Abstract
The incidence of acute complications is high in patients presenting late with acute myocardial infarction (AMI). We describe the case of a patient who presented late with anterior AMI that was complicated by left ventricular (LV) thrombus and electrical storm (ES). Temporary right ventricular pacing suppressed ES under extracorporeal membrane oxygenation support but reduced cardiac function. Immediately after returning to sinus rhythm (i.e. increase in cardiac function), free-floating LV thrombus was detected by echocardiography, resulting in cerebral embolism. Rapid improvement in cardiac function related to mechanical hemodynamic support may become a trigger for embolization in patients with LV thrombus.
<Learning objective: Patients presenting late with acute myocardial infarction have a high incidence of complications. In patients with severe left ventricular (LV) dysfunction who require venoarterial extracorporeal membrane oxygenation, right ventricular (RV) pacing can advocate cardiac dysfunction with insufficient aortic valve opening. When patients have LV thrombus in this situation, rapid improvement in cardiac function by the interruption of RV pacing can dislodge LV thrombus, leading to systematic embolism.>
Keywords: Acute myocardial infarction, Late hospital presentation, Left ventricular thrombus, Systemic embolism, Electrical storm
Introduction
In the era of primary percutaneous coronary intervention (PCI), the incidence of complications of acute myocardial infarction (AMI) has reduced and the outcomes have improved [1]. However, this does not apply to patients with longer delays from symptom onset to hospital presentation. The mortality in patients with acute complications remains high [1]. In the present report, we describe the case of a patient with anterior AMI with delayed hospital presentation that was complicated by congestive heart failure and left ventricular (LV) thrombus on admission. The patient suffered from refractory electrical storm (ES) after hospitalization, resulting in poor outcomes.
Case report
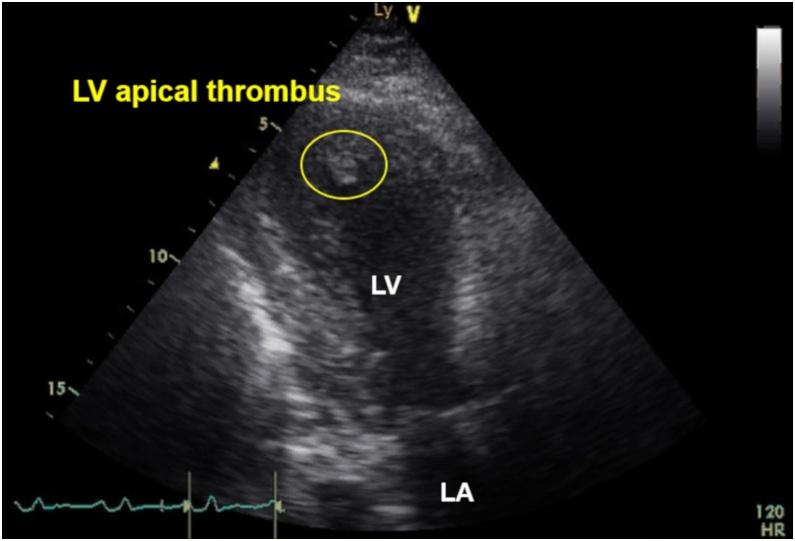
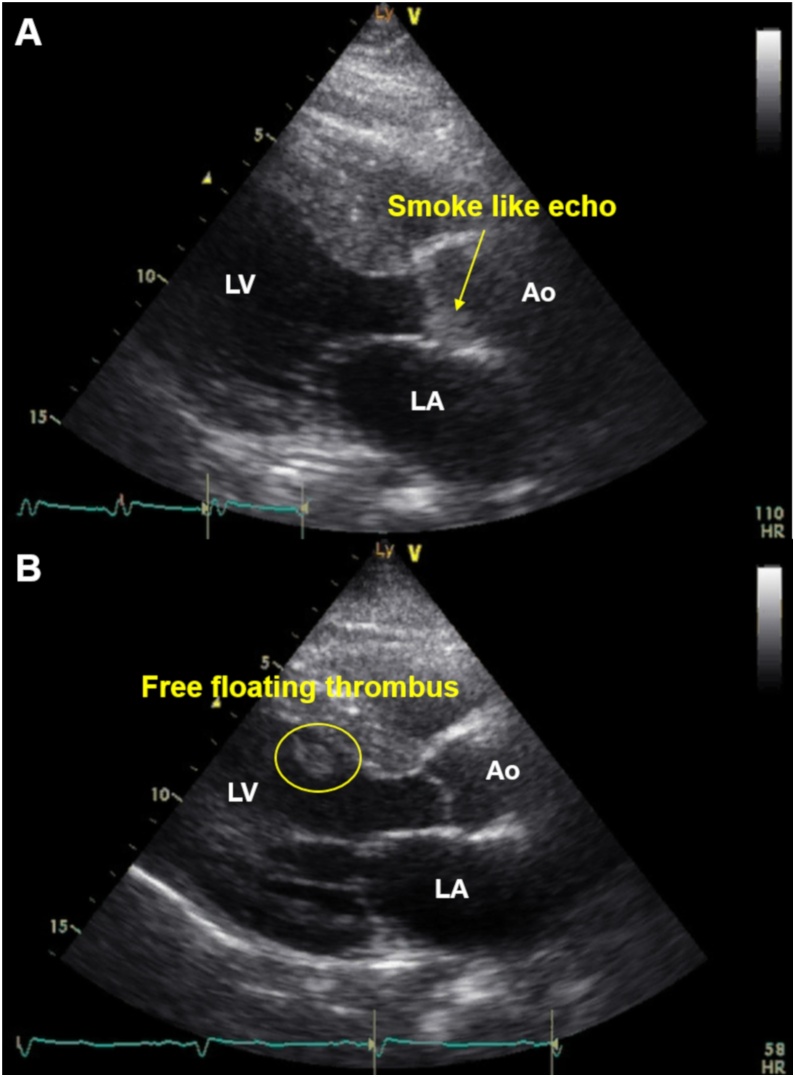
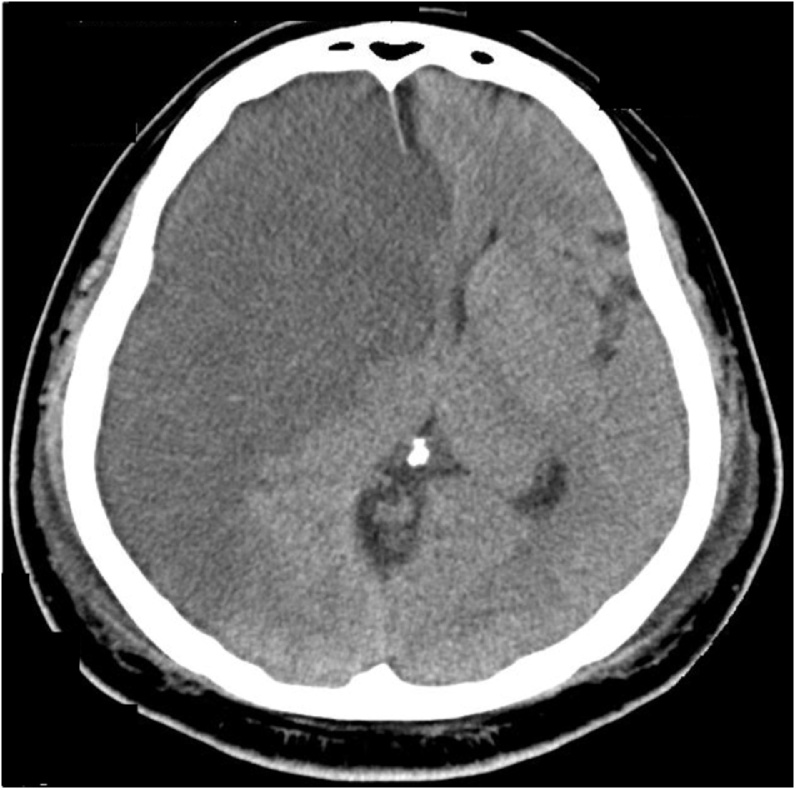
A 52-year-old man with untreated diabetes mellites (HbA1c, 11.7%) and dyslipidemia (low-density lipoprotein cholesterol, 252 mg/dL) presented to the hospital with chest pain 3 days previously with subsequent exercise-induced dyspnea. The electrocardiography on admission showed ST elevations and Q waves in leads V1–V5 and low voltage in the limb leads. The echocardiography on admission detected anterior wall asynergy and LV ejection fraction (LVEF) of 40% with LV apical thrombus (Fig. 1 and Video S1). The C-reactive protein (CRP) level on admission was remarkably elevated (30.5 mg/dL), and elevated D-dimer level on admission was mild (1.9 μg/mL). The condition of the patient was complicated by congestive heart failure (Killip II); therefore, diuretic treatment with anticoagulant therapy by heparin was performed. Although activated partial thromboplastin time was controlled within 40–60 s, LV thrombus did not disappear. The course of heart failure treatment was uneventful and oral administration for heart failure, including a small dose of beta-blocker, was provided. However, on day 5, ventricular fibrillation (VF) occurred from R-on-T phenomenon. The VF was terminated by direct current shock. Under deep sedation with intubation (i.e. mechanical ventilatory support), the intra-aortic balloon pump was inserted, and emergency coronary angiogram revealed severe stenosis of the proximal left anterior descending artery and right coronary artery. Both lesions were successfully treated with drug-eluting stents. Nevertheless, recurrent VF and ventricular tachycardia (VT) continued, even after the use of antiarrhythmic drugs, such as amiodarone, nifekalant, and landiolol. After the occurrence of ES, LVEF dropped to 15%. Although venoarterial extracorporeal membrane oxygenation (VA-ECMO) was inserted, ES was not suppressed. Under VA-ECMO support, activated coagulation time (ACT) was controlled within 180–200 s. On the day after the insertion of VA-ECMO (on day 6), temporary right ventricular (RV) pacing was inserted as an additional management of ES. RV overdrive pacing by a pacing rate of 110 bpm suppressed recurrent VF/VT but reduced cardiac function with insufficient aortic valve opening. The echocardiography detected spontaneous echo contrast, the so-called “smoke like echo,” into the coronary sinuses of Valsalva, suggesting blood stasis (Fig. 2A and Video S2). Therefore, on day 7, RV pacing was interrupted to avoid high LV filling pressure causing pulmonary congestion and new formation of thrombus. By a return to sinus rhythm, cardiac function returned (i.e. slightly increased) to the condition before RV pacing and aortic valve started to open again. However, at that time, echocardiography detected that LV thrombus flowed out of the LV outflow tract into the aorta (Fig. 2B and Video S3). When free-floating LV thrombus was observed, ACT was 195 s. After several hours, anisocoria was seen. Computed tomography confirmed a large right hemispheric cerebral infarction with midline shift (Fig. 3).
Fig. 1.
Electrocardiograph y on admission. Ball-like thrombus was observed in the apex of left ventricular cavity.
LA, left atrium; LV, left ventricle.
Fig. 2.
Changes in cardiac function and aortic valve opening according to temporary right ventricular (RV) overdrive pacing under extracorporeal membrane oxygenation.
(A) After temporary RV overdrive pacing, the aortic valve remained nearly closed due to reduced cardiac function. Spontaneous echo contrast, the so-called “smoke-like echo,” was observed into the coronary sinuses of Valsalva. (B) After the interruption of temporary RV overdrive pacing, cardiac function increased to its former condition and the aortic valve started to open again. However, just at that time, free-floating left ventricular thrombus was detected.
Ao, aorta; LA, left atrium; LV, left ventricle.
Fig. 3.
Cerebral embolism. Brain computed tomography image after the appearance of anisocoria showed a large right hemispheric cerebral infarction with midline shift.
Discussion
Although AMI patients with late hospital presentation have a high incidence of acute complications and poor outcomes even in the primary PCI era [2], current guidelines do not necessarily recommend primary PCI in patients with AMI with ST-segment elevation who present later than 12 h from symptom onset [3]. In AMI patients with late presentation, the search for acute complications on admission and careful observation in the acute phase is more important than in patients with successful early reperfusion. Multiple complications may develop in patients with late presentation, and the management is more difficult in such a situation. In this AMI patient with LV thrombus, primary PCI was not performed because of very late presentation (over 3 days after onset). However, myocardial ischemia was one of the triggers for ES and severe embolic cerebral infarction occurred during the management of refractory ES.
LV thrombus after AMI is a clinically important complication that can cause systemic embolization. Recent reports showed the incidence of LV thrombus was about 1.5%–3% in the primary PCI era [4], [5]. The risk factors associated with LV thrombus include delayed reperfusion, anterior AMI, large infarct size, aneurysmal change of LV apex, and cardiac dysfunction [5]. Although there are few data regarding embolic complications by LV thrombus in patients undergoing primary PCI, embolic complications in the thrombolytic era occurred in 2%−3% of patients with LV thrombus [6]. Ball-like thrombus, which is mobile and pedunculated, has a higher risk of embolization than mural type thrombus [7]. This patient had many risk factors of LV thrombus and embolism, such as those described above. More aggressive anticoagulant therapy might be considered early in the hospitalization.
ES is a life-threatening complication in AMI. It is commonly defined as the occurrence of three or more episodes of VT or VF occurring within 24 h and requiring intervention [8]. It was recently reported that the incidence of in-hospital ES in the era of primary PCI was about 0.9% among AMI patients [9]. The appearance of ES is related to the presence of heart failure on admission, large infarct size, elevated CRP level, and diabetes [9]. Late ES, which occurs ≥48 h after the onset of AMI, is an independent predictor of in-hospital death [9]. This patient was at high risk of ES as well as LV thrombus. The following methods should be considered for the management of ES: antiarrhythmic drugs, sedation, mechanical hemodynamic support, and catheter ablation [3]. Despite the above management, except for catheter ablation, being performed in addition to the improvement of myocardial ischemia, ES in this patient was refractory.
In this case, temporary RV overdrive pacing was also used for the reduction of the burden of ventricular tachyarrhythmias but advocated cardiac dysfunction with insufficient aortic valve opening. Although temporary overdrive pacing has been pointed to as an option for resistant ventricular tachyarrhythmias, RV pacing can diminish cardiac function [10]. Furthermore, in this case, an increase in the LV afterload by the retrograde aortic flow of VA-ECMO contributed to insufficient aortic valve opening. Although RV pacing was interrupted to avoid high LV filling pressure and new formation of thrombus, free-floating LV thrombus was observed at that time. Rapid improvement of cardiac function by the interruption of RV pacing may explain free-floating LV thrombus, as well as the mechanism by which the improvement of local cardiac wall motion by treatment (e.g. takotsubo syndrome and after coronary reperfusion) can also become a risk of systemic embolization. In other words, LV thrombus might have been dislodged when a return to sinus rhythm increased cardiac function. As a result, LV thrombus flowed into the aorta because the aortic valve started to open again at that time, leading to severe cerebral embolism. Free-floating thrombus might have been one that originally existed from admission or one that was newly formed while the aortic valve remained nearly closed. When RV pacing advocated cardiac dysfunction with insufficient aortic valve opening, we should have established the appropriate pacing rate with aortic valve opening. If it did not exist, we should have immediately interrupted RV pacing.
In conclusion, more attention should be paid to the management of acute complications, especially in AMI patients with late hospital presentation, even in the primary PCI era. In patients with severe LV dysfunction who require VA-ECMO, RV pacing can advocate cardiac dysfunction with insufficient aortic valve opening. When patients have LV thrombus in this situation, rapid improvement in cardiac function by the interruption of RV pacing can dislodge LV thrombus. Rapid improvement in cardiac function related to mechanical hemodynamic support may become a trigger for embolization in AMI patients with LV thrombus.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jccase.2020.02.009.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bajaj A., Sethi A., Rathor P., Suppogu N., Sethi A. Acute complications of myocardial infarction in the current era: diagnosis and management. J Investig Med. 2015;63:844–855. doi: 10.1097/JIM.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 2.McNair P.W., Bilchick K.C., Keeley E.C. Very late presentation in ST elevation myocardial infarction: predictors and long-term mortality. Int J Cardiol Heart Vasc. 2019;22:156–159. doi: 10.1016/j.ijcha.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura K., Kimura T., Ishihara M., Nakagawa Y., Nakao K., Miyauchi K. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2019;83:1085–1096. doi: 10.1253/circj.CJ-19-0133. [DOI] [PubMed] [Google Scholar]
- 4.Mao T.F., Bajwa A., Muskula P., Coggins T.R., Kennedy K., Magalski A. Incidence of left ventricular thrombus in patients with acute ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2018;121:27–31. doi: 10.1016/j.amjcard.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Choi U.L., Park J.H., Sun B.J., Oh J.K., Seong S.W., Lee J.H. Impaired left ventricular diastolic function is related to the formation of left ventricular apical thrombus in patients with acute anterior myocardial infarction. Heart Vessels. 2018;33:447–452. doi: 10.1007/s00380-017-1079-z. [DOI] [PubMed] [Google Scholar]
- 6.Delewi R., Zijlstra F., Piek J.J. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98:1743–1749. doi: 10.1136/heartjnl-2012-301962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratton J.R., Resnick A.D. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987;75:1004–1011. doi: 10.1161/01.cir.75.5.1004. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser S.H., Al-Khalidi H.R., Pratt C.M., Brum J.M., Tatla D.S., Tchou P. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006;27:3027–3032. doi: 10.1093/eurheartj/ehl276. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi Y., Tanno K., Ueno A., Fukamizu S., Murata H., Watanabe N. In-hospital electrical storm in acute myocardial infarction-clinical background and mechanism of the electrical instability. Circ J. 2018;83:91–100. doi: 10.1253/circj.CJ-18-0785. [DOI] [PubMed] [Google Scholar]
- 10.Aguiar Rosa S., Oliveira M., Valente B., Silva Cunha P., Almeida Morais L., Cruz Ferreira R. Ventricular electrical storm after acute myocardial infarction successfully treated with temporary atrial overdrive pacing. Med Intensiva. 2017;41:252–254. doi: 10.1016/j.medin.2016.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.