Abstract
A 76-year-old male was admitted to our hospital for progressive bilateral pleural effusion. Because of typical echocardiographic findings such as left ventricular (LV) hypertrophy, thickness of the mitral valve, and a granular sparkling appearance of the LV wall, amyloid cardiomyopathy was suspected. Regardless of up-titration of several diuretic agents, the bilateral pleural effusion did not improve. Because the histological findings of the right ventricular septum (direct-fast-scarlet staining) obtained by biopsy that demonstrated amyloid deposits in perivascular and pericellular lesions, amyloid cardiomyopathy was diagnosed. However, cardiac catheterization revealed normal right and left atrial pressure and normal right and left ventricular end-diastolic pressure. Therefore, hemodynamic deterioration was less likely to be the cause of persistent pleural effusion. Amyloid deposits were also detected in the pleural biopsy specimen, so pleural amyloidosis was diagnosed and may have played an important role in the refractoriness of the pleural effusion.
<Learning objective: Systolic and diastolic dysfunction of various degrees can occur in patients with amyloid cardiomyopathy, which is usually progressive and induces heart failure. In these patients, diuretics are key drugs for resolving fluid retention issues such as pleural effusion. In cases of refractory pleural effusion associated with amyloid cardiomyopathy despite aggressive diuretic therapy, these may be induced by pleural amyloidosis.>
Keywords: Systemic amyloidosis, Refractory pleural effusion, Pleural amyloidosis
Introduction
Amyloidosis is a family of diseases caused by the overexpression of proteins that deposit in tissues as insoluble β-pleated sheet configurations, disrupting organ function. In patients with primary systemic amyloidosis (amyloid L: AL), clonal plasma cells secrete monoclonal immunoglobulin light chains that deposit in the kidney, heart, nerves, and other tissues [1]. It has been reported that evidence of cardiac involvement is present in up to 50% of patients with AL amyloidosis — so-named amyloid cardiomyopathy, the most common manifestation of which is heart failure [2], [3].
Case report
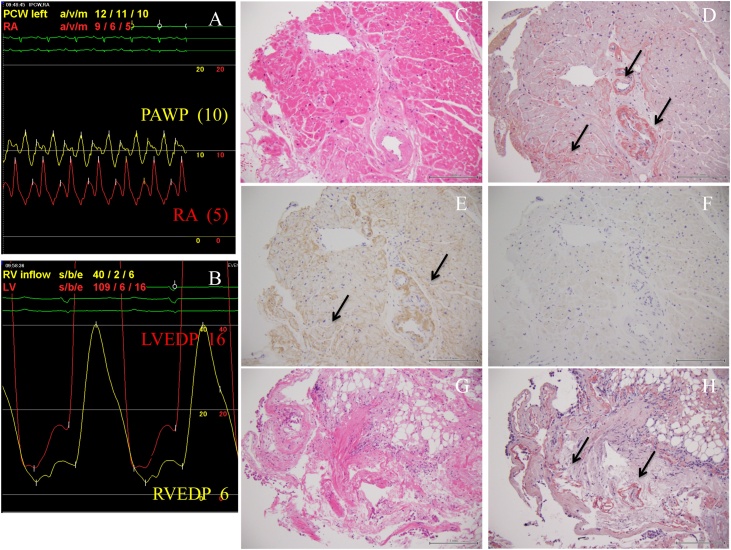
The subject was a 76-year-old male who suffered from dyspnea on exertion. At the age of 73 years, he became aware of bilateral leg edema. At the age of 75 years, left-side unilateral pleural effusion was diagnosed at another hospital (Fig. 1, Panel A). At the age of 76 years, left-side pleural effusion, which was transudative, gradually increased and right-side pleural effusion also appeared (Fig. 1, Panel B) and he was referred to our hospital. His physical examination revealed jugular vein distention and moderate edema in both legs. A SIII without cardiac murmur was auscultated at the lower left sternal border. His body weight was 62 kg, which had increased 3.0 kg over the previous three years. The electrocardiogram revealed low voltage in the extremity leads (Fig. 1, Panel C). The echocardiogram demonstrated left ventricular (LV) wall thickness (interventricular septum: 12.0 mm, posterior wall: 12.3 mm) with the typical granular sparkling appearance and a thickened mitral valve. LV systolic function was preserved at 66% of ejection fraction; however, LV diastolic function was impaired at 1.6 of the E/A ratio, 166 msec of mitral valve deceleration time, and 13.3 of the E/e’ ratio (Fig. 1, Panel D). The laboratory findings were as follows: 268 pg/ml of the plasma brain natriuretic peptide (BNP) level, 1.15 mg/dl of the serum creatinine level, and 3.7 g/dl of the serum albumin level. Bence Jones proteinuria was positive, suggesting monoclonal immunoglobulinemia. The pathophysiology was considered as heart failure induced by diastolic dysfunction of the left ventricle. Because of the typical echocardiographic findings and positive Bence-Jones proteinuria, amyloid cardiomyopathy was highly suspected as an underlying cardiac condition. To resolve fluid retention, oral furosemide was started and up-titrated to 80 mg; however the patient’s symptoms, bilateral pleural effusion and plasma BNP level did not improve (Fig. 2). Therefore, he was admitted to our hospital for further evaluation and treatment. After aggressive diuretic therapy, which included trichlormethiazide, spironolactone, and tolvaptan in addition to furosemide, his body weight decreased to 54.5 kg (−8.5 kg); however, bilateral pleural effusion was refractory and remained (Fig. 2). Cardiac catheterization showed not only normal right atrium and pulmonary artery wedge pressure (5 and 10 mmHg, respectively, Fig. 3, Panel A) but also normal end-diastolic pressure of the right and left ventricles (6 and 16 mmHg, respectively, Fig. 3, Panel B). The histology findings of the right ventricular septum obtained by biopsy (direct-fast-scarlet staining: DFS, which specifically dyes an amyloid material) demonstrated amyloid deposits in the perivascular and pericellular lesions (Fig. 3, Panel D). Moreover, immunofluorescence microscopy clearly showed the deposition of monoclonal Kappa (κ) light chain in AL amyloidosis in the same lesions (Fig. 3, Panel E). Therefore, amyloid cardiomyopathy was definitively diagnosed. However, lower biatrial pressure and biventricular end-diastolic pressure suggested that the elevated hydrostatics were not the principle mechanism of pleural fluid accumulation. In addition, serum albumin level was 3.7 g/dl, suggesting that hypoalbuminemia was not a cause of refractory pleural effusion. We hypothesized that amyloid deposits in the pleura, so-named pleural amyloidosis, was another cause of refractory pleural effusion; therefore, we performed a pleural biopsy guided by video-assisted thoracoscopy. The histological findings of the pleura stained by DFS also demonstrated amyloid deposits in the perivascular lesions and basement membrane (Fig. 3, Panel H). Finally, we definitively diagnosed systemic amyloidosis that involved both the heart and pleura. Pleural amyloid infiltration may have played a central role in the creation and persistence of the pleural effusion, which was refractory to aggressive diuretic therapy. Afterwards, bone marrow showed monoclonal proliferation of plasma cells, which confirmed the diagnosis of multiple myeloma and myeloma-associated systemic amyloidosis.
Fig. 1.
(A) Chest X-ray of the patient at age 75 years revealed left-side unilateral pleural effusion. (B) Left-side pleural effusion gradually increased and right-side pleural effusion also appeared at age 76 years. (C) The electrocardiogram revealed low voltage in the extremity leads. (D) The echocardiogram demonstrated left ventricular (LV) wall thickness (interventricular septum: 12.0 mm, posterior wall: 12.3 mm), in which a granular sparkling appearance was shown. A thickened mitral valve was also shown. LV systolic function was preserved at 66% of ejection fraction; however, LV diastolic function was impaired at 1.6 of the E/A ratio, 166 msec of mitral valve deceleration time, and 13.3 of the E/e’ ratio.
Fig. 2.
This figure illustrates the patient’s clinical course. Oral furosemide was started and up-titrated to 80 mg; however the patient’s symptoms, bilateral pleural effusion, and plasma BNP level did not improve so he was admitted to the hospital. After aggressive diuretic therapy, which included trichlormethiazide, spironolactone, and tolvaptan in addition to furosemide, his body weight decreased to 54.5 kg (−8.5 kg). However, his bilateral pleural effusion was refractory.
BNP, brain natriuretic peptide; BW, body weight.
Fig. 3.
(A) Cardiac catheterization revealed normal right atrium pressure and pulmonary artery wedge pressure (5 and 10 mmHg, respectively). (B) End-diastolic pressure of both the right and left ventricles was also normal (6 and 16 mmHg, respectively), which suggested that heart failure was not the main cause of the pleural effusion. (C) The histology findings of the right ventricular septum obtained by biopsy (hematoxylin–eosin staining). (D) The histology findings of the right ventricular septum (direct-fast-scarlet staining) showed amyloid deposits in the perivascular and pericellular lesions. (E) Immunofluorescence microscopy of the right ventricular septum clearly showed the deposit of monoclonal kappa light chain in AL amyloidosis in the perivascular and pericellular lesions. (F) In contrast, lambda light chain was negative. (G) The histological findings of the pleura obtained by video-assisted thoracoscopy-guided biopsy (hematoxylin–eosin staining). (H) The histological findings of the pleura stained by direct-fast-scarlet demonstrated amyloid deposits in the perivascular lesions and basement membrane. RA, right atrium; PAWP, pulmonary artery wedge pressure; RVEDP, right ventricle end-diastolic pressure; LVEDP, left ventricle end-diastolic pressure.
Discussion
Amyloidosis was first described in 1842 by Rokitansky et al. [4]. Pleural effusion is a rare complication of systemic amyloidosis. When present, it can significantly affect the clinical course of systemic amyloidosis. The prevalence of pleural effusion among patients with systemic amyloidosis is difficult to define. Berk et al. reported that 6% of 636 AL patients presented with large and refractory pleural effusions, with less significant effusion occurring in another 10–15% of cases [5], [6]. In contrast, Smith et al. reported that among 223 amyloidosis patients who underwent autopsy, none of the amyloid A patients were reported to have presented with pleural effusion [7]. In a comparison between 35 primary systemic amyloidosis patients with large and refractory pleural effusions and 120 primary systemic amyloid cardiomyopathy patients without pleural effusion, neither cardiac function, including systolic and diastolic dysfunction, nor renal function differed in the two groups. Therefore, the authors concluded that the elevated hydrostatic force was not the principle mechanism of pleural fluid accumulation and amyloid infiltration to the pleura did play an important role in the refractoriness of pleural effusion in patients with AL amyloidosis. It is speculated that amyloid deposits to the pleura may inhibit pleural fluid resorption through the lymphatic system, which leads to unresponsiveness to diuretic agents [5], [6]. Pleural amyloidosis is a rare complication of systemic amyloidosis. In Japan, only six definitive cases have been reported [8]. Treatment of refractory pleural effusion includes diuretics and direct drainage of the pleural space. Pleurodesis can be another palliative option most commonly used in patients with symptoms of malignant pleural effusion [9]. This can be adapted to prevent repeated pleural fluid collection in patients with pleural amyloidosis. However, the number of reported cases is limited [10]. Pleural effusion associated with pleural amyloidosis is refractory to diuretic agents. Our case highlights the importance of paying attention to the pleura of patients with amyloid cardiomyopathy who have treatment-resistant pleural effusion.
Conclusion
Pleural amyloidosis should be taken into consideration when treating patients with refractory pleural effusion associated with amyloid cardiomyopathy.
Financial support
This report received no specific grant from any funding agency, commercial, or not-for-profit entity.
Ethical standards
The patient provided informed consent for publication.
Conflict of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
None.
References
- 1.Kyle R.A., Gertz M.A. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 2.Dubrey S.W., Cha K., Anderson J., Chamarthi B., Reisinger J., Skinner M. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91:141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 3.Dubrey S.W., Hawkins P.N., Falk R.H. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97:75–84. doi: 10.1136/hrt.2009.190405. [DOI] [PubMed] [Google Scholar]
- 4.Glenner G.G., Page D.L. Amyloid, amyloidosis, and amyloidogenesis. Int Rev Exp Pathol. 1976;15:1–92. [PubMed] [Google Scholar]
- 5.Berk J.L., Keane J., Seldin D.C., Sanchorawala V., Koyama J., Dember L.M. Persistent pleural effusions in primary systemic amyloidosis: etiology and prognosis. Chest. 2003;124:969–977. doi: 10.1378/chest.124.3.969. [DOI] [PubMed] [Google Scholar]
- 6.Berk J.L. Pleural effusions in systemic amyloidosis. Curr Opin Pulm Med. 2005;11:324–328. doi: 10.1097/01.mcp.0000162378.35928.37. [DOI] [PubMed] [Google Scholar]
- 7.Smith R.R., Hutchins G.M., Moore G.W., Humphrey R.L. Type and distribution of pulmonary parenchymal and vascular amyloid. Correlation with cardiac amyloid. Am J Med. 1979;66:96–104. doi: 10.1016/0002-9343(79)90488-1. [DOI] [PubMed] [Google Scholar]
- 8.Tada M., Takayanagi N., Ishiguro T., Tokunaga D., Shimizu Y., Sugita Y. Pleural amyloidosis and multiple myeloma with pleural effusion as the initial symptom. Nihon Kokyuki Gakkai Zasshi. 2013;2:359–364. (in Japanese) [Google Scholar]
- 9.Mercer R.M., Hassan M., Rahman N.M. The role of pleurodesis in respiratory diseases. Expert Rev Respir Med. 2018;12:323–334. doi: 10.1080/17476348.2018.1445971. [DOI] [PubMed] [Google Scholar]
- 10.Masunaga A., Takeda N., Akaike K., Tsumori K., Goto E., Ichiyasu H. Successful treatment of pleurodesis for seemingly intractable pleural effusion in pleural amyloidosis with rheumatoid arthritis. Nihon Kokyuki Gakkai Zasshi. 2011;49:897–902. (in Japanese) [PubMed] [Google Scholar]