Abstract
The present study explored effects of L-glutamate (Glu) levels on growth, digestive and absorptive capability, and intestinal physical barrier functions of Jian carp (Cyprinus carpio). A total of 600 Jian carp (126.40 ± 0.21 g) were randomly distributed into 5 groups with 3 replicates each, fed diets containing graded levels of Glu (53.4 [control], 57.2, 60.6, 68.4, and 83.4 g/kg) for 63 d. Results showed compared with control diet, feed intake and percent weight gain (PWG) in fish fed 83.4 g of Glu/kg diet were increased and feed conversion ratio in fish fed 68.4 g of Glu/kg diet was decreased (P < 0.05). Similarly, body crude protein and lipid contents in fish fed 68.4 g of Glu/kg diet were higher (P < 0.05). The activities of trypsin and chymotrypsin in the hepatopancreas and intestine, and amylase, alkaline phosphatase (AKP), Na+, K+-ATPase (NKA), and creatine kinase (CK) in intestine were higher in fish fed 68.4 g of Glu/kg diet (P < 0.05). Dietary Glu (57.2 to 83.4 g/kg diet) decreased malondialdehyde (MDA) and protein carbonyl (PCO) contents in the intestine (P < 0.05). The activities of catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST) in the hepatopancreas and intestine were higher in fish fed 60.6 and 68.4 g of Glu/kg diets (P < 0.05). Intestinal the glutathione reductase (GR) activity and glutathione (GSH) content in fish fed 60.6, 68.4, and 83.4 g of Glu/kg diet were increased (P < 0.05). The GPx1a, GST, and nuclear factor erythroid 2-related factor 2 (Nrf2) mRNA expressions in the intestine were up-regulated in fish fed 60.6 and 68.4 g of Glu/kg diet (P < 0.05). The zonula occludens protein-1 (ZO-1), occludin1, and claudin3 mRNA expressions were also up-regulated in fish fed 83.4 g of Glu/kg diet (P < 0.05). Fish fed 68.4 g of Glu/kg diet had higher levels of claudin 2, claudin7, and protein kinase C (PKC) mRNA (P < 0.05). These results indicated that Glu improved fish growth, digestive and absorptive ability, and intestinal physical barrier functions. Based on the quadratic regression analysis of PWG, and MDA of the hepatopancreas and intestine, the optimal dietary Glu levels were estimated to be 81.97, 71.06, and 71.36 g/kg diet, respectively.
Keywords: L-glutamate, Growth, Digestive ability, Intestinal barrier function, Jian carp
1. Introduction
L-glutamate (Glu) is regarded as one of dispensable amino acids in fish (NRC, 2011). Recently, growing evidences show that some dispensable amino acids and their metabolites are important regulators of key metabolic pathways, which are necessary for maintenance, growth, feed intake, nutrient utilization, immunity, as well as resistance to environmental stressors and pathogenic organisms in various fishes (Wu et al., 2012, Wu et al., 2011, Xie et al., 2016, Xie et al., 2014). Beyond the role of protein synthesis, previous studies also demonstrated Glu has an important role in fish growth, feed utilization, and intestinal health (Caballero-Solares et al., 2015, Chika et al., 2016, Zhao et al., 2015). Dietary supplementation with 8 g of Glu/kg diet improves growth, protein and lipid utilization in grass carp (Ctenopharyngodon Idella) (Zhao et al., 2015), with 40 g of Glu/kg diet promotes protein and lipid utilization in gilthead seabream Sparus aurata (Caballero-Solares et al., 2015), with 20 g of Glu/kg diet increases growth in rainbow trout Oncorhynchus mykiss (Chika et al., 2016). The other study shows that dietary supplementation with 15 g of Glu/kg diet has no effect on growth and feed utilization in Atlantic salmon Salmo salar (Larsson et al., 2014). These results showed that dietary Glu level can affect the piscine growth and feed utilization rate, but different fish species might have different optimum levels. According to above studies, based on the better growth performance, dietary Glu supplementation level varies from 8 to 40 g/kg diet in different fish species (Caballero-Solares et al., 2015, Chika et al., 2016, Zhao et al., 2015).
Fish growth depends on the nutrient digestion and absorption, which is governed by the activities of enzymes including digestive and absorptive enzymes (Deng et al., 2010, Hakim et al., 2009). To our knowledge, there is only one report regarding the effects of dietary Glu on the digestive and absorptive capacity of fish, which showed that diet supplementation with 8 g of Glu/kg diet improved the intestinal digestive enzymes activities in grass carp, as well as the activities of intestinal brush-border enzymes, including alkaline phosphatase (AKP), creatine kinase (CK), Na+, K+-ATPase (NKA), and γ-glutamyl transpeptidase (γ-GT) (Zhao et al., 2015). Meanwhile, Glu is one of the most abundant amino acids, which is a truly functional amino acid (Brosnan and Brosnan, 2013). Nevertheless, despite its importance in fish nutrition, few studies have so far endeavored to determine the optimum level of dietary Glu for fish growth, digestive and absorptive function. Therefore, the 5 isoenergetic diets were formulated to supplement with 0 (53.4), 4 (57.2), 8 (60.6), 16 (68.4), 32 (83.4) g/kg Glu to evaluate the effect of dietary Glu on growth performance and digestive and absorptive capability in Jian carp.
Fish intestinal physical barrier function is closely related to the integrity of cellular structure (Zhang et al., 2002). At the same time, the structural integrity of fish intestine is the guarantee of its normal digestion and absorption. Intestinal antioxidant capacity plays a key role in maintaining piscine intestinal structural integrity and function (Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c). To prevent oxidative damage, fish have developed non-enzymatic and enzymatic antioxidant defense systems (Martínez-álvarez et al., 2005). Glutathione (GSH) is an important non-enzymatic antioxidant compound of fish (Hong et al., 2015). The Glu is the preferred source for mucosal GSH synthesis in the intestine (Reeds et al., 1997). The pathway for the synthesis of GSH in the enterocyte cytosol is probably limited by Glu availability (Blachier et al., 2009). As other aerobic organisms, fish developed diverse antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), glutathione reductase (GR), and glutathione peroxidase (GPx) (Zhang et al., 2004). The previous studies showed that dietary supplementation with 8 g of Glu/kg diet improved intestinal antioxidant capacity in grass carp (Zhao et al., 2015) and culture medium pre-supplementation with Glu enhanced antioxidant capacity and regulated antioxidant-related signaling molecule expression in fish enterocytes (Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c). These results suggested Glu plays an important role in fish intestines. Therefore, it is worthy to investigate the optimum level of dietary Glu on effect of intestinal antioxidant capacity and antioxidant-related signaling molecule expression in fish.
In addition to the intestinal antioxidant capacity, the structure integrity also plays a vital role in absorption capacity of intestines (Ballard et al., 1995). The intercellular structural integrity in the intestine depends largely on tight junction (TJ) proteins (such as zonula occludens protein-1 [ZO-1], the transmembrane protein occludin, members of the claudin family, and others) (Gonzalez-Mariscal et al., 2008). Intestinal mucosal absorption ability may be modified by modulating claudin expression specificity (Matsuhisa et al., 2012). The Ca2+ and Mg2+ absorption is increased by increasing expression of claudin2 (GaffneyStomberg et al., 2011) and claudin7 (Thongon and Krishnamra, 2012). Previous study also showed the integrity of TJ can be damaged by altered level of TJ protein expression (Landy et al., 2016). Dysfunction of TJ can lead to the disruption of intestinal barrier function. Emerging evidence showed the expression levels of TJ protein is modulated by valine, folic acid, and isoleucine in fish (Luo et al., 2014, Shi et al., 2016, Zhao et al., 2014). In piglets, dietary Glu supplementation increased the relative mRNA expression of ZO-1 and occludin in jejunal mucosa (Luo et al., 2014). However, information regarding the effects of Glu on TJ proteins in fish remains scarce.
Thus, this study was conducted to test the following hypothesis: dietary Glu improved growth, digestive and absorptive capability, and intestinal physical barrier function by enhancing digestion and absorption enzyme activities and TJ gene expressions in Jian carp.
2. Materials and methods
Feeding management of fish was conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Sichuan Agricultural University, China.
2.1. Experimental design and diets
Feed formulation and chemical composition of diets are presented in Table 1, Table 2. Crystalline amino acids (Donboo Amino Acid, Nantong, Jiangsu, China) were used to simulate the amino acid pattern of diets. Soybean meal, canola meal, corn gluten meal, and fish meal were used as dietary protein sources. All feed ingredients were purchased from Xinnong Feed Company (Shanghai, China). Soybean oil and wheat flour were used as dietary lipid and carbohydrate sources, respectively. The basal diet was formulated to meet the nutrient requirements of carp based on NRC (National Research Council) (2011). The 5 isoenergetic diets were supplemented with Glu (0, 4, 8, 16, 32 g/kg) to provide Glu at the concentrations of 53.4 (control), 57.2, 60.6, 68.4, and 83.4 g/kg diet. Increased Glu levels were compensated by decreasing equal levels of wheat flour. Differences in the amino acid composition of diets were negligible (Table 2), except for diets Glu. Dietary Glu content was analyzed via high performance liquid chromatography as described by Buentello and Gatlin (2002). All dry ingredients were ground through a 60-mesh screen. The diets were prepared by mixing the dry ingredients with the oil and water using a mixer. Then each diet was extruded in a twin-screw extruder (MY-165) with a 2-mm die (EXT50A, Yang gong Machine, China). The processing conditions were as follows: 100 r/min screw speed, 127 °C, and 3.04 to 4.56 MPa. Floating extruded pellets were air-dried and stored at 4 °C in plastic bags until being used.
Table 1.
Composition and nutrient contents of the experimental diets (g/kg, as fed basis).
| Item | Dietary L-glutamate level, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| Ingredients | |||||
| Soybean meal | 290 | 290 | 290 | 290 | 290 |
| Canola meal | 220 | 220 | 220 | 220 | 220 |
| Corn gluten meal | 60 | 60 | 60 | 60 | 60 |
| Fish meal | 60 | 60 | 60 | 60 | 60 |
| Wheat flour | 277 | 273 | 269 | 261 | 245 |
| Soy oil | 50 | 50 | 50 | 50 | 50 |
| Vitamin premix1 | 10 | 10 | 10 | 10 | 10 |
| Trace mineral premix2 | 10 | 10 | 10 | 10 | 10 |
| Choline chloride | 10 | 10 | 10 | 10 | 10 |
| Monocalcium phosphate | 2 | 2 | 2 | 2 | 2 |
| Lysine | 6 | 6 | 6 | 6 | 6 |
| Methionine | 2 | 2 | 2 | 2 | 2 |
| Threonine | 3 | 3 | 3 | 3 | 3 |
| L-glutamate | 0 | 4 | 8 | 16 | 32 |
| Total | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| Analyzed chemical composition | |||||
| Crude protein | 306.0 | 312.4 | 316.0 | 316.3 | 322.3 |
| Crude lipid | 60.9 | 61.9 | 61.5 | 60.1 | 60.0 |
| Crude ash | 70.6 | 71.4 | 73.1 | 70.4 | 69.9 |
Per kilogram of vitamin premix contained the following: 400,000 IU of vitamin A as retinyl acetate; 240,000 IU of vitamin D3; 10.000 g of vitamin E; 0.100 g of vitamin K as menadione sodium bisulfate; 0.010 g of cyanocobalamin; 0.100 g of D-biotin; 0.500 g of folic acid; 0.102 g of vitamin B1 as thiamin nitrate; 6.667 g of vitamin C as ascorbyl acetate; 2.800 g of niacin; 51.800 g of meso-inositol; 2.461 g of D-pantothenic acid as calcium-D-pantothenate; 0.500 g of riboflavine; 0.740 g of vitamin B6 as pyridoxine hydrochloride. All ingredients were diluted with corn starch to 1 kg.
Per kilogram of mineral premix contained the following: 13.730 g Fe of as FeSO4·7H2O; 0.300 g Cu of as CuSO4·5H2O; 4.869 g Zn of as ZnSO4·7H2O; 1.200 g Mn of as MnSO4·H2O; 0.110 g I of as KI; 0.025 g Se of as NaSeO3. All ingredients were diluted with CaCO3 to 1 kg.
Table 2.
Amino acid composition of experimental diets (g/kg, dry matter basis).
| Item | Dietary L-glutamate level, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| Essential amino acids | |||||
| Lysine | 20.7 | 20.7 | 20.6 | 20.6 | 20.6 |
| Methionine | 6.8 | 6.8 | 6.8 | 6.7 | 6.7 |
| Threonine | 15.3 | 15.3 | 15.3 | 15.3 | 15.2 |
| Arginine | 18.7 | 18.7 | 18.6 | 18.6 | 18.5 |
| Leucine | 26.4 | 26.4 | 26.4 | 26.3 | 26.2 |
| Histidine | 7.1 | 7.1 | 7.1 | 7.1 | 7.1 |
| Isoleucine | 13.6 | 13.5 | 13.5 | 13.5 | 13.4 |
| Phenylalanine | 14.5 | 14.5 | 14.5 | 14.5 | 14.5 |
| Valine | 14.2 | 14.2 | 14.2 | 14.2 | 14.2 |
| Non-essential amino acids | |||||
| Alanine | 14.6 | 14.5 | 14.5 | 14.5 | 14.4 |
| Aspartate | 25.4 | 25.4 | 25.4 | 25.3 | 25.2 |
| Cystine | 5.0 | 5.0 | 4.9 | 4.9 | 4.9 |
| Glutamine | 2.5 | 2.6 | 2.6 | 2.6 | 2.5 |
| Glutamate | 53.4 | 57.2 | 60.6 | 68.4 | 83.4 |
| Glycine | 14.6 | 14.6 | 14.5 | 14.5 | 14.4 |
| Proline | 16.5 | 16.5 | 16.4 | 16.3 | 16.2 |
| Serine | 14.8 | 14.8 | 14.8 | 14.8 | 14.7 |
| Tyrosine | 10.8 | 10.8 | 10.8 | 10.8 | 10.7 |
2.2. Fish management and feeding
The feeding trial was conducted at Experiment Station of Ya'an, Sichuan Agricultural University, China. Carp, obtained from the Tongwei fisheries (Sichuan, China), and were acclimatised to the experimental (outdoor) conditions for 4 wk. Fish were fed 3 times daily with the control diet to satiation during this period. A total of 600 fish with similar sizes (mean initial weight 126.40 ± 0.21 g) were randomly assigned into 15 outdoor concrete tanks (2 m × 1.5 m × 1 m), resulting in 40 fish in each tank. During the experimental period, fish were reared under natural light conditions. Water temperature was measured at 08:00, 13:00, and 18:00 daily. Average water temperature was 25.5 ± 3.0 °C. Continuous flowing water were maintained at the rate of 0.7 L/min in each tank. The pH was maintained at 7.0 ± 0.5. Water was continuously aerated using air stones to adjust the dissolved oxygen (>5.0 mg/L). Each of the 5 diets was fed to 3 replicates of fish 3 times daily (07:20, 13:20, and 18:20) to satiation for 63 d. Uneaten feed was collected 1 h after feeding, dried and weighed to calculate feed intake according to Zhao et al. (2015).
2.3. Sample collection and analysis
After a fasting period of 24 h, fish in each tank were weighed and counted at the initiation and termination of the feeding trail. Prior to sampling, fish were anaesthetized in benzocaine bath (50 mg/L). Fifteen fish from the same population before the experiment and 5 fish from each tank at the end of feeding trail were selected for determination of initial and final carcass proximate composition respectively. The proximate compositions of fish carcass and feed were measured according to AOAC (Horwitz, 2000). The hepatopancreas and intestines of another 6 fish from each tank were quickly removed, weighed and frozen in liquid nitrogen, then stored at −70 °C until analysis.
The intestine and hepatopancreas samples were each homogenized in 10 volumes (wt/vol) of ice-cold physiological saline solution and centrifuged at 6,000 × g for 20 min at 4 °C. The supernatant was collected for enzyme activity analysis. Trypsin, amylase, and lipase activities were determined according to Zhao et al. (2015). Trypsin activity was determined using ρ-toluenesulphonyl-l-arginine methyl esther as a substrate in 0.05-mol/L Tris–HCl buffer, pH = 9.0. Amylase was assayed using 1% soluble starch as a substrate in 0.02-mol/L phosphate buffer, pH = 8.0. Lipase activity was measured at 405 nm by the rate of methylresorufin formation at 37 °C using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The activities of alkaline phosphatase (AKP) and NKA are assayed according to Hong et al. (2015). To measure AKP activity, 50 μL of samples was mixed with 1.0 mL of reaction solution containing disodium phenyl phosphate. Absorbance was monitored at 520 nm. The NKA, creatine kinase (CK) and gamma-glutamyl transpeptidase (γ-GT) activities were determined by commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The intestine and hepatopancreas protein content was determined using the Coomassie Brilliant Blue dye binding technique with bovine serum albumin as the standard following the method of Bradford (1976). The intestinal malondialdehyde (MDA) content was analysed using the thiobarbituric acid reaction. The MDA forms a red adduct with thiobarbituric acid, with an absorbance at 532 nm. The protein carbonyl (PCO) content was determined according to the method described by Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c with a minor modification using 2,4-dinitrophenylhydrazine (DNPH) reagent. The GSH content and activities of SOD, CAT, and GR in the intestine were determined by the commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The intestinal superoxide anion (·O2−) (SAS) and hydroxyl radical (·OH−) (HRS) scavenging activities were measured as our previous study described by Jiang et al. (2015b). The SAS was determined by Superoxide Anion Free Radical Detection Kit. Superoxide radicals were generated by the action of xanthine and xanthine oxidase, with the electron acceptor added, a colouration reaction is developed using the griess reagent. The colouration degree is directly proportional to the quantity of superoxide anion in the reaction. The HRS was determined by a Hydroxyl Free Radical Detection Kit. Hydroxyl radicals are generated in the Fenton reaction, with the electron acceptor added, a colouration reaction is developed using the griess reagent. The colouration degree is directly proportional to the quantity of hydroxyl radicals in the reaction.
2.4. Real-time quantitative PCR
The procedures of RNA isolation, reverse transcription and real-time quantitative PCR were similar to the previous study (Jiang et al., 2016). The total RNA was extracted from the intestine using RNAiso Plus kit (TaKaRa, Dalian, China) according to the manufacturer's instructions followed by DNAse I treatment. The RNA purity and integrity were assessed spectrophotometric (A260:A280 ratio) analysis and agarose gel (1%) electrophoresis, respectively. Subsequently, the 2 μL of total RNA was used to synthesize cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). Specific primers for ZO-1 were designed according to sequences of Jian carp cloned in our laboratory (Table 3), and the primers for Cu/Zn superoxide dismutase (CuZnSOD), CAT, GPx1a, GPx1b, GST, GR, nuclear factor erythroid 2-related factor 2 (Nrf2), Keap1, occludin1, claudin2 (Jiang et al., 2016), claudin3, claudin7, and protein kinase C (PKC) were designed using the published sequences of Jian carp (Table 3). All of the real-time quantitative PCR analyses were performed in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Elongation factor 1A (EF1а) was used as a reference gene to normalize cDNA loading, according to the results of our preliminary experiment concerning the evaluation of internal control genes. The amount of the target gene was calculated based on the threshold cycle number (CT), and the CT for each sample was determined using the CFX Manager software. All of the primer amplification efficiencies were approximately 100%. The gene expression results were analyzed using the 2−ΔΔCT method according to Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c.
Table 3.
The primers and annealing temperature used for real-time quantitative PCR.
| Item | Sequence (5′-3′) | Annealing temperature, °C | GenBank ID |
|---|---|---|---|
| CuZnSOD | F: TGGCGAAGAAGGCTGTTTGT | 60.4 | JF342355 |
| R: TTCACTGGAGACCCGTCACT | |||
| CAT | F: CTGGAAGTGGAATCCGTTTG | 54 | JF411604 |
| R: CGACCTCAGCGAAATAGTTG | |||
| GPx1a | F: GTGACGACTCTGTGTCCTTG | 60.4 | JF411605 |
| R: AACCTTCTGCTGTATCTCTTGA | |||
| GPx1b | F: TATGTCCGTCCTGGCAATGG | 60.4 | JF411606 |
| R: ATCGCTCGGGAATGGAAGTT | |||
| GST | F: TCTCAAGGCACCCGTCTG | 56.6 | DQ411314.1 |
| R: TCTCCAAGTATCCATCCCACA | |||
| GR | F: GAGAAGTACGACACCATCCA | 56 | JF411607 |
| R: CACACCTATTGAACTGAGATTGAG | |||
| Nrf2 | F: TTCCCGCTGGTTTACCTTAC | 60 | JX462955 |
| R: CGTTTCTTCTGCTTGTCTTT | |||
| Keap1 | F: GCTCTTCGGAAACCCCT | 60 | JX470752 |
| R: GCCCCAAGCCCACTACA | |||
| ZO-1 | F: GCGAAATGACACGGGCTAT | 65 | KY290394 |
| R: CTCTGTTGTGGTTGAGTGTAGGC | |||
| Occludin1 | F: ATCGGTTCAGTACAATCAGG | 55.5 | KF975606 |
| R: GACAATGAAGCCCATAACAA | |||
| Claudin2 | F: CTGGAGTTGATGGGTTTCTTTTG | 63.5 | Syakuri et al. (2013) |
| R: AGACCTTTCATGCTTTCTACCG | |||
| Claudin3 | F: GCACCAACTGTATCGAGGATG | 56.6 | JQ767157.1 |
| R: GGTTGTAGAAGTCCCGAATGG | |||
| Claudin7 | F: CTTCTATAACCCCTTCACACCAG | 56 | JQ767155.1 |
| R: ACATGCCTCCACCCATTATG | |||
| PKC | F: AAATCCACCAAGCGACCT | 60 | JX470751.1 |
| R: CGAACCCTCCsCACAGACG | |||
| EF1а | F: TCACCATTGACATTGCTCTC | 56 | AF485331 |
| R: TGTTCTTGATGAAGTCTCTGT |
CuZnSOD = Cu/Zn superoxide dismutase; F = forward; R = reverse; CAT = catalase; GPx1a = glutathione peroxidase 1a; GPx1b = glutathione peroxidase 1b; GST = glutathione S-transferase; GR = glutathione reductase; Nrf2 = nuclear factor erythroid 2-related factor 2; ZO-1 = zonula occludens protein-1; PKC = protein kinase C; EF1а = elongation factor 1a.
2.5. Statistical analysis
Results were presented as means ± stand error (SE). The general linear models (GLM) procedure of SAS software (SAS Institute Inc., 2006) was used to determined treatment effects, and considered significant when P < 0.05 as described (Kabaroff et al., 2006). Orthogonal polynomial contrasts were used to test quadratic effects of dietary Glu level as described by Mahmoud et al. (2017). The quadratic regression analysis model was used to determine the optimal dietary Glu supplementation levels based on different indices, and R2 between 0.7 and 1 indicated a good fit of the regression equation to the data, according to Xu et al. (2018). Pearson correlation coefficient analysis was conducted using the Bivariate Correlation program.
3. Results
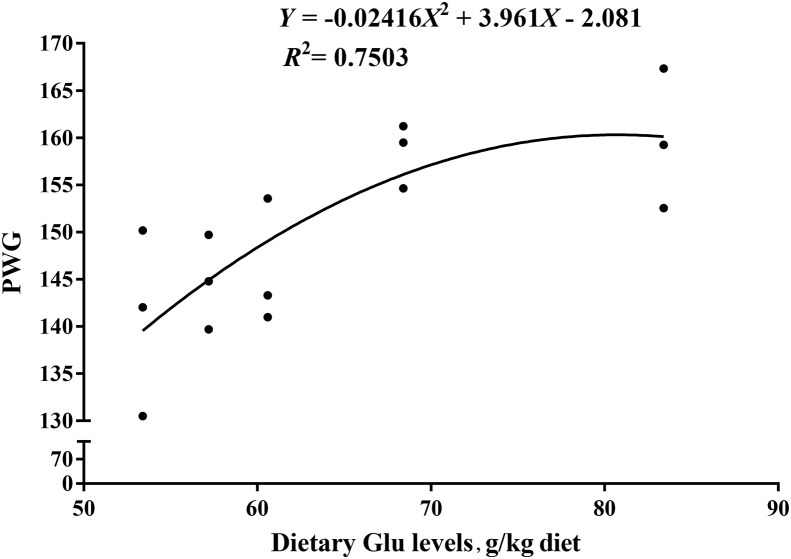
Dietary Glu did not have a significant effect on the survival rate of Jian carp (Table 4). No pathological signs were observed during the trail. Effect of graded levels of dietary Glu on growth parameters are presented in Table 4. The final body weight (FBW) and percent weight gain (PWG) were improved with increasing dietary Glu levels up to 83.4 g/kg diet (P < 0.05). The feed intake (FI) of fish was the highest for fish fed 83.4 g of Glu/kg diet (P < 0.05). The feed conversion ratio (FCR) was the highest for fish fed control diet, and significantly decreased with increasing dietary Glu levels up to 68.4 g/kg diet (P < 0.05). Body composition and net nutrient deposition of fish fed diets with graded levels of Glu are presented in Table 5. The body moisture of 57.2, 60.6, and 68.4 g of Glu/kg groups was significantly lower than that of the other groups (P < 0.05). Fish body crude protein and crude lipid contents, and lipid production value (LPV) were improved with increasing levels of dietary Glu up to 68.4 g/kg diet and depressed thereafter (P < 0.05). The ash content was significantly higher for fish fed Glu supplemented diets compared with control diet (P < 0.05). The protein production value (PPV) and ash production value (APV) were the highest for fish fed the diet with 68.4 and 83.4 g of Glu/kg diet, respectively (P < 0.05). Based on the quadratic regression analysis of PWG, the optimal dietary Glu levels were estimated to be 81.97 g/kg diet (Fig. 1).
Table 4.
The IBW, FBW, survival, FI, PWG, and FCR of carp fed diets with graded levels of L-glutamate for 9 weeks1.
| Item | Dietary L-glutamate levels, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| IBW, g/fish | 126.33 ± 0.17 | 126.33 ± 0.17 | 125.50 ± 0.00 | 126.33 ± 0.17 | 125.50 ± 0.00 |
| FBW, g/fish | 306.04 ± 8.97a | 309.17 ± 3.31ab | 311.12 ± 4.89abc | 326.49 ± 2.42bc | 328.52 ± 5.40c |
| Survival rate, % | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| FI, g/fish | 263.53 ± 3.01b | 248.51 ± 4.12a | 251.36 ± 4.21a | 257.15 ± 2.40ab | 278.36 ± 2.43c |
| PWG2 | 142.23 ± 6.83a | 144.74 ± 2.90a | 145.95 ± 3.86ab | 158.44 ± 1.97b | 159.70 ± 4.27b |
| FCR3 | 1.48 ± 0.09b | 1.36 ± 0.01ab | 1.36 ± 0.01ab | 1.28 ± 0.01a | 1.38 ± 0.03ab |
| Regression | |||
| YFBW = −0.03021X2 + 4.971X + 124.60 | R2 = 0.7566 | P = 0.07 | |
| YPWG = −0.02416X2 + 3.961X − 2.081 | R2 = 0.7503 | P = 0.079 | |
| YFCR = 0.000639X2 − 0.09038X + 4.467 | R2 = 0.7088 | P = 0.077 | |
IBW = initial body weight; FBW = final body weight; FI = feed intake; PWG = percent weight gain; FCR = feed conversion ratio.
a-c Mean values within the same row with different superscripts are significantly different (P < 0.05).
Values are means ± SE.
PWG = Weight gain (g)/Initial weight (g) × 100.
FCR = Feed intake (g)/Wet weight gain (g).
Table 5.
Body composition (% as wet tissue), PPV, LPV and APV of carp fed diets with graded levels of L-glutamate for 9 weeks1.
| Item | Dietary L-glutamate levels, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| Moisture | 73.83 ± 1.27b | 66.81 ± 1.29a | 66.54 ± 0.27a | 64.30 ± 0.32a | 71.84 ± 0.83b |
| Crude protein | 15.05 ± 0.11a | 17.24 ± 0.24c | 17.31 ± 0.07cd | 18.07 ± 0.35d | 16.29 ± 0.31b |
| Crude lipid | 7.95 ± 0.53a | 8.94 ± 0.24ab | 9.01 ± 0.28ab | 10.1 ± 0.08b | 8.14 ± 0.46a |
| Ash | 2.83 ± 0.04a | 3.08 ± 0.10b | 3.18 ± 0.04b | 3.21 ± 0.03b | 3.06 ± 0.08b |
| PPV2 | 34.28 ± 1.27a | 37.26 ± 1.04ab | 36.96 ± 0.42ab | 40.16 ± 1.61b | 37.24 ± 0.88ab |
| LPV3 | 124.30 ± 12.7a | 130.81 ± 2.91a | 131.60 ± 5.15a | 156.65 ± 1.82b | 128.34 ± 8.10a |
| APV4 | 25.91 ± 1.02a | 25.88 ± 1.08a | 26.96 ± 0.72a | 28.78 ± 0.22ab | 30.29 ± 1.04b |
| Regression | |||
| YMoisture = 0.03847X2 − 5.286X + 245.30 | R2 = 0.8734 | P = 0.089 | |
| YCrude protein = −0.01087X2 + 1.515X − 34.46 | R2 = 0.8590 | P = 0.110 | |
| YCrude lipid = −0.008351X2 + 1.151X − 29.72 | R2 = 0.8208 | P = 0.06 | |
| YAsh = −0.001326X2 + 0.1871X − 3.333 | R2 = 0.7771 | P = 0.104 | |
| YPPV = −0.01749X2 + 2.489X − 48.66 | R2 = 0.7259 | P = 0.10 | |
| YLPV = −0.1106X2 + 15.45X − 389.60 | R2 = 0.6080 | P = 0.228 | |
| YAPV = −0.002347X2 + 0.4815X + 6.515 | R2 = 0.8236 | P < 0.05 | |
PPV = protein production value; LPV = lipid production value; APV = ash production value.
a-d Mean values within the same row with different superscripts are significantly different (P < 0.05).
Values are means ± SE.
PPV = Fish protein gain (g)/Total protein intake (g) × 100.
LPV = Fish lipid gain (g)/Total lipid intake (g) × 100.
APV = Fish ash gain (g)/Total ash intake (g) × 100.
Fig. 1.
The quadratic regression analysis of percent weight gain (PWG) of Jian carp fed diets containing graded levels of L-glutamate (Glu). Values are means of 3 replicates, with 40 fish in each replicate.
The effects of dietary Glu on intestine somatic index (ISI), hepatosomatic index (HSI), relative gut length (RGL), and hepatopancreas protein (HPC) and intestinal protein contents (IPC) are shown in Table 6. The HPC significantly increased and obtained maximum values when Glu level was at 68.4 g/kg diet (P < 0.05). The ISI and IPC also showed similar trends. Fish fed diets containing 57.2, 60.6, and 68.4 g of Glu/kg diet had significantly higher RGL than those fed other diets (P < 0.05).
Table 6.
The HSI, HPC, ISI, RGL and IPC of carp fed diets with graded levels of L-glutamate for 9 weeks (%)1.
| Item | Dietary L-glutamate levels, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| Hepatopancreas | |||||
| HIS2 | 2.79 ± 0.15 | 2.89 ± 0.11 | 3.10 ± 0.16 | 2.73 ± 0.14 | 2.74 ± 0.23 |
| HPC3, % | 7.27 ± 0.88a | 9.74 ± 0.57ab | 9.60 ± 1.14ab | 10.99 ± 0.50b | 10.28 ± 0.88ab |
| Intestine | |||||
| ISI4 | 2.42 ± 0.11a | 2.67 ± 0.04b | 2.55 ± 0.03ab | 2.63 ± 0.07b | 2.61 ± 0.07b |
| RGL5 | 167.42 ± 3.00a | 196.63 ± 7.72b | 193.73 ± 4.15b | 188.19 ± 5.73b | 184.24 ± 6.58ab |
| IPC6, % | 4.25 ± 0.10a | 4.30 ± 0.31a | 4.36 ± 0.29ab | 6.10 ± 0.25c | 5.27 ± 0.25bc |
HSI = hepatosomatic index; HPC = hepatopancreas protein content; ISI = intestine somatic index; RGL = relative gut length; IPC = intestinal protein content.
a-c Mean values within the same row with different superscripts are significantly different (P < 0.05).
Values are means ± SE.
HSI = 100 × Wet hepatopancreas weight (g)/Wet body weight (g).
HPC = 100 × Hepatopancreatic protein (g)/Wet hepatopancreas weight (g).
ISI = 100 × Wet intestine weight (g)/Wet body weight (g).
RGL = 100 × Intestine length (cm)/Total body length (cm).
IPC = 100 × Intestinal protein (g)/Wet intestine weight (g).
The effects of dietary Glu on trypsin, chymotrypsin, lipase and amylase are presented in Table 7. Trypsin and chymotrypsin activities in the hepatopancreas were the highest for fish fed 68.4 g of Glu/kg diet (P < 0.05). Lipase activity in the hepatopancreas was the lowest for fish fed the control diet, and the highest for fish fed diet supplemented with 83.4 g/kg Glu (P < 0.05). Amylase activity in the hepatopancreas was significantly improved with increasing dietary Glu levels up to 60.6 g/kg diet and decreased thereafter (P < 0.05). The activities of trypsin and lipase in the intestine increased with increasing dietary Glu levels in up to 68.4 and 60.6 g/kg diets, respectively, and decreased thereafter (P < 0.05). Fish fed diets containing 68.4 and 83.4 g of Glu/kg diets had higher level of chymotrypsin activity in the intestine than those fed other levels diets (P < 0.05). Amylase activity in the intestine was the highest for fish fed 60.6 g of Glu/kg diet (P < 0.05).
Table 7.
The activities (U/g tissue) of trypsin, chymotrypsin, lipase, and amylase in the hepatopancreas and whole intestine of carp fed diets with graded levels of L-glutamate for 9 weeks1.
| Item | Dietary L-glutamate levels, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| Hepatopancreas | |||||
| Trypsin | 395.04 ± 7.61a | 581.17 ± 3.44b | 561.78 ± 9.67b | 695.05 ± 5.12d | 620.50 ± 11.3c |
| Chymotrypsin | 520.35 ± 47.20a | 740.59 ± 10.33b | 649.78 ± 21.19b | 898.72 ± 30.95c | 752.20 ± 35.50b |
| Lipase | 1.32 ± 0.16a | 2.51 ± 0.17bc | 1.87 ± 0.24ab | 2.50 ± 0.23bc | 3.19 ± 0.55c |
| Amylase | 2,001.53 ± 58.72a | 2,494.38 ± 38.63bc | 2,516.04 ± 42.86c | 2,142.84 ± 119.45ab | 2,242.13 ± 99.48abc |
| Intestine | |||||
| Trypsin | 25.24 ± 3.77a | 27.39 ± 2.41ab | 29.50 ± 2.42abc | 38.66 ± 0.93c | 36.03 ± 1.81bc |
| Chymotrypsin | 102.82 ± 1.03a | 96.34 ± 1.00a | 102.23 ± 5.85a | 124.41 ± 5.75b | 120.83 ± 4.09b |
| Lipase | 1.06 ± 0.02a | 1.45 ± 0.16ab | 2.90 ± 0.60c | 2.43 ± 0.33bc | 2.07 ± 0.20abc |
| Amylase | 419.39 ± 3.04b | 399.67 ± 20.81b | 518.15 ± 20.12c | 439.57 ± 11.94b | 324.98 ± 19.76a |
| Regression | |||
| YHpeatopancreas trypsin = −0.7840X2 + 113.9X − 3,428 | R2 = 0.8904 | P = 0.106 | |
| YHpeatopancreas chymotrypsin = −0.9685X2 − 139.9X − 4,173 | R2 = 0.7433 | P = 0.225 | |
| YHpeatopancreas lipase = −0.0008520X2 + 0.1681X − 4.931 | R2 = 0.6358 | P = 0.266 | |
| YIntestine trypsin = −0.02936X2 + 4.435X − 129.30 | R2 = 0.7925 | P = 0.087 | |
| YIntestine chymotrypsin = −0.03535X2 + 5.704X − 107.70 | R2 = 0.6516 | P = 0.288 | |
| YIntestine lipase = −0.005404X2 + 0.7691X − 24.54 | R2 = 0.5863 | P = 0.318 | |
| YIntestine amylase = −0.4199X2 + 54.39X − 1,294 | R2 = 0.6691 | P = 0.299 | |
a-d Mean values within the same row with different superscripts are significantly different (P < 0.05).
Values are means ± SE.
The brush border enzyme activities in proximal intestine (PI), mid intestine (MI) and distal intestine (DI) for Jian carp fed graded levels of Glu are presented in Table 8. The AKP activity in all intestinal segments was the highest for fish fed 83.4 g of Glu/kg diet (P < 0.05). The NKA activity in PI and DI was the highest for fish fed 68.4 g of Glu/kg diet (P < 0.05), and the lowest for fish fed control diet. The activity of NKA in MI was increased with increasing Glu levels up to 68.4 g/kg diet and decreased thereafter (P < 0.05). The γ-GT activity in MI was not different for fish fed diets containing ≤68.4 g of Glu/kg diet (P > 0.05), and was significantly increased in diet containing 83.4 g of Glu/kg diet (P < 0.05). The γ-GT activity in DI was the highest for fish fed 83.4 g of Glu/kg diet and the lowest for fish fed control diet (P < 0.05). The CK activity in PI, MI, and DI was significantly improved with increasing dietary Glu levels up to 68.4 g/kg diet (P < 0.05), where the response reached a plateau (P > 0.05).
Table 8.
The activities of AKP, NKA, γ-GT and CK in PI, MI, DI of carp fed diets with graded levels of L-glutamate for 9 weeks1.
| Item | Dietary L-glutamate levels, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| AKP, U/g tissue | |||||
| PI | 4.33 ± 0.26a | 3.89 ± 1.02a | 4.41 ± 0.44a | 5.68 ± 0.51ab | 8.22 ± 0.97b |
| MI | 2.72 ± 0.07a | 3.91 ± 0.73ab | 3.71 ± 0.48ab | 5.96 ± 0.92b | 5.97 ± 0.28b |
| DI | 1.47 ± 0.15a | 2.01 ± 0.41ab | 2.15 ± 0.04ab | 2.23 ± 0.10ab | 2.54 ± 0.45b |
| NKA, μmol of phosphorus released/g tissue per h | |||||
| PI | 160.21 ± 0.88a | 193.45 ± 12.6bc | 170.23 ± 2.20ab | 215.93 ± 4.13bc | 157.36 ± 6.21a |
| MI | 132.27 ± 2.13a | 160.47 ± 2.76b | 182.67 ± 2.83c | 185.44 ± 5.25c | 127.47 ± 0.80a |
| DI | 128.74 ± 5.93a | 174.46 ± 1.57c | 147.67 ± 0.75ab | 195.78 ± 2.89d | 156.49 ± 4.84bc |
| γ-GT, U/g tissue | |||||
| PI | 0.93 ± 0.00 | 0.97 ± 0.14 | 0.93 ± 0.10 | 0.96 ± 0.12 | 1.07 ± 0.26 |
| MI | 0.93 ± 0.16a | 1.13 ± 0.30a | 1.23 ± 0.10ab | 1.30 ± 0.11ab | 1.78 ± 0.24b |
| DI | 1.72 ± 0.03a | 2.02 ± 0.09ab | 1.78 ± 0.05a | 2.39 ± 0.15b | 2.52 ± 0.35b |
| CK, U/g tissue | |||||
| PI | 22.08 ± 1.74a | 23.37 ± 1.99a | 23.99 ± 1.03a | 35.20 ± 2.02b | 39.58 ± 2.04b |
| MI | 21.72 ± 1.23a | 22.73 ± 0.48a | 24.51 ± 0.78ab | 27.70 ± 1.25b | 27.79 ± 1.31b |
| DI | 14.97 ± 0.55a | 17.86 ± 0.79b | 17.96 ± 0.40b | 18.94 ± 0.86b | 17.96 ± 0.73b |
| Regression | |||
| YPI AKP = 0.003225X2 − 0.3003X + 10.88 | R2 = 0.8603 | P < 0.05 | |
| YMI AKP = −0.005623X2 + 0.8821X − 28.43 | R2 = 0.8124 | P = 0.079 | |
| YDI AKP = −0.001373X2 + 0.2180X − 6.118 | R2 = 0.6144 | P = 0.117 | |
| YPI NKA = −0.2034X2 + 27.79X − 744.10 | R2 = 0.6308 | P = 0.332 | |
| YMI NKA = −0.2640X2 + 35.84X − 1026 | R2 = 0.9654 | P < 0.05 | |
| YDI NKA = −0.1914X2 + 27.01X − 764 | R2 = 0.6240 | P = 0.365 | |
| YDI γ-GT = −0.0006641X2 + 0.1183X − 2.71 | R2 = 0.6853 | P = 0.175 | |
| YPI CK = −0.01106X2 + 2.164X − 63.58 | R2 = 0.8905 | P = 0.072 | |
| YMI CK = −0.01193X2 + 1.853X − 43.67 | R2 = 0.8691 | P < 0.05 | |
| YDI CK = −0.01156X2 + 1.659X − 40.11 | R2 = 0.7352 | P = 0.137 | |
AKP = alkaline phosphatase; NKA = Na+, K+-ATPase; γ-GT = γ-glutamyl transpeptidase; CK = creatine kinase; PI = proximal intestine; MI = mid intestine; DI = distal intestine.
a-d Mean values within the same row with different superscripts are significantly different (P < 0.05).
Values are means ± SE.
The effect of dietary Glu on antioxidant parameters in the hepatopancreas and intestine are displayed in Table 9. The contents of MDA in the hepatopancreas and intestine, and PCO in the intestine were significantly decreased with the increased dietary Glu levels up to 68.4 g/kg diet, and then increased with further increase of dietary Glu levels (P < 0.05). The PCO content in the hepatopancreas was reduced in fish fed diets supplemented with Glu (P < 0.05). The activities of CAT in the hepatopancreas, GST in the hepatopancreas, and HRS in the intestine were significantly increased with the increased dietary Glu levels up to 68.4 g/kg diet (P < 0.05), and plateaued thereafter (P > 0.05). The activities of GPx in the hepatopancreas and intestine, GST in the intestine and SAS in the intestine were significantly improved with increasing dietary Glu levels up to 68.4 and 60.6 g/kg diet, and decreased thereafter (P < 0.05). The GR activity and GSH content in the intestine were the highest for fish fed the diet with 83.4 and 60.6 g of Glu/kg diet, respectively (P < 0.05), and the lowest for fish fed control diet (P < 0.05). The HRS activity in the hepatopancreas was significantly increased in response to diet with 57.2 g of Glu/kg diet (P < 0.05). According to a quadratic regression analysis model run on MDA content in the hepatopancreas
| YHepatopancreas = 0.016 X2 – 2.274X + 85.791, R2 = 0.914, P < 0.05 |
and the intestine
| YIntestine = 0.014 X2 – 1.998X + 78.75, R2 = 0.943, P < 0.05, |
the optimal dietary Glu level was estimated to be 71.06 (22.5% of crude protein) and 71.36 g/kg diet (22.6% of crude protein).
Table 9.
The activities of MDA, PCO, SOD, CAT, GPx, GST, GR, GSH, SAS, HRS in the hepatopancreas and whole intestine of carp fed diets with graded levels of L-glutamate for 9 weeks1.
| Item | Dietary L-glutamate levels, g/kg diet |
||||
|---|---|---|---|---|---|
| 53.4 | 57.2 | 60.6 | 68.4 | 83.4 | |
| Hepatopancreas | |||||
| MDA, nmol/mg protein | 11.52 ± 0.09c | 9.36 ± 0.26b | 9.03 ± 0.11b | 6.81 ± 0.25a | 9.88 ± 0.93b |
| PCO, nmol/mg protein | 4.76 ± 0.30b | 2.78 ± 0.04a | 2.65 ± 0.39a | 2.68 ± 0.24a | 3.10 ± 0.42a |
| SOD, U/mg protein | 563.00 ± 1.79 | 560.12 ± 15.4 | 581.75 ± 5.96 | 582.13 ± 8.77 | 556.88 ± 7.93 |
| CAT, U/mg protein | 51.23 ± 3.47a | 59.23 ± 4.04ab | 65.92 ± 3.76bc | 76.41 ± 1.53d | 73.84 ± 0.86cd |
| GPx, U/mg protein | 4,280.54 ± 7.01ab | 4,360.52 ± 50.05b | 4,650.92 ± 26.93c | 4,708.27 ± 42.00c | 4,204.55 ± 11.85a |
| GST, U/mg protein | 66.72 ± 2.36a | 70.77 ± 4.62ab | 80.23 ± 1.01bc | 82.42 ± 2.49c | 79.40 ± 0.53bc |
| GR, U/g protein | 56.91 ± 0.16 | 61.70 ± 2.73 | 63.23 ± 0.05 | 63.98 ± 3.00 | 58.90 ± 2.57 |
| GSH, μmol/g protein | 31.96 ± 0.02 | 31.09 ± 1.44 | 33.73 ± 2.22 | 34.78 ± 1.06 | 32.38 ± 1.59 |
| SAS, U/g protein | 3.38 ± 0.01 | 3.49 ± 0.35 | 3.93 ± 0.16 | 3.62 ± 0.16 | 3.56 ± 0.26 |
| HRS, U/mg protein | 613.87 ± 10.31a | 709.42 ± 44.05b | 699.34 ± 4.85ab | 680.39 ± 16.68ab | 635.89 ± 12.25ab |
| Intestine | |||||
| MDA, nmol/mg protein | 11.64 ± 1.04c | 9.13 ± 0.00b | 8.67 ± 0.17b | 6.63 ± 0.22a | 8.09 ± 0.52ab |
| PCO, nmol/mg protein | 3.20 ± 0.14b | 2.57 ± 0.13a | 2.59 ± 0.11a | 2.48 ± 0.02a | 3.07 ± 0.06b |
| SOD, U/mg protein | 282.06 ± 5.46 | 295.86 ± 2.90 | 300.74 ± 3.84 | 300.77 ± 8.87 | 286.37 ± 11.94 |
| CAT, U/mg protein | 2.01 ± 0.03a | 2.85 ± 0.19b | 4.14 ± 0.07c | 4.41 ± 0.06c | 4.30 ± 0.15c |
| GPx, U/mg protein | 3,324.10 ± 99.56a | 3,409.41 ± 31.67ab | 3,563.85 ± 26.08b | 3,622.51 ± 60.46b | 3,248.96 ± 97.05a |
| GST, U/mg protein | 61.86 ± 0.31a | 67.29 ± 1.19b | 67.72 ± 0.30b | 69.87 ± 1.93b | 63.00 ± 0.10a |
| GR, U/g protein | 26.02 ± 0.73a | 44.75 ± 1.70b | 39.59 ± 1.72b | 45.69 ± 1.32b | 45.78 ± 2.83b |
| GSH, μmol/g protein | 17.21 ± 0.09a | 26.44 ± 1.70ab | 35.94 ± 2.74b | 34.60 ± 4.62b | 29.34 ± 0.37b |
| SAS, U/g protein | 14.06 ± 0.08a | 17.70 ± 0.86b | 17.92 ± 0.23b | 15.38 ± 0.39ab | 14.73 ± 0.73a |
| HRS, U/mg protein | 944.44 ± 4.67a | 997.33 ± 10.03ab | 1,025.80 ± 8.83b | 1,099.33 ± 8.40c | 1,085.20 ± 17.35c |
| Regression | |||
| YHpeatopancreas MDA = 0.016X2 − 2.274X + 85.791 | R2 = 0.914 | P < 0.05 | |
| YHpeatopancreas PCO = 0.00667X2 − 0.9472X + 35.79 | R2 = 0.6262 | P = 0.311 | |
| YHpeatopancreas CAT = −0.06043X2 + 9.038X − 259.6 | R2 = 0.9325 | P < 0.05 | |
| YHpeatopancreas GPX = −2.163X2 + 294.7X − 5,324 | R2 = 0.9162 | P = .068 | |
| YHpeatopancreas GST = −0.04764X2 + 6.942X − 168.3 | R2 = 0.8219 | P = 0.076 | |
| YIntestine MDA = 0.014X2 − 1.988X + 78.75 | R2 = 0.943 | P < 0.05 | |
| YIntestine PCO = 0.03029X2 − 0.4138X + 16.53 | R2 = 0.7863 | P = 0.15 | |
| YIntestine CAT = −0.00664X2 + 0.9811X − 31.38 | R2 = 0.9245 | P = 0.066 | |
| YIntestine GPx = −1.521X2 + 206.2X − 3,367 | R2 = 0.8365 | P < 0.05 | |
| YIntestine GST = −0.03305X2 + 4.532X − 85.18 | R2 = 0.8740 | P = 0.058 | |
| YIntestine GR = −0.04461X2 + 6.594X − 194.40 | R2 = 0.6523 | P = 0.323 | |
| YIntestine GSH = −0.06204X2 + 8.814X − 274.80 | R2 = 0.7774 | P = 0.149 | |
| YIntestine HRS = −0.3534X2 + 53.15X − 888.30 | R2 = 0.9698 | P < 0.01 | |
MDA = malondialdehyde; PCO = protein carbonyl; SOD = superoxide dismutase; CAT = catalase; GPx = glutathione peroxidase; GST = glutathione S-transferase; GR = glutathione reductase; GSH = glutathione; SAS = superoxide anion scavenging ability; HRS = hydroxyl radical scavenging ability.
a-d Mean values within the same row with different superscripts are significantly different (P < 0.05).
Values are means ± SE.
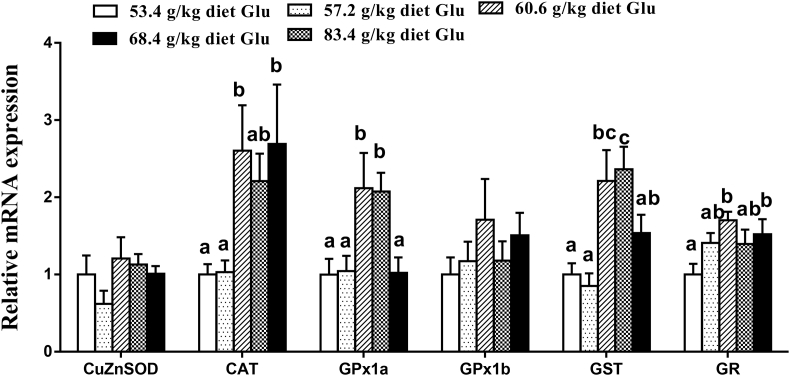
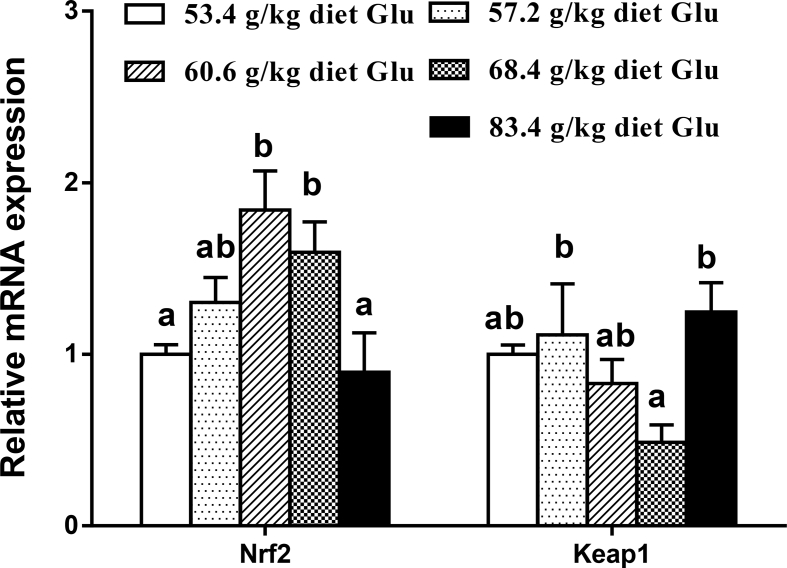
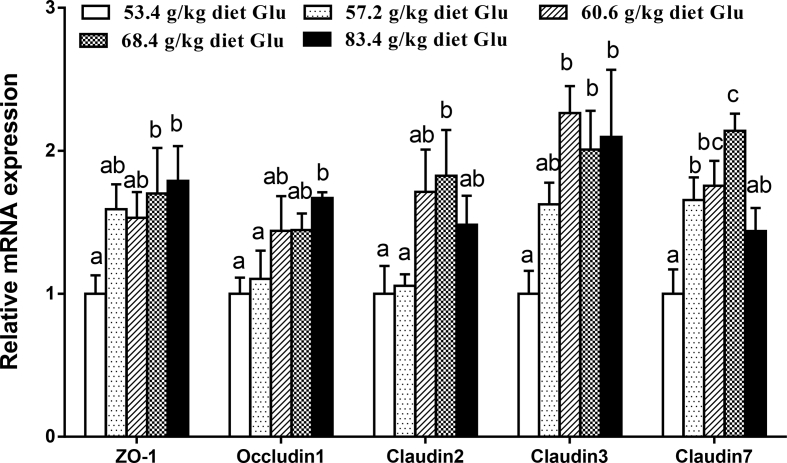
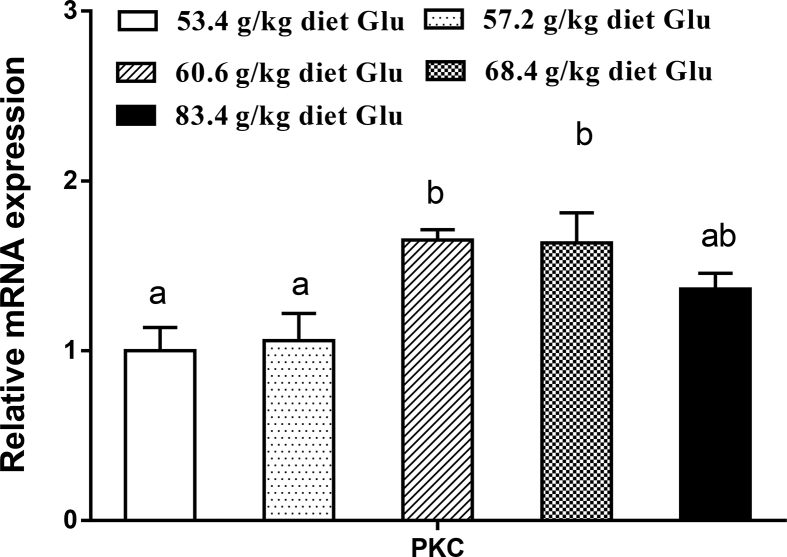
The gene expressions of antioxidant enzymes, Nrf2 and Keap1 in the intestine are displayed in Fig. 2, Fig. 3. The CAT and GR mRNA levels increased with increasing dietary Glu levels up to 60.6 g/kg diet (P < 0.05) and plateaued thereafter (P > 0.05). The GPx1a and GST mRNA levels gradually increased with increasing Glu levels up to 68.4 g/kg diet, and then decreased (P < 0.05). No significant differences were found in CuZnSOD and GPx1b mRNA expressions among groups (P > 0.05). The Nrf2 mRNA level was the highest for fish fed 60.6 g of Glu/kg diet (P < 0.05). The Keap1 mRNA level showed an inverse trend with respect to Nrf2 mRNA expression. As shown in Fig. 4, Fig. 5, ZO-1, occludin2, claudin3, and PKC mRNA levels increased with increasing dietary Glu levels up to 68.4 g/kg diet (P < 0.05) and plateaued thereafter (P > 0.05). Occludin1 mRNA level increased with increasing dietary Glu levels up to 83.4 g/kg diet (P < 0.05). Claudin7 mRNA level gradually increased with increasing Glu levels up to 68.4 g/kg diet, and then significantly decreased (P < 0.05).
Fig. 2.
Effects of dietary L-glutamate (Glu) on Cu/Zn superoxide dismutase (CuZnSOD), catalase (CAT), glutathione peroxidase 1a (GPx1a), glutathione peroxidase 1b (GPx1b), glutathione S-transferase (GST), and glutathione reductase (GR) gene expressions in the intestine of Jian carp. Values are means ± SE of 3 replicates with 6 fish in each replicate. Data columns with different letters denote significant difference (P < 0.05).
Fig. 3.
Effects of dietary L-glutamate (Glu) on nuclear factor erythroid 2-related factor 2 (Nrf2) and Keap1 gene expressions in the intestine of Jian carp. Values are means ± SE of 3 replicates with 6 fish in each replicate. Data column with different letters denote significant difference (P < 0.05).
Fig. 4.
Effects of dietary L-glutamate (Glu) on zonula occludens protein-1 (ZO-1), occludin1, claudin2, claudin3, and claudin7 gene expressions in the intestine of Jian carp. Values are means ± SE of 3 replicates with 6 fish in each replicate. Data columns with different letters denote significant difference (P < 0.05).
Fig. 5.
Effects of dietary L-glutamate (Glu) on protein kinase C (PKC) gene expressions in the intestine of Jian carp. Values are means ± SE of 3 replicates with 6 fish in each replicate. Data columns with different letters denote significant difference (P < 0.05).
Correlation analysis (Table 10) demonstrated that Glu was positively correlated with intestinal trypsin (r = 0.810, P = 0.096), AKP (rPI = 0.971, P < 0.01; rMI = 0.880, P < 0.05; rDI = 0.874, P = 0.053), γ-GT (rPI = 0.895, P < 0.05; rMI = 0.982, P < 0.01; rDI = 0.889, P < 0.05), and CK (rPI = 0.954, P < 0.05; rMI = 0.892, P < 0.05) activities. Secondly, weight gain appeared positive correlations with AKP (rPI = 0.913, P < 0.05; rMI = 0.960, P < 0.05; rDI = 0.817, P = 0.091), γ-GT (rPI = 0.756, P = 0.139; rMI = 0.878, P = 0.050; rDI = 0.939, P < 0.05), and CK (rPI = 0.996, P < 0.01; rMI = 0.968, P < 0.01). Thirdly, intestinal MDA was negatively correlated with intestinal trypsin (r = −0.897, P < 0.05), AKP (rMI = −0.881, P < 0.05; rPI = −0.840, P = 0.075), and CK (rMI = −0.870, P = 0.054) activities.
Table 10.
Correlation coefficient of some parameters.
| Independent parameters | Dependent parameters | Correlation coefficients | P-value |
|---|---|---|---|
| L-glutamate | Intestine trypsin | 0.810 | 0.096 |
| PI AKP | 0.971 | <0.01 | |
| MI AKP | 0.880 | <0.05 | |
| DI AKP | 0.874 | 0.053 | |
| PI γ-GT | 0.895 | <0.05 | |
| MI γ-GT | 0.982 | <0.01 | |
| DI γ-GT | 0.889 | <0.05 | |
| PI CK | 0.954 | <0.05 | |
| MI CK | 0.892 | <0.05 | |
| Intestine MDA | Intestine trypsin | −0.897 | <0.05 |
| MI AKP | −0.881 | <0.05 | |
| PI AKP | −0.840 | 0.075 | |
| MI CK | −0.870 | 0.054 | |
| Hepatopancreas MDA | Hepatopancreas trypsin | −0.886 | <0.05 |
| Hepatopancreas chymotrypsin | −0.895 | <0.05 | |
| Intestine trypsin | WG | 0.945 | <0.05 |
| Intestine chymotrypsin | WG | 0.942 | <0.05 |
| PI AKP | WG | 0.913 | <0.05 |
| MI AKP | WG | 0.960 | <0.05 |
| DI AKP | WG | 0.817 | 0.091 |
| PI γ-GT | WG | 0.756 | 0.139 |
| MI γ-GT | WG | 0.878 | 0.050 |
| DI γ-GT | WG | 0.939 | <0.05 |
| PI CK | WG | 0.996 | <0.01 |
| MI CK | WG | 0.968 | <0.01 |
PI = proximal intestine; AKP = alkaline phosphatase; MI = mid intestine; DI = distal intestine; γ-GT = γ-glutamyl transpeptidase; CK = creatine kinase; MDA = malondialdehyde; WG = weight gain.
4. Discussion
4.1. Effect of dietary glu on growth performance
In the present study, the growth performance of Jian carp was significantly influenced by dietary Glu levels. The FBW and PWG were improved in diet supplemented with 68.4 and 83.4 of Glu g/kg, which was in agreement with reports for grass carp (Zhao et al., 2015), Atlantic salmon (Oehme et al., 2010), and rainbow trout (Chika et al., 2016). The enhancement of fish growth may be attributed to the fact that feed efficiency was improved with appropriate dietary Glu. There is growing evidence that some of the traditionally classified nonessential amino acids (NEAA, e.g. glycine) are important regulators of key metabolic pathways, which play enormous roles in multiple signaling pathways, thereby regulating gene expression, intracellular protein turnover, nutrient metabolism, and oxidative defense (Xiao et al., 2014, Xie et al., 2016). In order to avoid the influence of other NEAA, all diets in the present study were not maintained isonitrogenous by supplementation of other NEAA as the study on glycine by Xie et al. (2014). Although a higher protein level was found in diet supplemented with Glu at 83.4 g/kg. However, a better growth performance is observed in diet supplemented with 81.97 g of Glu/kg. Therefore, a high level of Glu may result in adverse effects on growth performance. Fish weight gain is primarily attributed to the accretion of protein and fat (Bureau et al., 2000). The present study showed that Glu significantly enhanced carp body protein and lipid contents, and dietary protein and lipids unitization, which were in accordance with the results for grass carp (Zhao et al., 2015), gilthead seabream (Caballero-Solares et al., 2015), and rainbow trout (Chika et al., 2016). However dietary Glu supplementation has no effect on growth and feed utilization in Atlantic salmon S. salar (Larsson et al., 2014). One of reasons for this difference is that the ratio of dietary Glu to fat level is too low, which are both the main energy source of fish. Recent study indicates Glu contributes the major tissues of approximately 80% of ATP production in the liver, proximal intestine, and skeletal muscle of zebrafish (Danio rerio) and hybrid striped bass (Morone saxatilis ♀ + Morone chrysops ♂) (Jia et al., 2017). In grass carp (Zhao et al., 2015), gilthead seabream (Caballero-Solares et al., 2015), and rainbow trout (Chika et al., 2016), the ratios of dietary Glu to protein and fat level are 18.8% and 143.2%, 17.8% and 52.4%, and 23.3% and 74.5%, respectively. In Atlantic salmon, the ratio of dietary Glu to protein is 17.9%, but the ratio of dietary Glu to fat level only is 18% (Larsson et al., 2014). Therefore, it is needed to further study on the appropriate proportions of dietary fat and Glu. The present result also firstly showed the optimal dietary Glu level was 81.97 g/kg diet (116.5% of dietary crude lipid and 26.4% of dietary crude protein) for Jian carp growth.
4.2. Effect of dietary glu on digestive and absorptive capacity
Fish growth is related to the digestive and absorptive capacity (Gisbert et al., 2009), which can be reflected by digestive organ growth and development, as well as activities of intestinal enzymes related to digestion and absorption (Yan and Qiu-Zhou, 2006). In the present study, there were significant improvements in HPC, ISI, HSI, and RGL. These results indicated that Glu promoted the growth and development of the fish intestine and hepatopancreas. Intestinal growth and development are related with the intestinal cell proliferation and differentiation. In parenteral mice, Glu could prevent intestinal mucosal atrophy via promotion of intestine epithelial cell proliferation (Xiao et al., 2014). The previous studies showed Glu could improve the proliferation, differentiation, and function of fish enterocytes (Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c), and promote intestinal nucleotide synthesis and cell-proliferation in rainbow trout (Chika et al., 2016). Meanwhile, the activities of trypsin, chymotrypsin, amylase, and lipase in whole intestine, as well as trypsin, chymotrypsin, and lipase in the hepatopancreas were improved by dietary appropriate Glu levels. All these data above suggested that Glu enhanced the digestive capacity of Jian carp, which agreed with our previous study of grass carp (Zhao et al., 2015). Intestinal absorptive ability is also the basis of utilizing nutrient adequately (Wen et al., 2009). The enzymes located in the intestinal brush border section are responsible for the final stages of degradation and assimilation of the food. The AKP is considered to be involved in absorption of nutrients such as lipid, glucose, calcium, and inorganic phosphatase (Tengjaroenkul et al., 2000). The NKA (Gal-Garber et al., 2003, Geering, 1990) and γ-GT (Griffith and Meister, 1980, Ogawa et al., 1998) play a crucial role in absorption of most of amino acids and glucose. The CK has a key role in the energy metabolism of cells (Wallimann and Hemmer, 1994). In the present study, NKA and CK activities in all intestine segments, AKP activity in MI, γ-GT activity in DI significantly increased with increasing dietary Glu levels. Correlation analysis showed activities of intestinal brush-border enzymes was positively related to dietary Glu level. These results suggested that dietary Glu could improve the absorption ability in fish. Similarly, dietary Glu improved the activities of brush-border enzymes in grass carp (Zhao et al., 2015).
4.3. Effect of dietary glu on antioxidant capacity in the intestine
The structural integrity of fish intestines is the guarantee of its normal digestion and absorption. The structural and functional integrity of the intestine and enterocytes was closely related to the antioxidant enzyme activities (Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c, Jiang et al., 2013, Shoveller et al., 2005). The MDA is a by-product of lipid peroxidation induced by excessive reactive oxygen species (ROS) and a good marker of lipid peroxidation (Mourente et al., 2007). The PCO is one of the most extensively studied forms of proteins oxidative modification (Wang and Powell, 2010). The contents of MDA and PCO can reflect the antioxidant status of living organisms (Ghosh et al., 2008). In the present study, the contents of MDA and PCO were decreased with increasing dietary Glu levels up to certain values in both the intestine and hepatopancreas, suggesting depressions of the lipid peroxidation and protein oxidation. The Glu is a key transamination partner and is required for the synthesis of GSH in the intestine (Johnson et al., 2003). Previous study reported a strong negative correlation between GSH content and MDA level in the intestinal mucosa of weaned piglets (Wang and Li, 2012). The Glu ameliorated copper induced MDA generation in fish intestines (Jiang et al., 2016). Quadratic regression analysis against MDA content in the hepatopancreas and whole intestine showed the optimal dietary Glu level was 71.06 and 71.36 g/kg diet, respectively. Correlation analysis showed PWG was positively related to activities of trypsin and chymotrypsin in the intestine. Activities of trypsin and chymotrypsin were positively related to MDA contents in the hepatopancreas and intestine. However, there was no significant correlation between Glu and activities of trypsin and chymotrypsin. These results indicated Glu might improve the digestive ability by reducing oxidative damage in the hepatopancreas and increasing the ability of the pancreas to synthesize and secrete protease. The specific mechanism needs a further study.
In terrestrial animals, SOD, CAT, GPx, GST, GR, and GSH play a vital role in preventing cellular damage caused by ROS (David et al., 2008, Martínez-álvarez et al., 2005, Rong et al., 2012, Winston and Di Giulio, 1991, Wu et al., 2004). Our present results showed that Glu supplementation increased CAT, GPx, GST, GR activities and GSH content in the intestine. The SAS and HRS activities are 2 indexes used to evaluate the total capacity of scavenging superoxide and hydroxyl radical, respectively (Wu et al., 2004). The present results showed that Glu significantly increased the SAS and HRS activities in the intestine and hepatopancreas. These results indicated that the elevated anti-oxidative capacity of carp may, at least in part, be due to Glu induced the increment of the non-enzymatic content and enzymatic activities. Antioxidant enzymes are proteins, and their activities can be affected by the mRNA levels (Tiedge et al., 1997). In the present study, dietary Glu supplementation up-regulated CAT, GST, GR, and GPx1a mRNA expressions. These results matched a similar pattern to the respective enzyme activity changes. In eukaryote, as a nucleus transcription factor, the intranuclear Nrf2 can promote the transcription of antioxidant genes (Kwak et al., 2004) The Keap1 is identified as an Nrf2-binding protein that prevents Nrf2 translocation to the nucleus and promotes the ubiquitination-proteasomal degradation of Nrf2 (Ma, 2013). The present study showed Glu significantly up-regulated Nrf2 mRNA levels in the intestine of Jian carp. In contrast, Glu significantly decreased Keap1 mRNA expression. This result agreed with the previous study in grass carp that Glu supplementation alleviated oxidative damage induced by copper (Jiang et al., 2016). Thus, the positive effects of Glu on antioxidant enzymes mRNA expression may be partly ascribed to promote Nrf2 nuclear translocation by down-regulating Keap1 mRNA expression. However, the specific action of Glu affecting Nrf2 nuclear translocation needs a further study.
4.4. Effect of dietary glu on intestinal structure integrity
The previous study reported absorption capacity may be associated with TJ permeability of the intestine (Ballard et al., 1995). Our present results also showed that Glu supplementation significantly increased intestinal ZO-1, occludin1, claudin2, claudin3, and claudin7 mRNA expressions. This result was in good agreement with a report on piglet. The Glu supplementation improved intestinal integrity and up-regulated jejunum ZO-1 and occludin mRNA expression in weaning piglets (Lin et al., 2014). In addition, evidence from animals and cell studies also shows that glutamine is important for intestinal barrier function and regulation of TJ protein (Li and Neu, 2009). As well, it has been shown that prohibiting the conversion from glutamine to Glu inhibits enhancement effect of glutamine on TJ protein expression (Nose et al., 2010, Vermeulen et al., 2011). In fish enterocyte, the expression of glutaminase mRNA level is increased quickly and effectively by glutamine (Jiang et al., 2015a, Jiang et al., 2015b, Jiang et al., 2015c).. Thus, dietary Glu maybe improve intestinal barrier functions and nutrient absorptive capacities by up-regulating TJ gene expression. Furthermore, TJ permeability is mediated by PKC (Andreeva et al., 2006, Stenson et al., 1993, Stuart and Nigam, 1995). Previous study showed that dietary arginine and medium pre-supplementation with linolenic acid and docosahexaenoic acid affect TJ permeability in Jian carp and intestinal monolayer cells (Usami et al., 2003, Wang et al., 2016). In the current study, optimal dietary Glu caused an up-regulation of the PKC mRNA expression level in Jian carp. Nevertheless, how Glu interacts with PKC is unknown by now and requires further study.
5. Conclusions
The present study demonstrated that dietary Glu increased fish growth by enhancing digestive and absorptive enzyme activities and intestinal physical barrier functions by regulating antioxidant-related signaling molecule and TJ protein gene expressions in fish intestines. The beneficial actions of Glu in intestinal physical barrier functions are closely associated with its ability in increasing Nrf2 and PKC mRNA expression. The optimal dietary Glu levels for Jian carp (126 to 337 g) were estimated to be 81.97 g/kg diet based on growth performance (PWG), 71.06 and 71.36 g/kg diet based on antioxidant-related indices (MDA content in the hepatopancreas and whole intestine, respectively).
Conflict of Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This study was financially supported by National Key R&D Program of China (2019YFD0900200) and National Natural Science Foundation of China (31702362). The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Xiao-Qiu Zhou, Email: zhouxq@sicau.edu.cn.
Jun Jiang, Email: jjun3@foxmail.com.
References
- Andreeva A.Y., Piontek J., Blasig I.E., Utepbergenov D.I. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase c isoforms. Int J Biochem Cell Biol. 2006;38:222–233. doi: 10.1016/j.biocel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ballard S.T., Hunter J.H., Taylor A.E. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr. 1995;15:35–55. doi: 10.1146/annurev.nu.15.070195.000343. [DOI] [PubMed] [Google Scholar]
- Blachier F., Boutry C., Bos C., Tome D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr. 2009;90:814S–821S. doi: 10.3945/ajcn.2009.27462S. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brosnan J.T., Brosnan M.E. Glutamate: a truly functional amino acid. Amino Acids. 2013;45:413–418. doi: 10.1007/s00726-012-1280-4. [DOI] [PubMed] [Google Scholar]
- Buentello J.A., Gatlin D.M. Preliminary observations on the effects of water hardness on free taurine and other amino acids in plasma and muscle of channel catfish. N Am J Aquacult. 2002;64:95–102. [Google Scholar]
- Bureau D.P., Azevedo P.A., Tapia-Salazar M., Cuzon G. Pattern and cost of growth and nutrient deposition in fish and shrimp: potential implications and applications. Av Nutr Acuícola V. 2000;19:111–140. Memorias del V Simposium Internacional de Nutrición Acuícola. [Google Scholar]
- Caballero-Solares A., Viegas I., Salgado M.C., Siles A.M., Sáez A., Metón I. Diets supplemented with glutamate or glutamine improve protein retention and modulate gene expression of key enzymes of hepatic metabolism in gilthead seabream (Sparus aurata) juveniles. Aquaculture. 2015;444:79–87. [Google Scholar]
- Chika Y., Mayumi M., Makoto B., Takeshi Y. Glutamate promotes nucleotide synthesis in the gut and improves availability of soybean meal feed in rainbow trout. SpringerPlus. 2016;5:1021. doi: 10.1186/s40064-016-2634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Munaswamy V., Halappa R., Marigoudar S.R. Impact of sodium cyanide on catalase activity in the freshwater exotic carp, Cyprinus carpio (Linnaeus) Pestic Biochem Physiol. 2008;92:15–18. [Google Scholar]
- Deng J., Mai K., Ai Q., Zhang W., Tan B., Xu W. Alternative protein sources in diets for Japanese flounder Paralichthys olivaceus (Temminck and Schlegel): II. Effects on nutrient digestibility and digestive enzyme activity. Aquacult Res. 2010;41:861–870. [Google Scholar]
- GaffneyStomberg Cucchi, Carrie Benhua, Bataille Amy. Dietary protein increases intestinal calcium absorption in part by increasing tight junction expression of claudin-2. Faseb J. 2011;25:779–785. [Google Scholar]
- Gal-Garber O., Mabjeesh S.J., Sklan D., Uni Z. Nutrient transport in the small intestine: Na+, K+-ATPase expression and activity in the small intestine of the chicken as influenced by dietary sodium. Poultry Sci. 2003;82:1127–1133. doi: 10.1093/ps/82.7.1127. [DOI] [PubMed] [Google Scholar]
- Geering K. Subunit assembly and functional maturation of Na+, K+-ATPase. J Membr Biol. 1990;115:109–121. doi: 10.1007/BF01869450. [DOI] [PubMed] [Google Scholar]
- Ghosh J., Das J., Manna P., Sil P.C. Cytoprotective effect of arjunolic acid in response to sodium fluoride mediated oxidative stress and cell death via necrotic pathway. Toxicol Vitro. 2008;22:1918–1926. doi: 10.1016/j.tiv.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Gisbert E., Giménez G., Fernández I., Kotzamanis Y., Estévez A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture. 2009;287:381–387. [Google Scholar]
- Gonzalez-Mariscal L., Tapia R., Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Griffith O.W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. P Natl Acad Sci. 1980;77:3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim Y., Harpaz S., Uni Z. Expression of brush border enzymes and transporters in the intestine of European sea bass (Dicentrarchus labrax) following food deprivation. Aquaculture. 2009;290:110–115. [Google Scholar]
- Hong Y., Jiang W., Kuang S., Hu K., Tang L., Liu Y., Jiang J., Zhang Y., Zhou X., Feng L. Growth, digestive and absorptive capacity and antioxidant status in intestine and hepatopancreas of sub-adult grass carp Ctenopharyngodon idella fed graded levels of dietary threonine. J Anim Sci Biotechnol. 2015;6:34. doi: 10.1186/s40104-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz W., editor. Official methods of analysis of AOAC international. 17th. AOAC international; Gaithersburg, Md: 2000. [Google Scholar]
- Jia S., Li X., Zheng S., Wu G. Amino acids are major energy substrates for tissues of hybrid striped bass and zebrafish. Amino Acids. 2017;49:1–11. doi: 10.1007/s00726-017-2481-7. [DOI] [PubMed] [Google Scholar]
- Jiang W.D., Kuang S.Y., Liu Y., Jiang J., Hu K., Li S.H. Effects of myo-inositol on proliferation, differentiation, oxidative status and antioxidant capacity of carp enterocytes in primary culture. Aquacult Nutr. 2013;19:45–53. [Google Scholar]
- Jiang J., Feng L., Liu Y., Jiang W.D., Hu K., Li S.H. Molecular cloning and expression of kidney-type glutaminase from common carp (Cyprinus carpio) and its up-regulation by glutamine in primary culture enterocyte. Aquacult Nutr. 2015;20:731–740. [Google Scholar]
- Jiang J., Shi D., Zhou X.Q., Yin L., Feng L., Liu Y. Effects of glutamate on growth, antioxidant capacity, and antioxidant-related signaling molecule expression in primary cultures of fish enterocytes. Fish Physiol Biochem. 2015;41:1143–1153. doi: 10.1007/s10695-015-0076-3. [DOI] [PubMed] [Google Scholar]
- Jiang J., Shi D., Zhou X., Hu Y., Feng L., Liu Y. In vitro and in vivo protective effect of arginine against lipopolysaccharide induced inflammatory response in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 2015;42:457–464. doi: 10.1016/j.fsi.2014.11.030. [DOI] [PubMed] [Google Scholar]
- Jiang J., Wu X.Y., Zhou X.Q., Feng L., Liu Y., Jiang W.D. Glutamate ameliorates copper-induced oxidative injury by regulating antioxidant defences in fish intestine. Br J Nutr. 2016;116:70–79. doi: 10.1017/S0007114516001732. [DOI] [PubMed] [Google Scholar]
- Johnson A.T., Kaufmann Y., Luo S., Babb K., Hawk R., Klimberg V.S. Gut glutathione metabolism and changes with 7, 12-DMBA and glutamine. J Surg Res. 2003;115:242–246. doi: 10.1016/j.jss.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Kabaroff L.C., Rodriguez A., Quinton M., Boermans H., Karrow N.A. Assessment of the ovine acute phase response and hepatic gene expression in response to Escherichia coli endotoxin. Vet Immunol Immunopathol. 2006;113:113–124. doi: 10.1016/j.vetimm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kwak M., Wakabayashi N., Kensler T.W. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res Fund Mol Mech Mutagen. 2004;555:133–148. doi: 10.1016/j.mrfmmm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Landy J., Ronde E., English N., Clark S.K., Hart A.L., Knight S.C. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22:3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson T., Koppang E.O., Espe M., Terjesen B.F., Krasnov A., Moreno H.M. Fillet quality and health of Atlantic salmon (Salmo salar L.) fed a diet supplemented with glutamate. Aquaculture. 2014;426–427:288–295. [Google Scholar]
- Li N., Neu J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/AKT mediated pathway in Caco-2 cells. J Nutr. 2009;139:710–714. doi: 10.3945/jn.108.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Zhang B., Yu C., Li J., Zhang L., Sun H. L-glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PloS One. 2014;9 doi: 10.1371/journal.pone.0111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Feng L., Jiang W., Liu Y., Wu P., Jiang J. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 2014;40:197–207. doi: 10.1016/j.fsi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud H.K., Al-Sagheer A.A., Reda F.M., Mahgoub S.A., Ayyat M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture. 2017;475:16–23. [Google Scholar]
- Martínez-álvarez R.M., Morales A.E., Sanz A. Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish. 2005;15:75–88. [Google Scholar]
- Matsuhisa K., Kondoh M., Suzuki H., Yagi K. Comparison of mucosal absorption-enhancing activity between a claudin-3/-4 binder and a broadly specific claudin binder. Biochem Biophys Res Commun. 2012;423:229–233. doi: 10.1016/j.bbrc.2012.05.060. [DOI] [PubMed] [Google Scholar]
- Mourente G., Bell J.G., Tocher D.R. Does dietary tocopherol level affect fatty acid metabolism in fish? Fish Physiol Biochem. 2007;33:269–280. [Google Scholar]
- Nose K., Yang H., Sun X., Nose S., Koga H., Feng Y. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res. 2010;30:67–80. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council) The National Academy Press; Washington DC: 2011. Nutrient requirements of fish and shrimp; pp. 206–207. [Google Scholar]
- Oehme M., Grammes F., Takle H., Zambonino-Infante J., Refstie S., Thomassen M.S. Dietary supplementation of glutamate and arginine to Atlantic salmon (Salmo salar L.) increases growth during the first autumn in sea. Aquaculture. 2010;310:156–163. [Google Scholar]
- Ogawa M., Shiozawa M., Hiraoka Y., Takeuchi Y., Aiso S. Immunohistochemical study of localization of γ-glutamyl transpeptidase in the rat brain. Tissue Cell. 1998;30:597–601. doi: 10.1016/s0040-8166(98)80078-5. [DOI] [PubMed] [Google Scholar]
- Reeds P.J., Burrin D.G., Stoll B., Jahoor F., Wykes L., Henry J. Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am J Physiol. 1997;273:E408–E415. doi: 10.1152/ajpendo.1997.273.2.E408. [DOI] [PubMed] [Google Scholar]
- Rong S., Zhao Y., Bao W., Xiao X., Wang D., Nussler A.K. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ros generation and enhancing antioxidative capacity. Phytomedicine. 2012;19:545–550. doi: 10.1016/j.phymed.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Shi L., Feng L., Jiang W., Liu Y., Jiang J., Wu P. Immunity decreases, antioxidant system damages and tight junction changes in the intestine of grass carp (Ctenopharyngodon idella) during folic acid deficiency: regulation of NF-κB, Nrf2 and MLCK mRNA levels. Fish Shellfish Immunol. 2016;51:405–419. doi: 10.1016/j.fsi.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Shoveller A.K., Stoll B., Ball R.O., Burrin D.G. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J Nutr. 2005;135:1609–1612. doi: 10.1093/jn/135.7.1609. [DOI] [PubMed] [Google Scholar]
- Stenson W.F., Easom R.A., Riehl T.E., Turk J. Regulation of paracellular permeability in caco-2 cell monolayers by protein kinase C. Am J Physiol. 1993;265:G955. doi: 10.1152/ajpgi.1993.265.5.G955. [DOI] [PubMed] [Google Scholar]
- Stuart R.O., Nigam S.K. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syakuri H., Adamek M., Brogden G., Rakus K.A., Matras M., Irnazarow I. Intestinal barrier of carp (Cyprinus carpio L.) during a cyprinid herpesvirus 3-infection: molecular identification and regulation of the mRNA expression of claudin encoding genes. Fish Shellfish Immunol. 2013;34:305–314. doi: 10.1016/j.fsi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Tengjaroenkul B., Smith B.J., Caceci T., Smith S.A. Distribution of intestinal enzyme activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture. 2000;182:317–327. [Google Scholar]
- Thongon N., Krishnamra N. Apical acidity decreases inhibitory effect of omeprazole on Mg2+ absorption and claudin-7 and -12 expression in Caco-2 monolayers. Exp Mol Med. 2012;44:684. doi: 10.3858/emm.2012.44.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Usami M., Komurasaki T., Hanada A., Kinoshita K., Ohata A. Effect of γ-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition. 2003;19:150–156. doi: 10.1016/s0899-9007(02)00927-9. [DOI] [PubMed] [Google Scholar]
- Vermeulen M.A., de Jong J., Vaessen M.J., van Leeuwen P.A., Houdijk A.P. Glutamate reduces experimental intestinal hyperpermeability and facilitates glutamine support of gut integrity. World J Gastroenterol. 2011;17:1569–1573. doi: 10.3748/wjg.v17.i12.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem. 1994;133:193–220. doi: 10.1007/BF01267955. [DOI] [PubMed] [Google Scholar]
- Wang T., Li W.L. Application of glutathione in animal production. China Feed. 2012;23:19–21. [Google Scholar]
- Wang P., Powell S.R. Decreased sensitivity associated with an altered formulation of a commercially available kit for detection of protein carbonyls. Free Radical Biol Med. 2010;49:119. doi: 10.1016/j.freeradbiomed.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Feng L., Chen G., Jiang W., Liu Y., Kuang S. Jian carp (Cyprinus carpio var. Jian) intestinal immune responses, antioxidant status and tight junction protein mrna expression are modulated via Nrf2 and PKC in response to dietary arginine deficiency. Fish Shellfish Immunol. 2016;51:116–124. doi: 10.1016/j.fsi.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Wen Z.P., Zhou X.Q., Feng L., Jiang J., Liu Y. Effect of dietary pantothenic acid supplement on growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) Aquacult Nutr. 2009;15:470–476. [Google Scholar]
- Winston G.W., Di Giulio R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol. 1991;19:137–161. [Google Scholar]
- Wu G., Fang Y., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Burghardt R.C., Johnson G.A., Kim S.W., Knabe D.A., Li P., Li X., McKnight J.R., Satterfield M.C. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;40:1053–1063. doi: 10.1007/s00726-010-0715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Wu Z., Dai Z., Yang Y., Wang W., Liu C., Wang B., Wang J., Yin Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2012;44:1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- Xiao W., Feng Y., Holst J.J., Hartmann B., Yang H., Teitelbaum D.H. Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. Faseb J. 2014;28:2073–2087. doi: 10.1096/fj.13-238311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Tian L., Jin Y., Yang H., Liang G., Liu Y. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture. 2014;418–419:159–164. [Google Scholar]
- Xie S., Zhou W., Tian L., Niu J., Liu Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, anti-oxidative and immune ability of nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016;55:233–241. doi: 10.1016/j.fsi.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Xu J., Feng L., Jiang W., Wu P., Liu Y., Jiang J., Kuang S., Tang L., Zhou X. Different dietary protein levels affect flesh quality, fatty acids and alter gene expression of Nrf2-mediated antioxidant enzymes in the muscle of grass carp (Ctenopharyngodon idella) Aquaculture. 2018;493:272–282. [Google Scholar]
- Yan L., Qiu-Zhou X. Dietary glutamine supplementation improves structure and function of intestine of juvenile jian carp (Cyprinus carpio var. Jian) Aquaculture. 2006;256:389–394. [Google Scholar]
- Zhang C., Sheng Z., Hu S., Gao J., Yu S., Liu Y. The influence of apoptosis of mucosal epithelial cells on intestinal barrier integrity after scald in rats. Burns. 2002;28:731–737. doi: 10.1016/s0305-4179(02)00210-3. [DOI] [PubMed] [Google Scholar]
- Zhang J., Shen H., Wang X., Wu J., Xue Y. Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere. 2004;55:167–174. doi: 10.1016/j.chemosphere.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Zhao J., Feng L., Liu Y., Jiang W., Wu P., Jiang J., Zhang Y., Zhou X. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 2014;41:663–673. doi: 10.1016/j.fsi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Hu Y., Zhou X.Q., Zeng X.Y., Feng L., Liu Y., Jiang W.D., Li S.H., Li D.B., Wu X.Q. Effects of dietary glutamate supplementation on growth performance, digestive enzyme activities and antioxidant capacity in intestine of grass carp (Ctenopharyngodon idella) Aquacult Nutr. 2015;21:935–941. [Google Scholar]