Abstract
Purpose
There have been few recent studies regarding vascular aging and its relationship with left ventricular (LV) geometry. Moreover, the association of abnormal LV geometry with various kinds of vascular aging has not yet been systematically analyzed. Thus, this study aimed to further elucidate this relationship.
Materials and Methods
In this study, 3363 older participants (43.6% male, aged 71.1±5.9 years; 56.4% female, aged 71.1±6.1 years) derived from the Northern Shanghai Study were enrolled. Vascular aging criteria included arteriosclerosis, defined as carotid-femoral pulse wave velocity >10 m/s or brachial–ankle pulse wave velocity >1800 cm/s, and peripheral atherosclerosis, defined as ankle–brachial index <0.9, carotid artery intima-media thickness (cIMT) greater than 0.9 mm, or carotid plaque indicating carotid artery abnormality. Micro-albuminuria was defined as urinary albumin-to-creatinine ratio >30 mg/g. Decreased estimated glomerular filtration rate (eGFR) was defined as eGFR <60 mL/min/1.73 m2.
Results
When vascular aging parameters were respectively adjusted for age and sex, arteriosclerosis, micro-albuminuria, and peripheral atherosclerosis were significantly associated with concentric remodeling, eccentric LV hypertrophy (LVH), and concentric LVH (P<0.045) but not with decreased eGFR or abnormal cIMT and presence of plaque. Peripheral atherosclerosis was strongly associated with LV concentric geometry (LVCG) when considering other covariates (risk factors, diseases, and treatments) (P<0.012).
Conclusion
Vascular aging parameters such as arteriosclerosis, micro-albuminuria, and peripheral atherosclerosis are significantly and independently associated with LVCG in community-dwelling older Chinese population, suggesting the importance of vascular aging during early clinical assessment of abnormal LV geometry change and serious cardiovascular events.
Keywords: arteriosclerosis, micro-albuminuria, peripheral atherosclerosis, community-dwelling elderly
Introduction
Cardiovascular disease (CVD) is one of the most common causes of mortality and morbidity globally.1 Structural and/or functional cardiac and vascular aging is closely related to cardiovascular risk factors.2 A cohort study has shown that the alternation of left ventricular (LV) size was related to the incidence of major cardiovascular events.3,4 Increased LV mass (LVM) is considered an independent predictor of cardiovascular morbidity and mortality.5 Moreover, increased LVM is related to coronary artery disease (CAD).3,6,7
Changes in LV geometry, as hypertension-mediated organ damage,8 are closely related to hemodynamic and humoral and hormonal changes accompanying systemic hypertension.9–11 A previous study from a Northern Shanghai group suggested that as one of the hypertension-mediated organ damages, LV hypertrophy (LVH) is significantly associated with arteriosclerosis.12 Microvascular aging is common in hypertensive individuals, and people with micro-albuminuria showed higher prevalence of abnormal LV geometric patterns compared with those without micro-albuminuria.13–16 A recent study showed that coronary artery atherosclerosis is associated with LV concentric geometry (LVCG), suggesting the greater cardiovascular risk associated with concentric remodeling and LVH.17 A cohort study of 47,730 Koreans undergoing echocardiography revealed that mildly decreased estimated glomerular filtration rate (eGFR) had increased probability of LV geometry change represented by abnormal relative wall thickness (RWT).18 Increment in carotid intima-media thickness (cIMT) or presence of plaque considered as a hypertension-related vascular aging,8 better predicts cardiovascular events.19 In conclusion, LV geometric change may be related to systemic abnormalities in individuals who are at high risk of cardiovascular events; moreover, this may be caused by vascular aging which brings hemodynamic change to blood circulation and organ damage.
Thus, we aimed to investigate the association of vascular aging (arteriosclerosis, peripheral atherosclerosis, increment in cIMT or plaque, decreased eGFR and micro-albuminuria) with the LV geometry in community-dwelling older Chinese from the Northern Shanghai Study.
Patients and Methods
Study Design
The Northern Shanghai Study20 (NSS) is a registered (ClinicalTrials.gov Identifier: NCT02368938), prospective population study based on community-dwelling older population. The study enrolled a total of 3363 participants from local community in North Shanghai. The inclusion criteria were as follows: (1) aged ≥65 years, (2) local residents from urban communities, and (3) willing to sign the informed consent and available for long-term follow-up. The exclusion criteria were as follows: (1) had serious heart disease (New York Heart Association functional classification ≥IV) or end-stage renal disease (chronic kidney disease ≥4 stage), (2) suffered from cancer or had life expectancy <5 years, and (3) stroke history within 3 months.
The Northern Shanghai Study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital. Written informed consent was obtained from all participants.
Social, Clinical, and Biological Parameters
Medical and family histories were obtained from the standardized structured questionnaire, including age, sex, smoking behavior, history of hypertension, previous CVD, family history of premature CVD, history of diabetes mellitus, and use of anti-hypertensives and anti-diabetics. Body height and weight were measured in a regular method, and body mass index (BMI) was calculated in kg/m2. Waist and hip circumferences were measured, and waist-to-hip ratio was calculated. Blood and urine samples were obtained in a fasting state in the morning of the check-up day. Biological markers were tested in the lab of Shanghai Tenth People’s Hospital. Asian modified Chronic Kidney Disease Epidemiology Collaboration equation was used to determine eGFR.21
Echocardiography and Carotid Artery Ultrasound
Ultrasound parameters were measured by Mylab 30 Gold cardiovascular system (ESAOTE SpA, Genoa, Italy) by trained cardiologists. To obtain the LV diameters, M-mode and two-dimensional echocardiography were performed using a 3.5-MHz probe, according to the guidelines of the American Society of Echocardiography.6 LV end-diastolic diameter (LVEDd), interventricular septal (IVSd), and posterior wall thickness at end-diastole (PWTd) were measured from the parasternal view, respectively,6,22 and LVM, LVMI, and RWT were calculated using the following formulas.6,23
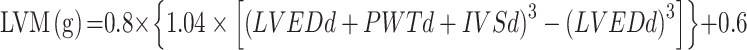 |
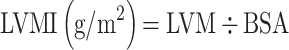 |
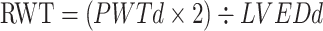 |
The cIMT and carotid plaque were measured with a 7.5-MHz probe using the same device, and average of three measurements was retained. Simultaneously, the presence or absence of plaques in the carotid arteries was recorded.24
Definition for LV Geometry
RWT (male and female) ≤0.42, LVMI (male) ≤115 g/m2, and LVMI (female) ≤95 g/m2 were considered normal geometry. LVMI (male) >115g/m2 or LVMI (female) >95 g/m2, and RWT > 0.42 were considered increased. LV geometry was defined, based on LVMI and RWT, into normal (normal LVMI and normal RWT), concentric remodeling (normal LVMI and increased RWT), concentric LVH (increased LVMI and RWT), and eccentric LVH (increased LVMI and normal RWT). Concentric remodeling and concentric LVH were defined with LVCG, while eccentric LVH and concentric LVH were defined with LVH.6
Measurements and Definitions of Vascular Aging
Blood pressure was measured using a calibrated mercury sphygmomanometer while participants were sitting in a chair. The average blood pressure was calculated after repeating the measurement three times in 2-min intervals.8 The carotid-femoral pulse wave velocity (cf-pwv) was measured by applanation tonometry (SphygmoCor, AtCor Medical, Sydney, Australia).25,26 The brachial–ankle PWV (ba-pwv) and ankle–brachial index (ABI) were recorded as automatically calculated by the VP1000 system (Omron, Kyoto, Japan).27 Both cf-PWV and ba-PWV were tested after a 5-min rest. Vascular aging criteria included arteriosclerosis, which was defined as cf-PWV above 10 m/s or ba-PWV above 1800 cm/s; peripheral atherosclerosis, which was defined as ABI below 0.9, and micro-albuminuria, defined as urinary albumin-to-creatinine ratio (UACR) above 30 mg/g,8 cIMT greater than 0.9 mm, or carotid plaque indicating carotid artery abnormality.24,28 Decreased eGFR was defined as eGFR less than 60 mL/min/1.73 m2.
Statistical Analysis
Continuous data were presented as means ± standard deviation and categorical variables by absolute numbers or percentage. Comparisons between sexes were done by independent-sample t test or chi-squared test. To investigate the correlation of conventional cardiovascular risk factors with LVMI and RWT, Pearson correlation analyses were applied. Binary logistic regressions were done to obtain the regression coefficients and odds ratios (ORs) of conventional cardiovascular risk factors in LVH and LVCG, such as age, sex, family history of premature CVD, smoking, waist-to-hip ratio, body mass index (BMI), fasting blood glucose, urine acid, triglycerides, high-density lipoprotein/low-density lipoprotein (HDL/LDL) cholesterol, systolic blood pressure, and heart rate. Further, multivariate linear regressions were conducted to investigate the relations of vascular aging parameters with LVMI and RWT, adjusted for age, sex, family history for CVD, smoking, waist-to-hip ratio, and BMI. Subsequently, multivariate logistic regressions were conducted to detect the regression coefficients and ORs of vascular aging parameters in LVH/LVCG, and three abnormal LV geometries. Model 1 was adjusted for age and sex; Model 2 was adjusted for age and sex, and vascular aging parameters were incorporated in the model; Model 3 was adjusted for conventional cardiovascular risk factors, diseases and treatments; and Model 4 was adjusted for conventional cardiovascular risk factors and diseases and treatments, and vascular aging parameters were incorporated in the model. Statistical analysis was performed using SAS software version 9.3 (SAS Institute, Cary, NC). P<0.05 was considered statistically significant.
Results
Participants
Participant characteristics according to sex are presented in Table 1, including cardiovascular risk factors and vascular and LV parameters. The 3363 participants included 1896 (56.4%) women, 749 (22.3%) with diabetes mellitus, 2148 (64.1%) with hypertension, of whom 1682 (50.0%) were taking antihypertensive agents. Compared with men, women had higher incidence of family history for premature CVD (23.4% vs 19.0%, P=0.002), smoked significantly less (1.9% vs 54.3%, P<0.001), and had lower urine acid (308.9±75.3 vs 363.9±83.9 μmol/L, P<0.001), higher triglyceride (1.68±1.06 vs 1.54±1.00 mmol/L, P<0.001), and higher HDL/LDL cholesterol ratio (0.49±0.21 vs 0.48±0.21, P=0.04). Additionally, women had lower waist/hip circumference ratio (0.88±0.07 vs 0.91±0.06, P<0.001) and higher heart rate (73±11 vs 71±11 beats/min, P<0.001). As for vascular and LV parameters, women had higher ba-pwv (1879.6±419.3 vs 1815.5±373.2 cm/s, P<0.001) and eGFR (84.8±14.0 vs 68.6±16.3 mL/min/1.73 m2, P<0.001), higher UACR (52.4±57.5 vs 46.6±60.7 mg/g, P=0.007), and lower LVMI (85.8±25.6 vs 88.6±25.2 mg/g, P=0.002). Moreover, women showed lower prevalence of incrassated cIMT or carotid plaque (64.3% vs 71.2%, P<0.001) and lower prevalence of peripheral atherosclerosis (11.8% vs 15.1%, P=0.005). Further, there are 1757 participants who have normal LV geometry, 741 have concentric remodeling, 537 have eccentric LVH, and 238 have concentric LVH, and women showed higher prevalence of LVH (73.7%).
Table 1.
Participant Characteristics
| Overall (n=3363) | Female (n=1896) | Male (n=1467) | P value | |
|---|---|---|---|---|
| Cardiovascular risk factors | ||||
| Age (years) | 71.1±6.0 | 71.1±6.1 | 71.1±5.9 | 0.93 |
| Family history of premature CVD, n (%) | 722 (21.5) | 443 (23.4) | 279 (19.0) | 0.002* |
| Smoking (n, %) | 823 (24.7) | 36 (1.9) | 797 (54.3) | <0.001* |
| Waist-to-hip ratio | 0.90±0.07 | 0.88±0.07 | 0.91±0.06 | <0.001* |
| BMI (kg/m2) | 24.55±3.58 | 24.57±3.81 | 24.53±3.26 | 0.73 |
| Fasting blood glucose (mmol/L) | 5.78±1.76 | 5.74±1.65 | 5.83±1.87 | 0.16 |
| Urine acid (μmol/L) | 332.6±83.5 | 308.9±75.3 | 363.9±83.9 | <0.001* |
| Triglycerides (mmol/L) | 1.62±1.02 | 1.68±1.06 | 1.54±1.00 | <0.001* |
| HDL/LDL Cholesterol | 0.49±0.21 | 0.49±0.21 | 0.48±0.21 | 0.04* |
| Systolic BP (mmHg) | 136±17 | 136±18 | 136±16 | 0.60 |
| Heart rate (beats/min) | 72±11 | 73±11 | 71±11 | <0.001* |
| Vascular and left ventricular parameters | ||||
| Cf-PWV (m/s) | 9.3±2.3 | 9.3±2.3 | 9.3±2.4 | 0.67 |
| Ba-PWV (cm/s) | 1876.0±413.9 | 1879.6±419.3 | 1815.5±373.2 | <0.001* |
| ABI<0.9 (n, %) | 445 (13.2) | 224 (11.8) | 221 (15.1) | 0.005* |
| cIMT>0.9 or Plaque (n, %) | 2265 (67.4) | 1220 (64.3) | 1045 (71.2) | <0.001* |
| eGFR (mL/min/1.73 m2) | 77.8±16.3 | 84.8±14.0 | 68.6±16.3 | <0.001* |
| UACR (mg/g) | 49.8±59.0 | 52.4±57.5 | 46.6±60.7 | 0.007* |
| LVMI (g/m2) | 87.0±25.5 | 85.8±25.6 | 88.6±25.2 | 0.002* |
| RWT | 0.39±0.08 | 0.39±0.07 | 0.39±0.08 | 0.368 |
| Diseases and treatment | ||||
| Hypertension, n (%) | 2148 (64.1) | 1210 (63.8) | 938 (63.9) | 0.93 |
| Diabetes, n (%) | 756 (22.5) | 419 (22.1) | 337 (23.0) | 0.55 |
| Anti-hypertension treatment (n, %) | 1682 (50.0) | 943 (49.7) | 739 (50.4) | 0.713 |
| Anti-diabetic treatment, n (%) | 573 (17.0) | 312 (16.5) | 261 (17.8) | 0.307 |
| Statin therapy (n, %) | 620 (18.4) | 371 (19.6) | 249 (17.0) | 0.054 |
Notes: Continuous data were presented as mean ± standard deviation and categorical variables by absolute numbers or percentage. Comparisons between genders were done by independent-sample t-test or chi-squared test. *P<0.05.
Abbreviations: CVD, cardiovascular disease; LVMI, left ventricular mass index; RWT, relative wall thickness; cf-PWV, carotid to femoral aortic pulse wave velocity; ba-pwv, brachial–ankle pulse wave velocity; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio; cIMT, carotid artery intima-media thickness; ABI, ankle–brachial index.
Correlation of Cardiovascular Risk Factors with LVMI and RWT
In the Pearson correlation analysis, Table 2 shows that LVMI is significantly correlated with age (r=0.155, P<0.001), waist/hip ratio (r=0.170, P<0.001), BMI (r=0.184, P<0.001), urine acid (r=0.116, P<0.001), systolic blood pressure (r=0.178, P<0.001), heart rate (r=−0.077, P<0.001), sex (r=0.054, P=0.002), smoking (r=0.037, P=0.034), and triglycerides (r=0.051, P=0.004). As for RWT, only age (r=0.051, P=0.003), fasting blood glucose (r=0.068, P<0.001), and heart rate (r=0.074, P<0.001) showed significant correlation.
Table 2.
Correlation of Cardiovascular Risk Factors with LVMI and RWT
| Cardiovascular Risk Factors | LVMI | RWT | ||
|---|---|---|---|---|
| r | P | r | P | |
| Age (years) | 0.155 | <0.001* | 0.051 | 0.003* |
| Gender (0=Male; 1=Female) | 0.054 | 0.002* | −0.016 | 0.368 |
| Family history of premature CVD (YES=1, NO=0) | −0.006 | 0.714 | −0.011 | 0.521 |
| Smoking (YES=1, NO=0) | 0.037 | 0.034* | −0.011 | 0.512 |
| Waist-to-hip ratio | 0.170 | <0.001* | −0.014 | 0.417 |
| BMI (kg/m2) | 0.184 | <0.001* | −0.008 | 0.663 |
| Fasting blood glucose (mmol/L) | 0.012 | 0.496 | 0.068 | <0.001* |
| Urine acid (μmol/L) | 0.116 | <0.001* | 0.010 | 0.576 |
| Triglycerides (mmol/L) | 0.051 | 0.004* | 0.017 | 0.318 |
| HDL/LDL cholesterol | −0.016 | 0.355 | 0.010 | 0.552 |
| Systolic BP (mmHg) | 0.178 | <0.001* | 0.002 | 0.903 |
| Heart rate (beats/min) | −0.077 | <0.001* | 0.074 | <0.001* |
Notes: Pearson correlation analyses were applied to investigate the correlation of conventional cardiovascular risk factors with LVMI and RWT. *P<0.05.
Abbreviations: LVMI, left ventricular mass index; RWT, relative wall thickness; CVD, cardiovascular disease.
Association of Cardiovascular Risk Factors with LVH and LVCG
As shown in Table 3, in binary logistic regressions, LVH is significantly associated with age (OR 1.043, 95% CI 1.028–1.058, P<0.001), sex (OR 3.035, 95% CI 2.365–3.893, P<0.001), BMI (OR 1.050, 95% CI 1.022–1.079, P<0.001), systolic blood pressure (OR 1.012, 95% CI 1.006–1.017, P<0.001), waist/hip ratio (OR 10.386, 95% CI 2.374–45.441, P=0.002), and heart rate (OR 0.992, 95% CI 0.984–1.000, P=0.043). Conversely, LVCG was only associated with age (OR 1.017, 95% CI 1.004–1.031, P=0.011), fasting blood glucose (OR 1.067, 95% CI 1.022–1.113, P=0.003), and heart rate (OR 1.012, 95% CI 1.005–1.019, P=0.001).
Table 3.
Logistic Regression Analysis of LVH and LVCG with Cardiovascular Risk Factors
| Cardiovascular Risk Factors | LVH | LVCG | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (years) | 1.043(1.028–1.058) | <0.001* | 1.017(1.004–1.031) | 0.011* |
| Gender (0=male; 1=female) | 3.035(2.365–3.893) | <0.001* | 1.073(0.874–1.318) | 0.499 |
| Family history of premature CVD (YES=1, NO=0) |
1.034(0.838–1.275) | 0.757 | 0.890(0.735–1.078) | 0.233 |
| Smoking (YES=1, NO=0) |
0.971(0.728–1.295) | 0.842 | 1.048(0.835–1.315) | 0.688 |
| Waist-to-hip ratio | 10.386(2.374–45.441) | 0.002* | 0.391(0.098–1.559) | 0.183 |
| BMI (kg/m2) | 1.050(1.022–1.079) | <0.001* | 0.986(0.961–1.012) | 0.297 |
| Fasting blood glucose (mmol/L) | 0.982(0.931–1.035) | 0.495 | 1.067(1.022–1.113) | 0.003* |
| Urine acid (μmol/L) | 1.001(1.000–1.002) | 0.194 | 1.000(0.999–1.001) | 0.388 |
| Triglycerides (mmol/L) | 1.014(0.929–1.106) | 0.760 | 1.036(0.958–1.120) | 0.377 |
| HDL/LDL cholesterol | 1.395(0.904–2.153) | 0.132 | 0.941(0.635–1.395) | 0.763 |
| Systolic BP (mmHg) | 1.012(1.006–1.017) | <0.001* | 0.997(0.992–1.002) | 0.188 |
| Heart rate (beats/min) | 0.992(0.984–1.000) | 0.043* | 1.012(1.005–1.019) | 0.001* |
Notes: Binary logistic regressions were done to obtain the regression coefficients and odds ratios (ORs) of conventional cardiovascular risk factors in LVH and LVCG. *P<0.05.
Abbreviations: LVH, left ventricular hypertrophy; LVCG, left ventricular concentric geometry; LVMI, left ventricular mass index; RWT, relative wall thickness; CVD, cardiovascular disease.
Association of LVMI and RWT with Vascular Parameters
In Table 4, five vascular parameters (cf-pwv, ba-pwv, eGFR, UACR and cIMT) were separately analyzed in multivariate linear regressions, showing that LVMI and RWT were significantly associated with cf-PWV, ba-PWV, and UACR, and cIMT is significantly related only to LVMI, after adjustment for conventional cardiovascular risk factors and diseases and treatments.
Table 4.
Multivariate Linear Regression Analyses of LVMI and RWT with Vascular Parameters
| LVMI | RWT | |||||
|---|---|---|---|---|---|---|
| β±SE | T | P | β±SE | T | P | |
| Cf-PWV (m/s) | 0.743±0.224 | 3.313 | 0.001* | 0.001±0.001 | 0.746 | 0.018* |
| Ba-PWV (cm/s) | 0.003±0.001 | 2.204 | 0.028* | 0.000006±0.000004 | 1.459 | 0.011* |
| eGFR (mL/min/1.73 m2) | 0.055±0.034 | 1.586 | 0.113 | −0.000081±0.000106 | −0.766 | 0.444 |
| UACR (mg/g) | 0.041±0.008 | 5.233 | <0.001* | 0.000043±0.000024 | 2.578 | 0.009* |
| cIMT (mm) | −10.962±2.530 | −4.334 | <0.001* | −0.002±0.008 | −0.248 | 0.804 |
Notes: Multivariate linear regressions were conducted to investigate the relations of vascular ageing parameters with LVMI and RWT. Adjusted for conventional cardiovascular risk factors and diseases and treatments, respectively. *P<0.05.
Abbreviations: LVMI, left ventricular mass index; RWT, relative wall thickness; cf-PWV, carotid to femoral aortic pulse wave velocity; ba-pwv, brachial–ankle pulse wave velocity; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio.
Association of Abnormal LV Geometry with Vascular Aging Parameters
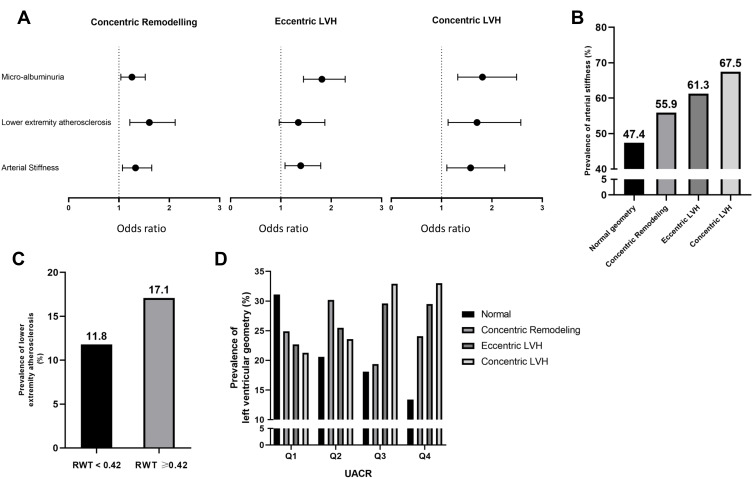
Table 5 shows logistic regressions analysis results for LVH, LVCG, and three kinds of abnormal LV geometry, using four different models to determine the regression coefficients and ORs for vascular aging parameters (arteriosclerosis, micro-albuminuria, peripheral atherosclerosis, decreased eGFR, and cIMT or presence of plaque) in different kinds of LV geometry. Results showed that when vascular aging parameters were adjusted for age and sex, arteriosclerosis, micro-albuminuria, and peripheral atherosclerosis were significantly associated with LVH and LVCG. Furthermore, they were significantly associated with concentric remodeling, eccentric LVH, and concentric LVH (P<0.045, Model 1). When vascular aging parameters were adjusted for age and sex and incorporated into the model, except for micro-albuminuria, other parameters were still statistically significant (P<0.026, Model 2). When adjusted for conventional cardiovascular risk factors and diseases and treatments, only arteriosclerosis and micro-albuminuria remained associated with LVH (P<0.018, Model 3), and only arteriosclerosis and peripheral atherosclerosis remained associated with LVCG (P<0.006, Model 3). Meanwhile, as for LV geometry, only eccentric LVH did not show more significant association with peripheral atherosclerosis. Subsequently, after adjustment for conventional cardiovascular risk factors, diseases and treatments, and incorporation into the model, Model 4 showed similar results with Model 3 (Figure 1A). Thus, arteriosclerosis was strongly and continuously associated with LVH, and with LVCG in more detail, concentric remodeling, eccentric LVH, and concentric LVH; micro-albuminuria was strongly and continuously associated with abnormal LV geometry; as for peripheral atherosclerosis, it was strongly associated with LVCG when other covariates (risk factors, diseases, and treatments) were considered. Figure 1B shows that the prevalence of arteriosclerosis is >50% in abnormal LV geometry. Furthermore, the prevalence of peripheral atherosclerosis was higher in LVCG (Figure 1C). To concretize the effect of micro-albuminuria on LV geometry, we used the interquartile range of UACR. As shown in Figure 1D, the prevalence of concentric and eccentric LVH increased rapidly after the second quartile (Q2 ranged from 16.3 to 32.2 mg/g), and both concentric and eccentric LVH showed nearly equal prevalence in the third and fourth quartiles; however, the concentric remodeling showed nearly 30% of prevalence in the second quartile.
Table 5.
Multivariate Logistic Regression Analysis of Left Ventricular Geometry with Vascular Ageing Parameters
| LVH | LVCG | Left Ventricular Geometry | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentric Remodelling (n =741) | Eccentric LVH (n =537) | Concentric LVH (n =238) | ||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Model 1 | ||||||||||
| Arteriosclerosis | 1.417(1.184–1.696) | <0.001* | 1.332(1.134–1.564) | <0.001* | 1.359(1.131–1.634) | 0.001* | 1.477(1.193–1.829) | <0.001* | 1.880(1.378–2.563) | <0.001* |
| Peripheral atherosclerosis | 1.345(1.058–1.709) | 0.015* | 1.505(1.214–1.865) | <0.001* | 1.524(1.179–1.968) | 0.001* | 1.376(1.020–1.855) | 0.036* | 2.016(1.392–2.920) | <0.001* |
| Micro-albuminuria | 1.744(1.444–2.105) | <0.001* | 1.177(1.004–1.379) | 0.044* | 1.261(1.051–1.512) | 0.013* | 1.968(1.590–2.437) | <0.001* | 1.888(1.397–2.550) | <0.001* |
| Decreased eGFR | 0.869(0.707–1.068) | 0.181 | 0.849(0.699–1.033) | 0.102 | 1.206(0.960–1.516) | 0.107* | 0.885(0.691–1.132) | 0.330 | 1.007(0.712–1.423) | 0.970 |
| cIMT>0.9 or plaque | 0.929(0.777–1.111) | 0.419 | 1.017(0.864–1.197) | 0.839 | 1.050(0.869–1.267) | 0.615* | 0.947(0.765–1.173) | 0.617 | 0.889(0.661–1.196) | 0.436 |
| Model 2 | ||||||||||
| Arteriosclerosis | 1.343(1.112–1.622) | 0.002* | 1.290(1.090–1.528) | 0.003* | 1.320(1.088–1.602) | 0.005* | 1.406(1.125–1.759) | 0.003* | 1.731(1.253–2.393) | 0.001* |
| Peripheral atherosclerosis | 1.376(1.070–1.770) | 0.013* | 1.526(1.219–1.910) | <0.001* | 1.583(1.211–2.069) | 0.001* | 1.440(1.054–1.968) | 0.022* | 2.019(1.370–2.976) | <0.001* |
| Micro-albuminuria | 1.829(1.525–2.194) | <0.001* | 1.150(0.979–1.352) | 0.089 | 1.229(1.022–1.479) | 0.025* | 1.969(1.585–2.446) | <0.001* | 1.888(1.390–2.564) | <0.001* |
| Model 3 | ||||||||||
| Arteriosclerosis | 1.282(1.046–1.572) | 0.017* | 1.301(1.085–1.561) | 0.005* | 1.347(1.094–1.659) | 0.005* | 1.369(1.075–1.742) | 0.011* | 1.615(1.143–2.282) | 0.007* |
| Peripheral atherosclerosis | 1.236(0.954–1.602) | 0.109 | 1.459(1.163–1.831) | 0.001* | 1.523(1.164–1.991) | 0.002* | 1.307(0.951–1.797) | 0.099 | 1.681(1.127–2.506) | 0.011* |
| Micro-albuminuria | 1.690(1.398–2.043) | <0.001* | 1.170(0.991–1.382) | 0.064 | 1.243(1.028–1.504) | 0.025* | 1.812(1.446–2.270) | <0.001* | 1.783(1.299–2.447) | <0.001* |
| Model 4 | ||||||||||
| Arteriosclerosis | 1.291(1.044–1.598) | 0.019* | 1.271(1.051–1.537) | 0.013* | 1.329(1.069–1.651) | 0.010* | 1.393(1.083–1.791) | 0.010* | 1.577(1.104–2.254) | 0.012* |
| Peripheral atherosclerosis | 1.276(0.973–1.673) | 0.078 | 1.515(1.197–1.918) | 0.001* | 1.603(1.215–2.116) | 0.001* | 1.346(0.967–1.874) | 0.079 | 1.704(1.129–2.573) | 0.011* |
| Micro-albuminuria | 1.695(1.401–2.051) | <0.001* | 1.172(0.990–1.386) | 0.065 | 1.257(1.038–1.522) | 0.019* | 1.815(1.447–2.277) | <0.001* | 1.812(1.320–2.489) | <0.001* |
Notes: Multivariate logistic regressions were conducted to detect the regression coefficients and odds ratios (ORs) of vascular ageing parameters in LVH/LVCG, and 3 abnormal LV geometry. Model 1: adjusted for age and gender, respectively. Model 2: adjusted for age and gender, then vascular ageing parameters were incorporated in the model. Model 3: adjusted for conventional cardiovascular risk factors and diseases and treatments, respectively. Model 4: adjusted for conventional cardiovascular risk factors and diseases and treatments, then vascular ageing parameters were incorporated in the model. *P<0.05.
Abbreviations: LVH, left ventricular hypertrophy; LVCG, left ventricular concentric geometry, eGFR, estimated glomerular filtration rate; cIMT, carotid artery intima-media thickness.
Figure 1.
(A) Odds ratio of vascular ageing parameters (arteriosclerosis, peripheral atherosclerosis, and micro-albuminuria), adjustment for conventional cardiovascular risk factors and diseases and treatments (Model 4). (B) Prevalence of arteriosclerosis in four different types of LV geometry. (C) Prevalence of peripheral atherosclerosis in LV concentric geometry compared to normal. (D) Prevalence of LV geometry from the first to fourth quartiles of UACR (Q2=16–32 mg/g).
Abbreviations: UACR, urine albumin-to-creatinine ratio; LVH, left ventricular hypertrophy.
Discussion
The major finding of this research was that vascular aging parameters such as arteriosclerosis, micro-albuminuria, and peripheral atherosclerosis are significantly and independently associated with LVCG in community-dwelling older Chinese population.
Hypertension is one of the causes of LVH,8 a hypertension-mediated organ damage. In theory, increased arteriosclerosis may cause elasticity loss in the arteries, which increase systolic blood pressure.29 In turn, an elevation of blood pressure induces vessel wall structure remodeling and vessel dysfunction, which exacerbate the arteriosclerosis.29–31 Previous studies demonstrated a strong correlation between arteriosclerosis and LV geometric change.32–34 The differences in geometric adaptation of the left ventricle and associated cardiovascular risks may reflect the differential effects of conventional risk factors and arteriosclerosis on the left ventricle.35 In our study, we found that not only LVH is associated with arteriosclerosis, and LVCG is associated. This may because of the LV compensative reaction to afterload in the blood circulation, bringing alteration to LV thickness.
Micro-albuminuria is considered an identifier to hypertensive patients at higher risk of developing cardiovascular diseases.8,36 Numerous studies have provided evidence that increased urinary albumin excretion is a concomitant of cardiac37 and vascular aging,38 as well as a powerful, independent predictor of cardiovascular events.13,39,40 Previous studies suggested that micro-albuminuria showed higher prevalence of abnormal LV geometric patterns compared with those without micro-albuminuria among hypertensive individuals; furthermore, they suggested that the absence of micro-albuminuria resulted in normal LV geometric pattern, while its presence is associated with inappropriate LVM and concentric LV hypertrophy pattern.13,14 In our study, statistics suggested that not only LVM or concentric LVH, but also all three kinds of LV abnormal geometry were strongly related to micro-albuminuria. Meanwhile, the prevalence of micro-albuminuria in concentric remodeling is approximately 30%; however, the range of UACR was 16–32 mg/g. This may alter the position of micro-albuminuria in predicting and preventing major cardiovascular events and confirms that it is a useful tool for early prediction of LV geometric change, considering that concentric remodeling tended to become to LVH.41
Computed tomography coronary angiography (CTCA) can be a modality to better detect CAD among patients with peripheral artery disease, further suggesting that low ABI and abnormal IMT are associated with more extensive CAD and higher burden of high‑risk coronary artery plaques.42 A multicenter study indicated that in patients undergoing coronary angiography, LVCG is associated with a greater prevalence of coronary stenosis, and the RWT value is associated with the severity of coronary disease: that is, the higher the RWT value, the more coronary arteries were burdened.42 Moreover, we found that when adjusted only according to age and sex, peripheral atherosclerosis was significantly associated with abnormal LV geometry. However, when conventional cardiovascular risk factors along with diseases and treatments are adjusted, ABI below 0.9 was associated with LVCG only. Thus, to the best of our knowledge, we assume that peripheral atherosclerosis may share a similar pathological alteration on coronary arteries, and assessing of reduction in ABI may be feasible in the early assessment of LVCG and coronary stenosis, along with echocardiography.
Other studies indicated that the presence of concentric LVH was associated with carotid artery abnormality, possibly contributing to the worse prognosis.43 Salvetti et al17 also suggested that cIMT is a factor influencing LVCG; however, our study does not show evidence of cIMT or plaque associated with LVMI or RWT or any types of LV geometry, and this may be interpreted by higher age (≥65 years) and high incidence of abnormal cIMT or carotid plaque (67.4%). Similarly, no evidence supported the relationship between abnormal LV geometry and decreased eGFR.
This study has some limitations. The Northern Shanghai study includes only community-dwelling older Chinese participants (aged ≥65 years), making our results not extrapolatable to other age groups. Therefore, our results should be generalized with caution to other populations and ethnic groups. Our participants are all elderly people with vascular aging, but we did not further distinguish the healthy vascular aging (physical aging) and non-healthy vascular aging (pathophysiological aging) in our analysis. Further studies are warranted.
Conclusion
Vascular aging resulting from pathological changes, such as arteriosclerosis, peripheral atherosclerosis, and micro-albuminuria, is significantly and independently associated with LVCG in community-dwelling older Chinese population. This finding suggests the importance of vascular aging during early clinical assessment of abnormal LV geometry change and serious cardiovascular events.
Data Sharing Statement
The participant data of this study/trial are not to be shared due to some limitations, and no other study-related document will be made available.
Acknowledgments
We would like to thank all the participants and staff of the Northern Shanghai Study. This study was conducted in accordance with the Declaration of Helsinki.
Funding Statement
This study was financially supported by the Shanghai Municipal Government (Grant ID. 2013ZYJB0902; 15GWZK1002) and the National Key Technology R&D (2017YFC0111800). Dr. Yi Zhang was also supported by the National Nature Science Foundation of China (81670377) and the Shanghai Excellent Young Scholars Program (2017YQ065). The funders did not participate in the design of the study, the collection, analysis, interpretation of the data, or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114(25):2850–2870. doi: 10.1161/CIRCULATIONAHA.106.655688 [DOI] [PubMed] [Google Scholar]
- 3.Bluemke DA, Kronmal RA, Lima JAC, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study Of Atherosclerosis) study. J Am Coll Cardiol. 2008;52(25):2148–2155. doi: 10.1016/j.jacc.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneyama K, Venkatesh BA, Bluemke DA, et al. Cardiovascular magnetic resonance in an adult human population: serial observations from the multi-ethnic study of atherosclerosis. J Cardiovasc Magn Reson. 2017;19(1):52. doi: 10.1186/s12968-017-0367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong AC, Gidding S, Gjesdal O, et al. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC. 2012;5(8):837–848. doi: 10.1016/j.jcmg.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 7.Kishi S, Magalhaes TA, George RT, et al. Relationship of left ventricular mass to coronary atherosclerosis and myocardial ischaemia: the CORE320 multicenter study. Eur Heart J Cardiovasc Imaging. 2015;16(2):166–176. doi: 10.1093/ehjci/jeu217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 9.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19(7):1550–1558. doi: 10.1016/0735-1097(92)90617-V [DOI] [PubMed] [Google Scholar]
- 10.de Simone G. Concentric or eccentric hypertrophy: how clinically relevant is the difference? Hypertension. 2004;43(4):714–715. doi: 10.1161/01.HYP.0000121363.08252.a7 [DOI] [PubMed] [Google Scholar]
- 11.Schmieder RE. The role of non-haemodynamic factors of the genesis of LVH. Nephrol Dialysis Transplant. 2005;20(12):2610–2612. doi: 10.1093/ndt/gfi190 [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Zhu, M, Bai B., et al. Comparison of carotid-femoral and brachial-ankle pulse-wave velocity in association with target organ damage in the community-dwelling elderly chinese: the northern shanghai study. J Am Heart Assoc. 2017; 6(2);6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wachtell K, Palmieri V, Olsen MH, et al. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Losartan intervention for endpoint reduction. Am Heart J. 2002;143(2):319–326. doi: 10.1067/mhj.2002.119895 [DOI] [PubMed] [Google Scholar]
- 14.Tsioufis C, Stefanadis C, Toutouza M, et al. Microalbuminuria is associated with unfavourable cardiac geometric adaptations in essential hypertensive subjects. J Hum Hypertens. 2002;16(4):249–254. doi: 10.1038/sj.jhh.1001379 [DOI] [PubMed] [Google Scholar]
- 15.Ratto E, Leoncini G, Viazzi F, et al. Inappropriate left ventricular mass is associated with microalbuminuria independently of left ventricular hypertrophy in primary hypertension. J Hypertens. 2008;26(2):345–350. doi: 10.1097/HJH.0b013e3282f2b149 [DOI] [PubMed] [Google Scholar]
- 16.Hemmati R, Gharipour M, Shemirani H, et al. Urine albumin to creatinine ratio and echocardiographic left ventricular structure and function in patients with essential hypertension. Am Heart Hosp J. 2011;9(2):90–94. doi: 10.15420/ahhj.2011.9.2.90 [DOI] [PubMed] [Google Scholar]
- 17.Salvetti M, Paini A, Facchetti R, et al. Relationship between vascular damage and left ventricular concentric geometry in patients undergoing coronary angiography: a multicenter prospective study. J Hypertens. 2019;37(6):1183–1190. doi: 10.1097/HJH.0000000000002052 [DOI] [PubMed] [Google Scholar]
- 18.Park SK, Jung JY, Kang JG, et al. Mildly decreased renal function and its relation to left ventricular geometry change. Circ j. 2019;83(11):2236–2241. doi: 10.1253/circj.CJ-19-0353 [DOI] [PubMed] [Google Scholar]
- 19.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–1607. doi: 10.1016/j.jacc.2009.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Xiong J, Yu S, et al. Northern Shanghai Study: cardiovascular risk and its associated factors in the Chinese elderly-a study protocol of a prospective study design. BMJ Open. 2017;7(3):e013880. doi: 10.1136/bmjopen-2016-013880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji H, Zhang H, Xiong J, et al. eGFRs from Asian-modified CKD-EPI and Chinese-modified CKD-EPI equations were associated better with hypertensive target organ damage in the community-dwelling elderly Chinese: the Northern Shanghai Study. Clin Interv Aging. 2017;12:1297–1308. doi: 10.2147/CIA.S141102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Protogerou AD, Iaria P, et al. Prognosis in the hospitalized very elderly: the PROTEGER study. Int J Cardiol. 2013;168(3):2714–2719. doi: 10.1016/j.ijcard.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458. doi: 10.1016/0002-9149(86)90771-X [DOI] [PubMed] [Google Scholar]
- 24.Touboul PJ, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the advisory board of the 3rd and 4th watching the risk symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovascular dis. 2007;23:75–80. [DOI] [PubMed] [Google Scholar]
- 25.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 26.Ji H, Xiong J, Yu S, et al. Measuring the carotid to femoral pulse wave velocity (Cf-PWV) to evaluate arterial stiffness. J Visualized Exp. 2018; 135. doi: 10.3791/57083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng CS, Li Y, Li L-H, et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64(5):1124–1130. doi: 10.1161/HYPERTENSIONAHA.114.04063 [DOI] [PubMed] [Google Scholar]
- 28.Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European society of cardiology working group on peripheral circulation: endorsed by the association for research into arterial structure and physiology (ARTERY) society. Atherosclerosis. 2015;241(2):507–532. doi: 10.1016/j.atherosclerosis.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Huang B, Liu M, et al. Effects of different types of antihypertensive agents on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis. 2015;7(12):2339–2347. doi: 10.3978/j.issn.2072-1439.2015.12.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace SM, Yasmin McEniery CM, Mäki-Petäjä KM, et al. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50(1):228–233. doi: 10.1161/HYPERTENSIONAHA.107.089391 [DOI] [PubMed] [Google Scholar]
- 31.Payne RA, Wilkinson IB, Webb DJ, et al. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010;55(1):9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464 [DOI] [PubMed] [Google Scholar]
- 32.Darne B, Girerd X, Safar M, et al. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13(4):392–400. doi: 10.1161/01.HYP.13.4.392 [DOI] [PubMed] [Google Scholar]
- 33.Girerd X, Laurent S, Pannier B, et al. Arterial distensibility and left ventricular hypertrophy in patients with sustained essential hypertension. Am Heart J. 1991;122(4):1210–1214. doi: 10.1016/0002-8703(91)90941-A [DOI] [PubMed] [Google Scholar]
- 34.Ohyama Y, Ambale-Venkatesh B, Noda C, et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2016;9(7). doi: 10.1161/CIRCIMAGING.115.004426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toprak A, Reddy J, Chen W, et al. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the bogalusa heart study). Am J Cardiol. 2009;103(7):978–984. doi: 10.1016/j.amjcard.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 36.Viazzi F, Parodi D, Leoncini G, et al. Optimizing global risk evaluation in primary hypertension: the role of microalbuminuria and cardiovascular ultrasonography. J Hypertens. 2004;22(5):907–913. doi: 10.1097/00004872-200405000-00011 [DOI] [PubMed] [Google Scholar]
- 37.Pontremoli R, Ravera M, Bezante GP, et al. Left ventricular geometry and function in patients with essential hypertension and microalbuminuria. J Hypertens. 1999;17(7):993–1000. doi: 10.1097/00004872-199917070-00016 [DOI] [PubMed] [Google Scholar]
- 38.Leoncini G, Sacchi G, Ravera M, et al. Microalbuminuria is an integrated marker of subclinical organ damage in primary hypertension. J Hum Hypertens. 2002;16(6):399–404. doi: 10.1038/sj.jhh.1001408 [DOI] [PubMed] [Google Scholar]
- 39.Jager A, Kostense PJ, Ruhé HG, et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19(3):617–624. doi: 10.1161/01.ATV.19.3.617 [DOI] [PubMed] [Google Scholar]
- 40.Jensen JS, Feldt-Rasmussen B, Strandgaard S, et al. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35(4):898–903. doi: 10.1161/01.HYP.35.4.898 [DOI] [PubMed] [Google Scholar]
- 41.Lieb W, Gona P, Larson MG, et al. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC: Cardiovascular Imaging. 2014;7(9):870–878. doi: 10.1016/j.jcmg.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miszalski-Jamka T, Lichołai S, Karwat K, et al. Computed tomography characteristics of coronary artery atherosclerosis in subjects with lower extremity peripheral artery disease and no cardiac symptoms. Pol Arch Med Wewn. 2013;123(12):657–663. doi: 10.20452/pamw.2005 [DOI] [PubMed] [Google Scholar]
- 43.Muiesan ML, Salvetti M, Zulli R, et al. Structural association between the carotid artery and the left ventricle in a general population in Northern Italy: the Vobarno study. J Hypertens. 1998;16(12):1805–1812. doi: 10.1097/00004872-199816120-00014 [DOI] [PubMed] [Google Scholar]