filled out after informed consent was given.
The Dutch CRC screening programme provided all invitees with an FIT kit by postal mail 14–21 days after the pre-announcement. Invitees who declined participation in FIT screening were asked to inform the screening organisation. Seven-to-14 days after sending the FIT kit, a reminder study invitation letter was sent. Invitees who had not responded to the FIT invitation received a reminder 56 days after the pre-announcement.
FIT analysis and communication with participants regarding the FIT result were according to the logistics of the national CRC screening programme. The positivity threshold of the FIT was set at 47 µg haemoglobin/g faeces in the Dutch national CRC screening programme. All participants received a letter with the FIT result, either a positive or negative result, within 7–14 days after returning the device. Participants with a positive test result received an appointment for an intake visit for colonoscopy.
Family history questionnaire
The online FHQ was based on a previously validated questionnaire, with high sensitivity and specificity in identifying individuals qualifying for referral because of suspected Lynch syndrome or familial CRC syndrome (Supplementary Information: Family History Questionnaire in Dutch).19 Questionnaire responses were automatically evaluated against the national referral criteria for genetic testing for Lynch syndrome and surveillance colonoscopies in case of a familial CRC syndrome (Supplementary Information Table 1). As an additional quality control measure, all generated results were manually checked by the research fellow (V.H.R.). A second research fellow (F.G.J.K.) performed additional verification of 10% of the sample.
In case an FHQ participant returned the FIT, FHQ results were sent to the participant within 7 days after receiving the FIT result. For participants who did not return the FIT, the FHQ result was sent 15 weeks after the pre-announcement letter. All participants who received a positive FHQ result were contacted by telephone by the research fellow (V.H.R.) within 10 days after receiving the FHQ result. To identify potential false-positive results, this trained researcher verified all answers with the participant. Once the positive CRC family history was confirmed, an intake at the Department of Clinical Genetics was arranged. During this intake, a clinical geneticist or genetic counsellor decided whether genetic testing or a surveillance advice was indicated.
Colonoscopy
All participants with a positive FIT and/or a positive FHQ, confirmed after genetic counselling, were referred for colonoscopy. Colonoscopies after a positive FIT and/or a confirmed familial CRC syndrome were performed in one nationally accredited colonoscopy centre (Bergman Clinics Amsterdam), unless the participant preferred another centre. In participants with Lynch syndrome, a colonoscopy was performed in a dedicated tertiary colonoscopy centre (Academic Medical Centre). All colonoscopies in participants with a positive FIT were performed by accredited screening gastroenterologists, according to the national CRC screening programme standards, who had performed at least 200 colonoscopies per year.20 Colonoscopies in those with a familial or hereditary CRC syndrome were performed by gastroenterologists with similar adenoma detection rates. Colonoscopies were conducted according to international standard quality criteria.21 Resected lesions were evaluated by experienced pathologists according to the World Health Organisation classification and Vienna criteria.22,23 Advanced adenomas were defined as adenomas having a diameter ≥10 mm and/or with ≥25% villous component and/or high-grade dysplasia. Depending on the findings, surveillance colonoscopies were advised according to national guideline criteria.24
Statistical analysis
The primary study outcome was diagnostic yield, defined as the number of participants in whom AN was detected relative to the number of invitees. We compared the diagnostic yield of a combined approach, selecting participants for colonoscopy based on FIT and family history, to that of (hypothetical) selection based on FIT only, in the same group of participants. Secondary outcomes were participation rate and positive predictive values. The positive predictive value of the screening instrument was defined as the number of participants in whom AN was detected relative to the number of participants testing positive. Differences in diagnostic yield and participation rate were tested for statistical significance using McNemar test statistic. A difference in positive predictive value between the two strategies was assessed for statistical significance with Chi-square test statistic.
Additional outcomes were positivity rate, number of colonoscopies performed, and detection rate. A significance level of 5% was used in all statistical tests. All statistical analyses were performed using R Studio version 1.1.383.
Sample size calculation
Our sample size calculation was based on the anticipated gain in diagnostic yield when using the combined strategy.18 With a study group of 6000 individuals, we expected to detect 130 individuals with AN after a positive FIT (21.7 per 1000 invitees). By adding the FHQ, we anticipated to detect an additional number of 11 individuals with AN (23.5 per 1000 invitees): an increase in diagnostic yield of 1.8 per 1000 invitees. We then would have a power of at least 91% in rejecting the null hypothesis of no gain, using McNemar test statistic.
Ethical considerations
Ethical approval for this study embedded in the national screening programme was obtained from the Dutch National Health Council. The study was performed in accordance with the Declaration of Helsinki.25
Results
Screening population
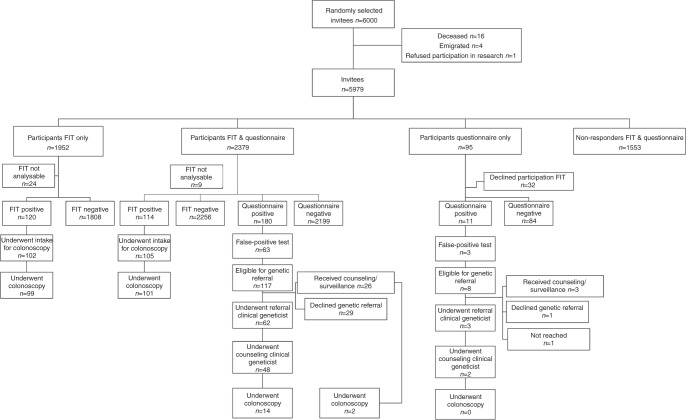
Of the 6000 initially selected, randomly sampled invitees, 16 were deceased, 4 had emigrated prior to the invitation for the study and 1 individual refused participation in research. Between April 2016 and January 2017, 5979 invitees received both an FIT and the FHQ (Fig. 1).
Fig. 1.
Flowchart of study population.
Participation
Of the 5979 invitees, 1952 (32.6%) completed the FIT only, 2379 (39.8%) completed both the FIT and the FHQ and 95 (1.6%) invitees completed the FHQ only; 1553 (26.0%) invitees neither returned an FIT nor completed the FHQ.
Table 1 lists key characteristics of invitees and four subgroups: participants who completed FIT, FHQ or both and non-participants. There were more people born in 1941 in the FIT-only group, compared to the other participants. The FIT-only group had more females, whereas there was a small majority of males among non-participants. In the small group who completed the FHQ only, without returning the FIT, more participants could be classified in the very high SES category, compared to the other groups.
Table 1.
Demographics.
| Invitees, n = 5979 | Participants FIT only, n = 1952 | Participants FIT and FHQ, n = 2379 | Participants FHQ only, n = 95 | Non-participants, n = 1553 | p Valuea | |
|---|---|---|---|---|---|---|
| Year of birth | ||||||
| 1941 | 769 (12.9%) | 290 (14.8%) | 256 (10.8%) | 10 (10.5%) | 213 (13.7%) | 0.02 |
| 1945 | 967 (16.2%) | 314 (16.1%) | 385 (16.2%) | 18 (18.9%) | 250 (16.1%) | |
| 1953 | 1329 (22.2%) | 429 (22.0%) | 564 (23.7%) | 21 (22.1%) | 315 (20.3%) | |
| 1955 | 1422 (23.8%) | 441 (22.6%) | 584 (24.5%) | 24 (25.3%) | 373 (24.0%) | |
| 1957 | 1492 (24.9%) | 478 (24.5%) | 590 (24.8%) | 22 (23.2%) | 402 (25.9%) | |
| Sex | ||||||
| Male | 2957 (49.5%) | 906 (46.4%) | 1180 (49.6%) | 47 (49.5%) | 824 (53.1%) | 0.002 |
| Female | 3022 (50.5%) | 1046 (53.6%) | 1199 (50.4%) | 48 (50.5%) | 729 (46.9%) | |
| Socioeconomicb status | ||||||
| Low | 193 (3.2%) | 74 (3.8%) | 55 (2.3%) | 5 (5.3%) | 59 (3.8%) | 0.007 |
| Average | 1657 (27.7%) | 542 (27.7%) | 677 (28.5%) | 19 (20.0%) | 419 (27.0%) | |
| High | 1543 (25.8%) | 513 (26.3%) | 588 (24.7%) | 22 (23.2%) | 420 (27.0%) | |
| Very high | 2401 (40.2%) | 763 (39.1%) | 1000 (42.0%) | 48 (50.5%) | 590 (38.0%) | |
| Missing | 185 (3.1%) | 60 (3.1%) | 59 (2.5%) | 1 (1.0%) | 65 (4.2%) | |
ap Values were calculated using Chi-square test statistic, comparing participants in the three groups and non-participants, excluding missing.
bBased on postal code areas.
Combined versus FIT-only strategy
For the comparison of the two strategies, we included all 4426 invitees who returned the FIT and the FHQ, FIT only or the FHQ only in the combined strategy (invitations for colonoscopy based on FIT and/or FHQ). Similarly, we included all 4331 invitees who returned the FIT only or the FIT and FHQ in the FIT-only strategy (invitations for colonoscopy based on FIT only). This implies that the participation rate of the combined strategy was marginally but significantly higher (4426/5979; 74.0%) compared to a FIT-only strategy (4331/5979; 72.4%; p < 0.001). Figure 2 shows the details of the comparison.
Fig. 2.
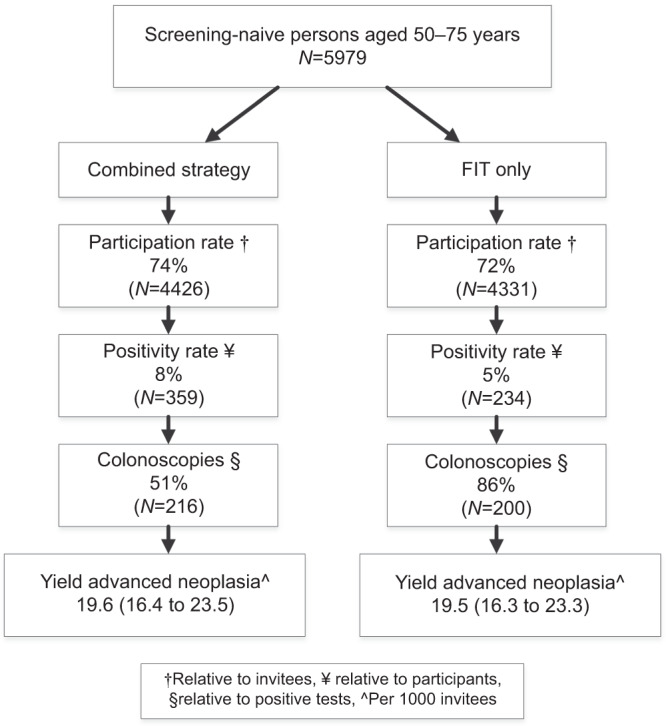
Study enrollment.
The FHQ was initially found to be positive in 191 (7.7%) of the 2474 participants who had returned the FHQ. After a telephone check, 66 of these (34.6%) appeared to be false positives (Fig. 1). Thirty-three of the 4331 (0.8%) returned FIT results could not be analysed, of whom 9 had also participated in the FHQ. Taking into account the 24 unanalysable tests for the combined strategy and the 33 unanalysable FIT results, the positivity rate for the combined strategy was significantly higher compared to that of the FIT-only strategy: 359 of 4402 (8.2%) versus 234 of 4298 (5.4%; p < 0.001).
In 14 individuals, CRC was detected during colonoscopy, all through a positive FIT result. Sixteen colonoscopies were performed in participants with a negative FIT but because of a positive FHQ. One additional advanced adenoma was detected in this group (Table 2). This resulted in a significantly higher positive predictive value for advanced adenomas and neoplasia for the FIT-only strategy compared to the combined strategy.
Table 2.
Colonoscopy outcomes: combined strategy versus FIT-only strategy.
| Combined strategy (n/N (%)) | FIT-only strategy (n/N (%)) | p Valuea | |
|---|---|---|---|
| Detection rate (%)b | |||
| CRC | 14/216 (6.5%) | 14/200 (7.0%) | 0.99 |
| Advanced adenoma | 103/216 (47.7%) | 102/200 (51.0%) | 0.56 |
| Advanced neoplasia | 117/216 (54.2%) | 116/200 (58.0%) | 0.49 |
| Non-advanced lesions | |||
| Non-advanced adenoma | 49/216 (22.4%) | 42/200 (21%) | 0.77 |
| SSL+others | 21/216 (9.7%) | 18/200 (9.0%) | 0.93 |
| No lesions | 29/216 (13.4%) | 24/200 (12.0%) | 0.77 |
| Positive predictive value (%)c | |||
| CRC | 14/359 (4% (2–6%)) | 14/234 (6% (3–10%)) | 0.33 |
| Advanced adenoma | 103/359 (29% (24–34%)) | 102/234 (44% (37–50%)) | <0.01 |
| Advanced neoplasia | 117/359 (33% (28–38%) | 116/234 (50% (43–56%)) | <0.01 |
CRC colorectal cancer, SSL sessile serrated lesion.
ap Values were calculated using the Chi-square test.
bDetection rates were defined as the most advanced lesion per participant relative to all colonoscopies performed.
cPositive predictive value was defined as the number of participants with CRC, advanced adenoma or advanced neoplasia relative to the number of positive tests.
Eventually, the yield for AN was 19.6 per 1000 invitees (95% confidence interval (CI): 16.4–23.5) for the combined strategy versus 19.5 per 1000 invitees (95% CI: 16.3–23.3) for FIT-only strategy, a non-significant difference of 0.1 per 1000 invitees (p value = 1.0; Fig. 2).
FHQ results
Of these participants eligible for referral for genetic counselling, 60 were not referred, because they had already received genetic counselling and/or received an advice for surveillance (n = 29), because they declined referral (n = 30) or were not reached (n = 1). Reported reasons for declining referral for genetic counselling were: financial reasons (n = 5), other comorbidities (n = 4), no perceived added value of counselling (n = 7), and no reason mentioned (n = 14). Fifty of the 65 (76.9%) participants referred for genetic counselling underwent counselling.
Genetic testing and counselling
Of the 50 participants who underwent genetic counselling, 26 underwent genetic testing because of suspicion of Lynch syndrome (Fig. 3). This resulted in the diagnosis of Lynch syndrome (MSH2 mutation) in one participant and the detection of two participants with gene variants of unknown clinical significance in the MLH1 gene and the MSH6 gene, appointed to a 2 yearly and 5 yearly surveillance interval, respectively. Lynch syndrome was excluded by genetic testing in the 23 other participants, of whom 10 were newly diagnosed with familial CRC syndrome, 7 were previously diagnosed with familial CRC syndrome, 2 participants were advised to undergo a one-time colonoscopy and 4 were advised to return to the national population-based FIT screening programme. In seven participants, referral of family members was advised to undergo genetic testing, of whom one was newly diagnosed, one previously diagnosed with familial CRC syndrome and five were advised to return to the national population-based FIT screening programme. In 14 participants, information was requested to exclude Lynch syndrome, of whom 3 were newly diagnosed with familial CRC, 3 were previously diagnosed with familial CRC syndrome and 8 were advised to return to the national population-based FIT screening programme. Three participants had declined genetic testing after genetic counselling, of whom one turned out to have serrated polyposis syndrome at colonoscopy for a newly diagnosed familial CRC syndrome and two were advised to return to the national population-based FIT screening programme.
Fig. 3.
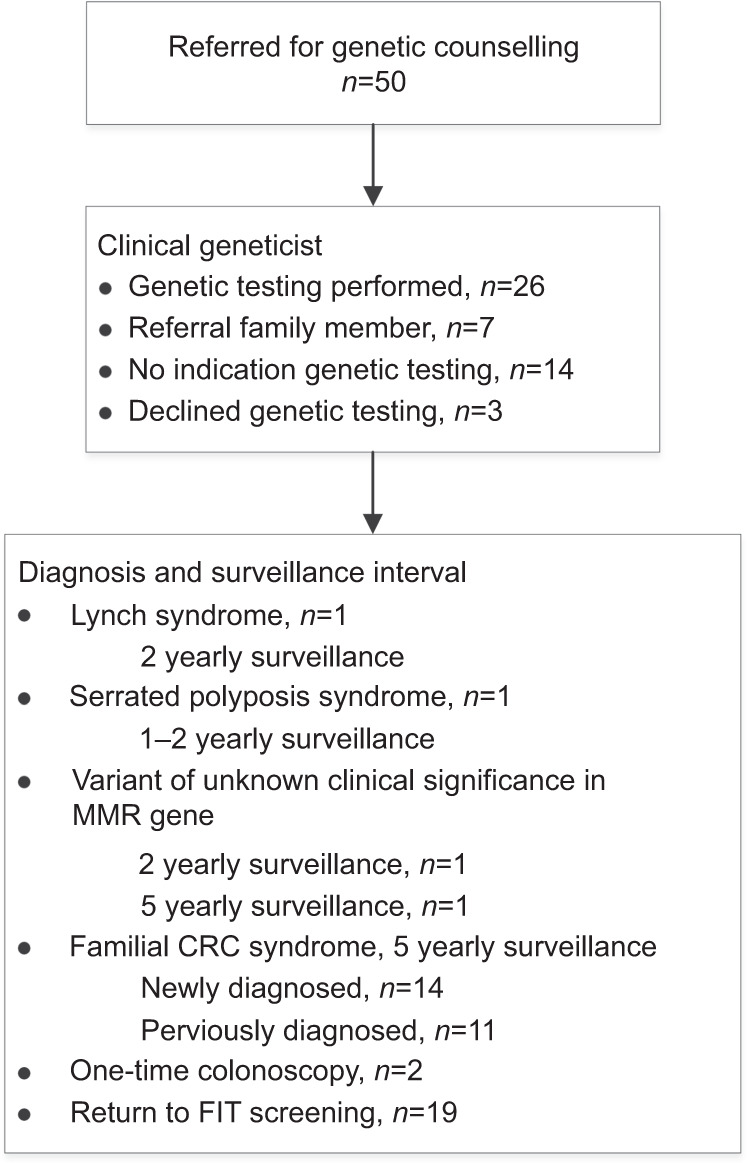
Genetic counselling results.
Colonoscopy surveillance instead of FIT screening
In addition to the colonoscopies after a positive FIT, 16 colonoscopies were performed as a result of a positive FHQ. Indications for colonoscopy were Lynch syndrome (n = 1), a carrier of a variant of unknown clinical significance in the mismatch repair (MMR) gene appointed to a 2-year surveillance interval (n = 1), newly diagnosed familial CRC syndrome (n = 11), one-time colonoscopy after exclusion of Lynch syndrome (n = 1), and previously diagnosed familial CRC syndrome withdrawn from surveillance (n = 2). One of the participants who underwent colonoscopy because of a newly diagnosed familial CRC syndrome turned out to have serrated polyposis syndrome. The second participant, carrier of a gene variant of unknown clinical significance in the MSH6 gene appointed to a 5 yearly surveillance interval, was already undergoing surveillance for familial CRC syndrome. Two patients with a new diagnosis of familial CRC syndrome declined to undergo a colonoscopy and two of them underwent the colonoscopy because of a concurrent positive FIT result. One participant advised to undergo a one-time colonoscopy after exclusion of Lynch syndrome had declined a colonoscopy because of his age.
In the end, we identified 19 individuals who had an indication to undergo a regular surveillance colonoscopy instead of participating in a FIT-based screening programme. These included 1 participant diagnosed with Lynch syndrome, 1 carrier of a variant of unknown clinical significance in the MMR gene appointed to a 2-year surveillance interval, 1 with serrated polyposis syndrome, 14 newly diagnosed individuals with familial CRC syndrome and 2 participants who had withdrawn from surveillance for familial CRC. Fifteen of these 19 (78.9%) participants actually underwent a colonoscopy because of the study and 2 (10.5%) because of a concurrent positive FIT. Two participants had declined surveillance for familial CRC syndrome (10.5%).
Discussion
In this study, we observed no significant incremental yield in detecting AN after adding a validated online FHQ on family history of CRC and Lynch syndrome-associated tumours to one round of FIT screening. In the almost 6000 invitees, 16 additional colonoscopies were performed for a positive FHQ result, but only 1 additional participant with AN was detected. Despite, we identified 19 individuals who had an indication to undergo colonoscopy surveillance instead of participating in a FIT-based screening programme.
An unfavourable result of this study was the high false positivity rate (35%) using the online FHQ, despite previous validation in patients scheduled for colonoscopy in a private colonoscopy centre.19 This prior validation had showed a comparable number of false positives but no false-negative results, when referrals based on questionnaire data were compared with referrals based on data collected in a telephone interview. Therefore, we had integrated the telephone check in the current online FHQ tool and had accepted the risk of having a false-positive result, while adequately detecting all participants at risk. Second, the SES in our sample was slightly higher than that of the national programme, even though the selected regions were considered comparable to the national SES distribution. Nevertheless, the slightly higher SES and addition of the FHQ did not affect the FIT participation rate, FIT positivity rate or AN detection rate.4 The higher SES may have influenced participation in the FHQ in a positive way.26 We observed that invitees with a lower SES were less likely to return the FHQ. As participants depend on their health insurance for reimbursement of the genetic follow-up examination, and financial reasons were often reported as a reason for declining genetic testing, costs may have been a reason for lower SES invitees not to return the FHQ.
A strength of this study is that it is the first to evaluate the addition of an online FHQ to a national organised FIT-based screening programme assessing the effect in participants with a positive as well as those with negative FIT result. The large randomly selected cohort was screen-naive and consisted of an average-risk population with an age and sex distribution similar to that of the national screening programme. Furthermore, the quality standards of the colonoscopy performed after a positive FHQ were comparable to that of the population screening colonoscopies. Lastly, positive FHQs were verified by a genetic counsellor or clinical geneticist.
In the Netherlands, in the year 2016 the incidence of CRC was 15,306 with a corresponding mortality of 5154 in a population of 16,980,000 individuals.1 A previous study in the Dutch population reported that 10% of the affected and 2.3% of the unaffected respondents reported to fulfil the Dutch referral criteria for genetic testing for Lynch syndrome or surveillance for familial CRC syndrome.27 These numbers indicate that, in our country, in total almost 400,000 individuals fulfil the criteria for Lynch syndrome or familial CRC syndrome. Although the exact numbers of patients diagnosed with Lynch syndrome and familial CRC syndrome are lacking, the majority of these patients are unaware of their diagnosis and at great risk for developing CRC.28 Therefore, the implementation of an FHQ as a screening tool could be beneficial.
Compared with a previously performed study in the Dutch population, the current participation rate of the FHQ was considerably lower.27 This could have perhaps been the result of the online nature of the FHQ as predominantly younger invitees with a higher SES had participated in the FHQ. Despite the fact that positivity rates of the FHQ were comparable to previously published studies7,17,29,30 and considerable evidence of a higher neoplastic yield among individuals with a family history of CRC, the yield of the combined screening strategy was limited.31,32 This may be explained by the low referral rate to the clinical geneticist of only 40% compared to a recently reported uptake of pre-symptomatic genetic testing for Lynch syndrome according to genetic centres of 41–94%.33 A large proportion of our patients actively declined referral (24%), did not show up at their appointment or cancelled it (12%). Compliance to appointments is known to be related to perceived urgency of medical clinic visits and associated with a strong recommendation by the physician.34 Given the screening situation, participants could have perceived this advice as less urgent and therefore may have declined referral to a clinical geneticist. Lengthy waiting time might also have caused participants to change their minds. Future research should evaluate whether different strategies, for instance without verification by the clinical geneticist, may lead to an increase in diagnostic yield.
Another explanation for the limited diagnostic yield is that a positive family history of CRC considers a life-long risk that is not necessarily associated with an increase in the diagnostic yield in just one single colonoscopy. Future research should investigate whether addition of an FHQ changes the yield after several round of FIT screening.
As in our study only one third of the participants with a positive FHQ result had already received genetic counselling and/or surveillance advice, there seems still room for improvement in the identification of individuals with familial or hereditary CRC syndromes. In the FIT information leaflet, there is no mention of exclusion from participation in FIT screening for patients with a positive family history for CRC. We observed that patients who had already undergone colonoscopy surveillance for a positive family history also participated in the FIT screening programme, indicating that the information for invitees on who should undergo FIT screening could be improved.
In conclusion, we observed no increase in yield after introducing a validated, online FHQ for detecting AN in this study that was embedded in the Dutch FIT-based CRC screening programme. Addition of this FHQ resulted in the identification of a small number of participants at increased risk of developing CRC who should undergo periodic colonoscopy surveillance and not participate in a screening programme based on FIT screening.
Supplementary information
Supplementary Information Family History Questionnaire in Dutch
Supplementary Information Table 1 Referral criteria for genetic testing and colonoscopy surveillance
Acknowledgements
We thank the foundation of Population Screening Mid-West for helping us to conduct this study, in particular Erwin Humer, Maja Groothuis and Sravasa Diemeer. Furthermore, we acknowledge Else-Mariëtte van Heijningen for her contributions to the data analysis.
Author contributions
Planning and/or conducting the study: all authors. Collecting and/or interpreting data: V.H.R., P.M.M.B., E.D. Drafting of the manuscript: V.H.R. Critical revision of the article for important intellectual content: F.G.J.K., M.v.d.V., C.M.A., P.M.M.B., E.D.
Ethics approval and consent to participate
Ethical approval for this study embedded in the national screening programme was obtained from the Dutch National Health Council (B21748-140305-PG).
Consent to publish
Informed consent was given by participants prior to filling out the validated online family history questionnaire.
Data availability
The authors are willing to collaborate in further analyses, on request.
Competing interests
E.D. has an endoscopic equipment on loan from FujiFilm and receives a research grant from FujiFilm. He has received a honorarium for consultancy from FujiFilm, Olympus, Tillots, GI Supply and CPP-FAP and a speakers’ fee from Olympus, Roche and GI Supply. Also he is on the supervisory board of eNose. The other authors declare no competing interests.
Funding information
This study was sponsored by The Dutch Digestive Foundation, who had no role in the study design, collection, analysis and interpretation of the data, writing the report or the submission of the paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-0832-8.
References
- 1.Integraal Kankercentrum Nederland. Incidence of cancer of the colon, rectosigmoid, rectum. www.cijfersoverkanker.nl (2017).
- 2.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eramus MC–NKI/AvL. Landelijke Monitoring Bevolkingsonderzoek Darmkanker 2016. https://www.rivm.nl/documenten/landelijke-monitoring-bevolkingsonderzoek-darmkanker-2016 (2016).
- 5.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann. Intern. Med. 2014;160:171. doi: 10.7326/M13-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152:1217.e3–1237.e3. doi: 10.1053/j.gastro.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Ng SC, Ching JY, Chan V, Wong MC, Suen BY, Hirai HW, et al. Diagnostic accuracy of faecal immunochemical test for screening individuals with a family history of colorectal cancer. Aliment. Pharmacol. Ther. 2013;38:835–841. doi: 10.1111/apt.12446. [DOI] [PubMed] [Google Scholar]
- 8.Cha JM, Lee JI, Joo KR, Shin HP, Park JJ, Jeun JW, et al. First-degree relatives of colorectal cancer patients are likely to show advanced colorectal neoplasia despite a negative fecal immunochemical test. Digestion. 2012;86:283–287. doi: 10.1159/000341738. [DOI] [PubMed] [Google Scholar]
- 9.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015;110:223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J. Clin. Oncol. 2015;33:209–217. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomaki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/S0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 13.Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331:1047. doi: 10.1136/bmj.38606.794560.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ait Ouakrim D, Lockett T, Boussioutas A, Hopper JL, Jenkins MA. Screening participation for people at increased risk of colorectal cancer due to family history: a systematic review and meta-analysis. Fam. Cancer. 2013;12:459–472. doi: 10.1007/s10689-013-9658-3. [DOI] [PubMed] [Google Scholar]
- 15.Dekker N, Hermens RP, Nagengast FM, van Zelst-Stams WA, Hoogerbrugge N. Familial colorectal cancer risk assessment needs improvement for more effective cancer prevention in relatives. Colorectal Dis. 2013;15:e175–e185. doi: 10.1111/codi.12117. [DOI] [PubMed] [Google Scholar]
- 16.Foo W, Young JM, Solomon MJ, Wright CM. Family history? The forgotten question in high-risk colorectal cancer patients. Colorectal Dis. 2009;11:450–455. doi: 10.1111/j.1463-1318.2009.01898.x. [DOI] [PubMed] [Google Scholar]
- 17.Worthley DL, Smith A, Bampton PA, Cole SR, Young GP. Many participants in fecal occult blood test population screening have a higher-than-average risk for colorectal cancer. Eur. J. Gastroenterol. Hepatol. 2006;18:1079–1083. doi: 10.1097/01.meg.0000231754.35340.fa. [DOI] [PubMed] [Google Scholar]
- 18.Kallenberg FG, Vleugels JL, de Wijkerslooth TR, Stegeman I, Stoop EM, van Leerdam ME, et al. Adding family history to faecal immunochemical testing increases the detection of advanced neoplasia in a colorectal cancer screening programme. Aliment. Pharmacol. Ther. 2016;44:88–96. doi: 10.1111/apt.13660. [DOI] [PubMed] [Google Scholar]
- 19.Kallenberg FG, IJ JE, Bossuyt PM, Aalfs CM, Dekker E. Validation of an online questionnaire for identifying people at risk of familial and hereditary colorectal cancer. Fam. Cancer. 2015;14:401–410. doi: 10.1007/s10689-015-9792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronzwaer MES, Depla A, van Lelyveld N, Spanier BWM, Oosterhout YH, van Leerdam ME, et al. Quality assurance of colonoscopy within the Dutch national colorectal cancer screening program. Gastrointest. Endosc. 2019;89:1–13. doi: 10.1016/j.gie.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United Eur. Gastroenterol. J. 2017;5:309–334. doi: 10.1177/2050640617700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton, S. R. & Altonen, A. E. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System (IARC Press, Lyon, 2000).
- 23.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dekker, E., van Leerdam, M., Hazewinkel, Y., Sanduleanu, S., Vasen, H. F., Lansdorp-Vogelaar, I. et al. Dutch Guideline for Colonoscopy Surveillance (Dutch Association of Gastroenterology Liver Physicians, Rotterdam, 2013).
- 25.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA310, 2191–2194 (2013). [DOI] [PubMed]
- 26.Goyder J, Warriner K, Miller S. Evaluating socio-economic status (SES) bias in survey nonresponse. J. Off. Stat. 2002;18:1–12. [Google Scholar]
- 27.de Jong AE, Vasen HF. The frequency of a positive family history for colorectal cancer: a population-based study in the Netherlands. Neth. J. Med. 2006;64:367–370. [PubMed] [Google Scholar]
- 28.Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev. Res. (Phila.) 2011;4:1–5. doi: 10.1158/1940-6207.CAPR-10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekker N, van Rossum LG, Van Vugt-van Pinxteren M, van Stiphout SH, Hermens RP, van Zelst-Stams WA, et al. Adding familial risk assessment to faecal occult blood test can increase the effectiveness of population-based colorectal cancer screening. Eur. J. Cancer. 2011;47:1571–1577. doi: 10.1016/j.ejca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Navarro M, Binefa G, Blanco I, Guardiola J, Rodriguez-Moranta F, Peris M. Colorectal cancer screening: strategies to select populations with moderate risk for disease. Rev. Esp. Enferm. Dig. 2009;101:855–860. doi: 10.4321/S1130-01082009001200005. [DOI] [PubMed] [Google Scholar]
- 31.Armelao F, Paternolli C, Franceschini G, Franch R, Orlandi PG, Miori G, et al. Colonoscopic findings in first-degree relatives of patients with colorectal cancer: a population-based screening program. Gastrointest. Endosc. 2011;73:527–34.e2. doi: 10.1016/j.gie.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Quintero E, Carrillo M, Leoz ML, Cubiella J, Gargallo C, Lanas A, et al. Risk of advanced neoplasia in first-degree relatives with colorectal cancer: a large multicenter cross-sectional study. PLoS Med. 2016;13:e1002008. doi: 10.1371/journal.pmed.1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menko, F. H., Ter Stege, J. A., van der Kolk, L. E., Jeanson, K. N., Schats, W., Moha, D. A., et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam. Cancer18, 127–135 (2019). [DOI] [PubMed]
- 34.Humphreys L, Hunter AG, Zimak A, O’Brien A, Korneluk Y, Cappelli M. Why patients do not attend for their appointments at a genetics clinic. J. Med. Genet. 2000;37:810–815. doi: 10.1136/jmg.37.10.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Family History Questionnaire in Dutch
Supplementary Information Table 1 Referral criteria for genetic testing and colonoscopy surveillance
Data Availability Statement
The authors are willing to collaborate in further analyses, on request.