Abstract
Within a given free-range flock, some hens prefer to spend the majority of their time in the shed (stayers), while others frequently access the range (rangers). Laying performance has been associated not only with the development of these sub-populations but also with different body weights (BW). The purpose of this study was to determine if range usage, BW or a combination of both is associated with energy metabolism and as such contribute to improved hen performance. Forty-eight Lohmann Brown hens at 74 wk of age were selected from a commercial free-range farm based on their BW and range usage over a 56-week period. Using a 2 × 2 factorial arrangement, hens were either classified as heavy (mean ± SEM; 2.01 ± 0.02 kg, n = 24) or light (1.68 ± 0.01 kg, n = 24), and also classified as rangers (accessed the range for 84.1% of available days, 242 ± 3.75 d; n = 24) or stayers (accessed the range for 7.17% of available days; 23.4 ± 6.08 d, n = 24). Stayers had significantly higher metabolizable energy (ME) intake per metabolic BW per d (0.852 vs. 0.798 MJ/kg BW0.75 per d; P = 0.025), higher heat production (0.637 vs. 0.607 MJ/kg BW0.75 per d; P = 0.005), higher heat increment (0.267 vs. 0.237 MJ/kg BW0.75 per d; P = 0.005) and retained more nitrogen (1.59 vs. 1.46 g/hen per d; P = 0.023) compared to the rangers. Light hens had significantly higher metabolic energy intake per metabolic BW (0.854 vs. 0.796 MJ/kg BW0.75 per d; P = 0.018), net energy (NE) intake (0.595 vs. 0.551 MJ/kg BW0.75 per d; P = 0.032), and retained energy (0.225 vs. 0.181 MJ/kg BW0.75 per d; P = 0.032), as well as lower heat production (0.936 vs. 1.003 MJ/hen per d; P = 0.002) compared to heavier hens. An interaction was observed across levels of analysis i.e. between light stayers and light rangers. The light rangers had significantly higher NE intake compared to the light stayers (9.77 vs. 9.27 MJ/kg BW0.75 per d; P = 0.024). In conclusion, light hens were more energy efficient compared to heavy hens. Moreover, light rangers had a more efficient feed utilisation compared to the light stayers.
Keywords: Egg production, Health, Husbandry, Metabolizable energy, Poultry nutrition, Welfare
1. Introduction
Cage-free husbandry systems such as barn or free-range facilities allow hens to exhibit a variety of behaviors including running, flying, dust and solar bathing, beak cleaning, preening, and stretching (Miao et al., 2005). This freedom of movement and locomotion can affect metabolic energy needs (Armstrong and Cermak, 1989). In free-range systems, the metabolic need of individual hens may vary even further due to the exposure of some hens to adverse weather conditions on the range (e.g. temperatures and humidity above or below the thermoneutral zone) (Olson et al., 1972, Peguri and Coon, 1993; Yahav et al., 1996). In addition, free-range hens may have an opportunity to forage and ingest a variety of pastures, shrubs, insects and other materials present in the extended environment which can interfere with the digestion of their formulated diet (Glatz and Ru, 2001, Glatz and Ru, 2002, Svihus, 2012). Within a given free-range flock, some hens prefer to spend most of their time in the shed (stayers), while others frequently access the range (rangers) (Gebhardt-Heinrich et al., 2014). The development of these sub-populations has been associated with different hen performance. For example, hens that range frequently come significantly earlier into lay (Sibanda and Ruhnke, 2018). Singh et al. (2016) and Iqbal et al. (2017) showed that hens with access to pasture are frequently heavier compared to stayers or hens without pasture access, indicating that the range or pasture access per se may contribute to a better nutrient utilisation. This is most likely due to the additional intake of the structural fibre component present in pasture as fibre in the diets have shown to have beneficial effects on the digestive organ development, starch digestibility and consequently feed efficiency (Hetland and Svihus, 2001, Amerah et al., 2009). To date it remains unclear to what degree body (and organ) weight as such, or/and the range access contributes to energy requirements and if there is a need to manage this for improved egg production. It has previously been observed that hens housed in loose husbandry systems have higher feed conversion ratios (FCR) than caged hens, and also that lighter hens are more feed efficient than heavy ones (Tiller, 2001, Akter et al., 2019). While the higher activity level of non-caged hens is considered responsible for the higher maintenance energy of free-range and barn housed hens, lighter hens seem to utilise their nutrients for egg production rather than body fat content (Tiller, 2001, Akter et al., 2019). The evaluation of the efficiency of energy utilisation in laying hens provides a comprehensive method of optimizing production (Reid et al., 1978, de Almeida Brainer et al., 2012). The net energy (NE) is the metabolizable energy (ME) of the feed corrected for the energy losses in the heat increment due to digestion and cellular processes and is directly available for maintenance, growth and production. Therefore, NE is a more accurate reflection of the energy requirement (Pirgozliev and Rose, 1999). While body weight (BW) and range usage vary widely in free-range housing conditions, the aim of this study was to determine the impact of BW and range usage on NE utilisation of commercial free-range laying hens.
2. Materials and methods
2.1. Experimental animals
This research was approved by the Animal Ethics Committee (AEC17-120), University of New England, NSW, Australia. Forty-eight Lohmann Brown hens at 74 wk of age were selected from a commercial free-range farm based on their BW as well as their range usage recorded over a 56-week period. Prior selection, the experimental animals were housed amongst a 40,000-hen flock and traced for range usage using a custom-made RFID system (Science and Engineering workshop at the University of New England, Armidale, NSW, Australia) as previously described by Sibanda et al. (2019). At time of depopulation, the 48 hens subject to this research were selected by screening for hens that spent more than 75% or less than 10% of their available days on the range and met also the selection criteria of presenting a BW either above 1.90 kg or below 1.70 kg. Cut off points for range access and BW were determined based on the 90th percentile results obtained from the selection cohort. The 48 hens were either classified as heavy (mean ± SEM; 2.01 ± 0.02 kg, n = 24) or light (1.68 ± 0.01 kg, n = 24), and also classified as rangers (accessed the range for 84.1% of available days, 242 ± 3.75 d; n = 24) or stayers (accessed the range for 7.17% of available days; 23.4 ± 6.08 d, n = 24).
2.2. NE measurements
On the day of hen selection, hens were transported to the experimental facilities at the University of New England and placed in calorimetry chambers for 10 d with ad libitum feed and water allowing for acclimatisation to the new, climate-controlled environment. The diet offered was identical with the one fed on the commercial farm prior to hen relocation (Table 1). Hens were assigned to 1 of the 4 treatment groups according to a 2 × 2 factorial arrangement with range use (stayers or rangers) and BW (heavy or light) as 2 factors resulting in the following: 12 hens classified as Rangers Heavy (RH), 12 hens classified as Rangers Light (RL), 12 hens classified as Stayers Heavy (SH), and 12 hens classified as Stayers Light (SL). Three hens of the same category were placed together in one closed-circuit calorimetry chamber (Barzegar et al., 2019). The cage was considered to be a replicate and 4 replicates per treatment were applied.
Table 1.
Composition and calculated nutrient content of the diet fed to the experimental hens (as fed, %).
| Item | Content |
|---|---|
| Ingredients | |
| Sorghum | 45.8402 |
| Soybean meal | 14.2115 |
| Wheat | 10.0 |
| Barley | 9.00 |
| Meat meal | 7.50 |
| Lime grit | 7.4276 |
| Canola meal | 2.00 |
| Vegetable oil | 1.00 |
| Opticell | 0.8333 |
| Single-cell protein | 0.6667 |
| Blood meal | 0.50 |
| Methionine hydroxide-liquid | 0.2616 |
| Layer premix1 | 0.20 |
| Salt | 0.1333 |
| Sodium bicarbonate | 0.10 |
| Lysine-HCl | 0.09 |
| Choline Cl liquid | 0.0667 |
| Synthetic emulsifier2 | 0.04 |
| Egg yolk pigments3 | 0.06 |
| L-threonine | 0.0359 |
| Liquid xylanase | 0.0333 |
| Nutrient content of the diet | |
| ME, kcal/kg | 2,728.9 |
| ME including enzyme activity, kcal/kg | 2,775 |
| Crude protein | 18.9 |
| Lysine | 0.92 |
| Methionine | 0.49 |
| Methionine + Cysteine | 0.79 |
| Threonine | 0.69 |
| Isoleucine | 0.7 |
| Leucine | 1.58 |
| Tryptophan | 0.21 |
| Arginine | 1.11 |
| Histidine | 0.45 |
| Valine | 0.88 |
| T. Dig. Lysine | 0.83 |
| T. Dig. Methionine | 0.45 |
| T. Dig. Methionine + Cysteine | 0.70 |
| T. Dig. Threonine | 0.60 |
| T. Dig. Isoleucine | 0.64 |
| T. Dig. Leucine | 1.40 |
| T. Dig. Tryptophan | 0.18 |
| T. Dig. Arginine | 1.00 |
| T. Dig. Valine | 0.79 |
| Crude fat | 4.10 |
| Linoleic acid | 1.21 |
| Crude fibre | 3.41 |
| Starch | 38.5 |
| Total xanthophyll | 0.001 |
| Red xanthophyll | 0.001 |
| Phytate phosphate | 0.23 |
| Ash | 12.4 |
| Calcium | 3.90 |
| Av. phosphate | 0.63 |
| Total phosphate | 0.76 |
| Sodium | 0.21 |
| Chloride | 0.21 |
| Potassium | 0.65 |
| Vitamin and mineral premix1 | 0.2 |
T. Dig. = total digestible; Av. = available.
Layer premix, vitamin and mineral premix per tonne diet: vitamin A, 8 MIU; vitamin E, 30 g; thiamin B1, 2 g; niacin (vitamin B3), 30 g; pantothenic acid (vitamin B5), 6.44 g; folate (vitamin B9), 1.5 g; biotin (vitamin H), 300 mg; pyridoxine (vitamin B6), 4.5 g; vitamin B12, 30 mg; vitamin D, 3 MIU; vitamin K, 3.5 g; copper, 3.74 g; zinc, 30 g; selenium, 0.2 g; manganese, 30 g; chromium, 0.15 g; iron, 11.25 g. MIU = million international units.
Synthetic emulsifier: each 1 kg contains 100% Glycerol polyethyleneglycol ricinoleate (Bredol, Akzo, Nobel, Sweden).
Egg yolk pigments: xanthophylls and canthaxanthin provided by Jabiru yellow and Jabiru Red L (Jabiru, Bowral, NSW, Australia).
After the 10-d acclimatization period, hens were maintained an additional 4 d in the closed-circuit calorimetry chamber with air pumps running. After this period, the closed-circuit calorimetry chambers were sealed airtight with a lid over a trough filled with water and their feed intake, excreta output, egg production, oxygen intake and carbon dioxide output were measured in 24 h intervals for the duration of 3 d following an established protocol (Swick et al., 2013, Wu et al., 2019, Barzegar et al., 2019). All parameters involving energy balance, nitrogen retention (RN) and egg number were calculated as previously described (Barzegar et al., 2019).
2.3. pH and organ weight
Immediately before and after completion of the NE chamber measurement, all the hens were individually weighted before being placed in the energy chamber and at the completion of the NE chamber measurement. Hens were humanely sacrificed, and gross necropsy performed at the end of the experiment. The pH of the individual gastrointestinal segments from crop to cecum were measured directly by inserting the spear tip probe of a pH meter (Ecoscan, Eutech instruments, Singapore) into the digesta. The empty organ weights of crop, gizzard, pancreas and liver (after visceral fat was removed) were obtained using a commercial scale (Shinko Denshi, RoHS Compliant, Japan) and were expressed as the percentages relative to the BW of the hens. The values were calculated using the following equation:
| Relative organ weight = (Organ weight/BW) × 100% |
2.4. Statistical analysis
Statistical analysis was performed using SPSS statistics v.24 (IBM Corp., Armonk, NY, USA). Two-way factorial ANOVA was conducted to compare the main effects of range usage and BW and their interactions. Further analysis of interaction was followed using One-way ANOVA within and between the levels of the analysis. The graphs were created using JMP Statistics software (v14 IBM SAS Institute Inc., Cary, NC). The level of significance was set at P < 0.05.
3. Results
3.1. Energy and nitrogen balance
The stayers had significantly higher ME intake (P = 0.025), higher heat production (P = 0.005), and higher heat increment per metabolic BW (P = 0.005) compared to the rangers (Table 2). Similarly, stayers retained a greater fraction of nitrogen intake (P = 0.023) compared to the rangers. The light hens had significantly higher metabolic energy intake (P = 0.018), NE intake (P = 0.032), and retained energy per metabolic BW (P = 0.032) whereas lower heat production (P = 0.002) compared to the heavy hens as shown in Table 2. An interaction was observed between BW and range where rangers had significantly higher NE intake compared to the stayers only in light birds (P = 0.024; Table 3) but not in heavy birds.
Table 2.
Energy utilisation of commercial free-range laying hens.
| Main effect |
GE intake, MJ | ME (DM feed basis), MJ/kg | MEi, kg/BW0.75 | RE, kg/BW0.75 | NE (DM basis), MJ/kg | HP/hen per d, MJ/kg | HP/hen per d, MJ/kg BW0.75 | HI, MJ/kg BW0.75 | NEi, MJ/kg BW0.75 | NE/ME | RN/hen per d, g | RN, % | Total egg number/3 hens per 3 d | LR, % | Total egg mass/hen per d, g | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RU | BW | |||||||||||||||
| Rangers | 15.07 | 13.67 | 0.7975a | 0.1904 | 9.59 | 0.965 | 0.607b | 0.237a | 0.5604 | 0.70 | 1.46a | 45.6 | 8.210 | 91.30 | 60.80 | |
| Stayers | 15.44 | 13.69 | 0.8517b | 0.2150 | 9.41 | 0.9738 | 0.637a | 0.267b | 0.5850 | 0.71 | 1.59b | 48.4 | 7.790 | 86.70 | 63.20 | |
| Light | 15.09 | 13.67 | 0.8537b | 0.2245b | 9.52 | 0.935a | 0.629 | 0.259 | 0.5945b | 0.69 | 1.54 | 48.2 | 8.460 | 94.00 | 63.19 | |
| Heavy | 15.41 | 13.69 | 0.7955a | 0.1809a | 9.48 | 1.003b | 0.615 | 0.245 | 0.5509a | 0.69 | 1.49 | 45.7 | 7.540 | 84.00 | 60.81 | |
| Two-way interaction (RU × BW) | ||||||||||||||||
| Rangers | Light | 14.64 | 13.80b | 0.8243 | 0.2143 | 9.77b | 0.9193 | 0.610 | 0.2400 | 0.5843 | 0.70 | 1.49 | 47.9 | 8.670 | 96.30 | 61.34 |
| Rangers | Heavy | 15.50 | 13.54ab | 0.7708 | 0.1665 | 9.42ab | 1.0113 | 0.6045 | 0.2345 | 0.5365 | 0.69 | 1.42 | 43.2 | 7.750 | 86.30 | 60.27 |
| Stayers | Light | 15.55 | 13.54a | 0.8830 | 0.1953 | 9.27a | 0.9515 | 0.6483 | 0.2783 | 0.6048 | 0.69 | 1.61 | 48.5 | 8.250 | 91.80 | 60.29 |
| Stayers | Heavy | 15.32 | 13.85ab | 0.8203 | 0.2348 | 9.55ab | 0.9940 | 0.6247 | 0.2547 | 0.5653 | 0.70 | 1.57 | 48.2 | 7.330 | 81.70 | 66.12 |
| P-values | ||||||||||||||||
| RU | 0.477 | 0.850 | 0.025 | 0.192 | 0.190 | 0.652 | 0.005 | 0.005 | 0.192 | 0.318 | 0.023 | 0.076 | 0.373 | 0.373 | 0.988 | |
| BW | 0.539 | 0.834 | 0.018 | 0.032 | 0.795 | 0.002 | 0.107 | 0.107 | 0.032 | 0.130 | 0.302 | 0.101 | 0.067 | 0.067 | 0.304 | |
| RU × BW | 0.298 | 0.044 | 0.830 | 0.816 | 0.040 | 0.155 | 0.296 | 0.296 | 0.816 | 0.470 | 0.750 | 0.145 | 1.000 | 1.000 | 0.981 | |
RU = range usage; BW = body weight; GE = gross energy; ME = metabolizable energy; MEi = metabolizable energy intake; RE = retained energy; NE = net energy; HP = heat production; HI = heat increment; NEi = net energy intake; RN = retained nitrogen; LR = laying rate.
Means were obtained from 4 replicate cages of 3 birds each.
a, b Within a column, means with no common superscript are significantly different at P < 0.05.
Table 3.
One-way ANOVA analysis of 4 different sub groups1 obtained from a commercial free-range farm kept in net energy chambers (DM basis, MJ/kg).
| Item | ME | NE | |
|---|---|---|---|
| Treatment groups1 | RL | 13.80 | 9.77 |
| RH | 13.54 | 9.42 | |
| SL | 13.54 | 9.27 | |
| SH | 13.85 | 9.55 | |
| P-values | RH × RL | 0.172 | 0.094 |
| RH × SH | 0.109 | 0.519 | |
| RH × SL | 0.988 | 0.404 | |
| RL × SL | 0.168 | 0.024 | |
| SH × SL | 0.106 | 0.171 | |
ME = metabolizable energy; NE = net energy; DM = dry matter; RL = Rangers Light; RH = Rangers Heavy; SL = Stayers Light; SH = Stayers Heavy.
RL, hens with light BW which frequently access the range; RL, hens with heavy BW which frequently access the range; SL, hens with light BW which prefer to spend the majority of their time in the shed; SH, hens with heavy BW which prefer to spend the majority of their time in the shed, within a given free-range flock.
3.2. Organ size or weight, and pH
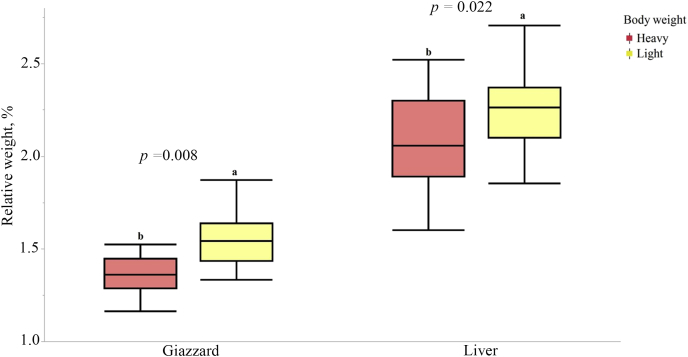
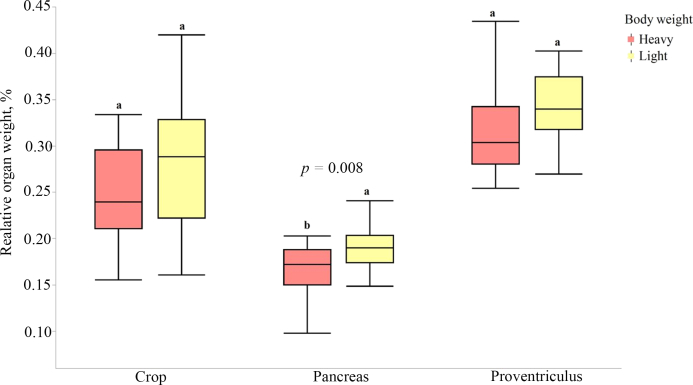
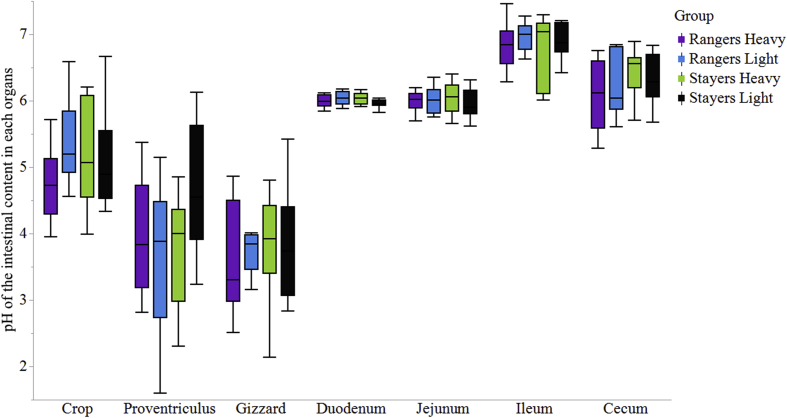
The total gizzard, liver and pancreas weight were found to be different in heavy and light hens where heavy hens had significantly smaller relative gizzard (1.40% vs. 1.53%; P = 0.008), liver (2.08% vs. 2.3%; P = 0.022) and pancreas (0.17% vs. 0.19%; P = 0.008) weight compared to the light hens (Fig. 1, Fig. 2). Organ weight was found to be significantly different based on BW but not on range usage. The pH of the organs did not differ significantly (P > 0.05) among the 4 treatment groups (Fig. 3).
Fig. 1.
Relative gizzard and pancreas weight of the heavy and light hens from a commercial free-range farm kept in net energy chambers. a, b Means with different superscripts indicate difference (P < 0.05).
Fig. 2.
Relative crop, pancreas and proventriculus weight of the heavy and light hens from a commercial free-range farm kept in net energy chambers. a, b Means with different superscripts indicate difference (P < 0.05).
Fig. 3.
pH of the intestinal content in each organ of the stayers- and rangers-groups from a commercial free-range farm kept in net energy chambers. RL = Rangers Light; RH = Rangers Heavy; SL = Stayers Light; SH = Stayers Heavy. RL, hens with light BW which frequently access the range; RL, hens with heavy BW which frequently access the range; SL, hens with light BW which prefer to spend the majority of their time in the shed; SH, hens with heavy BW which prefer to spend the majority of their time in the shed, within a given free-range flock.
4. Discussion
The current study demonstrates that the NE utilisation differs between heavy and light stayers and rangers when being confined in the closed-circuit NE chamber. This clearly indicates that the capacity to use energy differs among the investigated flock subpopulations.
The ME and NE intakes expressed in metabolic BW (kg BW0.75) were significantly higher in light hens compared to heavy hens. On contrary, heavy hens had significantly higher heat production compared to light hens (Table 2) which indicates that the more ME might have been used for heat production rather than maintenance of egg production in heavy hens. This is in line with the research performed by Akter et al. (2019) investigating Isa Brown caged hens, where light hens were significantly more feed efficient than heavy hens. Moreover, Parkinson et al. (2015) found lighter hens to exhibit superior laying persistency compared to heavier hens. However, it was observed that the relative weight of the organs associated with metabolism such as liver, pancreas, and gizzard were significantly higher in the light hens subject to this study. The visceral organs mass can have a high energy expenditure rate and as such increase the total energy requirement (Ferrell and Koong, 1986; Jorgensen et al., 1996). However, this was not observed in the hens subject to our research where lighter individuals were more efficient despite the higher relatively organ weights. Carre et al. (2008) reported that inefficient hens were heavier and had greater fat deposition compared to lean highly efficient hens. This has also been observed in the light caged hens (which were also the most feed efficient hens) of Akter et al. (2019), who had significantly less intraabdominal fat compared to their heavy counterparts. The higher relative gizzard weight observed in this research was suspected to lead to greater nutrient availability in the small intestine and therefore greater feed efficiency (Akter et al., 2019). This might indicate that the light hens are more energy efficient compared to the heavy hens and therefore, there is a need to determine the energy requirements and the lower BW threshold limit for feed formulation and better energy efficiency. In order to distinguish more precisely between energy deposited in organ development, egg production or fat storage, the intraabdominal fat pad and the carcass composition should be measured in all future NE studies.
The stayers had a significantly higher ME intake per metabolic BW compared to the rangers when being confined in the NE chambers. While this may lead to the conclusion that the increased ME intake in stayers might be related to their higher requirement for maintenance, it may also be possible that the ME was used for heat production, indicated by the higher heat increment and heat production (Table 2). Care must be taken to consider that the confined situation in the NE chambers provides a different environment than the free range shed and the energy expenditure that we observed in the experimental conditions may be significantly different when the hens were located in the field. Interestingly, light rangers had significantly higher ME and NE intake which showed that they were able to metabolize their energy more efficiently, indicating their ability to better derive energy per unit of feed than the light stayers. Moreover, the ME for the stayers might have been lost as heat for maintenance. Kolakshyapati et al., 2019a, Kolakshyapati et al., 2019b has shown that the stayers are more fearful compared to the rangers. Moreover, there was an increase in corticosterone level in the stayers compared to rangers (Kolakshyapati et al., 2019a, Kolakshyapati et al., 2019b). Any form of stress in birds such as poor weather, predation, social stress etc. can result in increased levels of corticosterone that modifies body metabolism (Wingfield and Kitaysky, 2002, Downing and Bryden, 2008). This might have been the case in the field situation, resulting in the development of different coping styles (Campbell et al., 2016), allowing stayers and rangers to respond differently to the transport and NE chamber confinement. Therefore, it might be possible that the stayers are more stressed than rangers which might impact their energy metabolism.
In a study by Singh et al. (2016), ranging hens at 63 wk of age showed increased gizzard and intestinal weight which might contribute to better nutrient utilisation. On contrary, there was no difference in the organ weight between rangers and stayers in hens subject to the present research. Range use provides opportunities for hens to explore and ingest a variety of materials such as forage, insects and others (Glatz and Ru, 2001, Glatz and Ru, 2002, Svihus, 2012) that might alter the microbial composition of these hens. The different microbial population in the gastrointestinal tract plays an important role in the energy efficiency and is different in high and low performing birds (Stanley et al., 2012). Therefore, it will be interesting to investigate the microbial composition and their metabolites in rangers and stayers.
5. Conclusion
In conclusion, the light rangers were significantly more efficient in feed utilisation compared to light stayers and the energy from feed was more available for production. This information is important for poultry nutritionists, formulating diets of various nutrient composition. If feed formulation is based on NE requirements, the percentage of a flock that utilises the range as well as the expected BW should be taken into account. Further research is required to determine a minimum BW threshold on NE metabolism of the modern laying hen. Alternative methodology to measure energy utilisation by free range birds without confining them to chambers would be beneficial in future research studies to allow more precise data collection when investigating rage use.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
We would like to thank Australian Eggs and the Poultry Cooperative Reserach Centre, established and supported under the Australian Government’s Cooperative Research Centres Program for funding the background research (project number 1UN151) that allowed us to select hens with known range use. We also thank the commercial farm involved for providing access to its infrastructure, hens and diet. We would also like to thank Jessica de Souza Vilela, Sosthene Musigwa, Andrew Cohen-Barnhouse, Shahram Barzegar, Jenny Wittig, Mark Porter, Dr. Chake Keerqin, Dr. Mehdi Toghyani Khorasgani, Matt Hilliar, David Trennery, and Gary Taylor for assistance in conducting the work.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Akter Y., Groves P.J., Liu S.Y., Moss A.F., Anene D., O'Shea C.J. Association of feed to egg efficiency with body weight and digestive organ characteristics in laying hens. Proc Aust Poult Sci Symp. 2019:249–252. [Google Scholar]
- Amerah A.M., Ravindran V., Lentle R.G. Influence of insoluble fibre and whole wheat inclusion on the performance, digestive tract development and ileal microbiota profile of broiler chickens. Br Poultry Sci. 2009;50(3):366–375. doi: 10.1080/00071660902865901. [DOI] [PubMed] [Google Scholar]
- Armstrong B., Cermak J.P. Review of some developments in animal housing systems-pigs and poultry. Br Vet J. 1989;145:426–435. doi: 10.1016/0007-1935(89)90050-X. [DOI] [PubMed] [Google Scholar]
- Barzegar S., Wu S., Noblet J., Choct M., Swick R.A. Energy efficiency and net energy prediction of feed in laying hens. Poultry Sci. 2019;98(11):5746–5758. doi: 10.3382/ps/pez362. [DOI] [PubMed] [Google Scholar]
- Campbell D.L.M., Hinch G.N., Downing J.A., Lee C. Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. Appl Anim Behav Sci. 2016:73–77. [Google Scholar]
- Carre B.S., Mignon-Grasteau S., Juin H. Breeding for feed efficiency and adaptation to feed in poultry. World’s Poult Sci J. 2008;64:377–390. [Google Scholar]
- de Almeida Brainer M.M., Rabello C.B.V., Lopes C.C., Lima R.A., Arruda E.M.F., Neto R.F. Metabolizable energy requirements for maintenance and egg production of free-range laying hens by multiple linear equation. World’s Poult Sci J. 2012;(1):5–9. [Google Scholar]
- Downing J.A., Bryden W.L. Determination of corticosterone concentrations in egg albumen: a non-invasive indicator of stress in laying hens. Physiol Behav. 2008;9:381–387. doi: 10.1016/j.physbeh.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ferrell C., Koong K.J. Influence of plane of nutrition on body composition, organ size and energy utilization of Sprague-Dawley rats. J Nutr. 1986;12:2525–2535. doi: 10.1093/jn/116.12.2525. [DOI] [PubMed] [Google Scholar]
- Gebhardt-Heinrich S.G., Toscano M.J., Frohlich E.K.F. Use of outdoor ranges by laying hens in different sized flocks. Appl Anim Behav Sci. 2014;155:74–81. [Google Scholar]
- Glatz P.C., Ru Y.J. Eco shelter housing of free-range poultry integrated into a pasture/crop rotation system in the wheat belt of Australia. In: Oester H., Wyss C., editors. Australian proceedings of the 6th European symposium on poultry. 2001. pp. 299–301. September 1-4, 2001. [Google Scholar]
- Glatz P.C., Ru Y.J. Free-range poultry in a pasture/crop rotation system. Proceedings of Poultry Information Exchange. 2002:7–10. [Google Scholar]
- Hetland H., Svihus B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br Poultry Sci. 2001;42(3):354–361. doi: 10.1080/00071660120055331. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Roberts J., Perez-Maldonado R.A., Goodarzi Boroojeni F., Swick R.A., Ruhnke I. Pasture, multi-enzymes, benzoic acid and essential oils positively influence performance, intestinal organ weight and egg quality in free-range laying hens. Br Poultry Sci. 2017;59(2):180–189. doi: 10.1080/00071668.2017.1403566. [DOI] [PubMed] [Google Scholar]
- Jorgensen B.H., Zhao X.Q., Knudsen K.E.B., Eggum B.O. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br J Nutr. 1996;75:379–395. doi: 10.1079/bjn19960141. [DOI] [PubMed] [Google Scholar]
- Kolakshyapati M., Sibanda T.Z., Taylor P.S., Ruhnke I. Proceedings of the 53rd congress of the international society of applied ethology. 2019. Association of fearfulness at the end of lay with range visits at 18-22 weeks of age in commercial laying hens; p. 342. [Google Scholar]
- Kolakshyapati M., Sibanda T.Z., Downing J., Schneider D., Boshoff J., Welch M., Ruhnke I. Egg corticosterone concentrations after acute stress exposure in free range hens with different range usage. Aust Poult Sci Symp. 2019;30:259. [Google Scholar]
- Miao Z.H., Glatz P.C., Ru Y.J. Free-range poultry production - a review. Asian-Australian J Anim Sci. 2005;18(1):113–132. [Google Scholar]
- Olson D.W., Sunde M.L., Bird H.R. The effect of temperature in metabolizable energy determination and utilisation by the growing chick. Poultry Sci. 1972;51:1915–1922. [Google Scholar]
- Parkinson G.B., Roberts J., Horn R. Pullet and layer flock uniformity, persistency and longevity: an epidemiological, industry-based approach to improve feed efficiency. AECL Publication. 2015;1UN112:xi. [Google Scholar]
- Peguri A., Coon C. Effect of feather coverage and temperature on layer performance. Poultry Sci. 1993;72:1318–1329. [Google Scholar]
- Pirgozliev V., Rose S.P. Net energy systems for poultry feeds: a quantitative review. World’s Poult Sci J. 1999;55:23–36. [Google Scholar]
- Reid B.L., Valencia M.E., Maiorino M. Energy utilisation by laying hens. I. Energetic efficiencies of maintenance and production. Poultry Sci. 1978;57:461–465. doi: 10.3382/ps.0570461. [DOI] [PubMed] [Google Scholar]
- Sibanda T.Z., Ruhnke I. Proceedings of the Poultry Information Exchange and Australasian Milling Conference. -; 2018. Nutritional management of laying hens; pp. 147–148. [Google Scholar]
- Sibanda T.Z., Walkden-Brown S.W., Kolakshyapati M., Dawson B., Schneider D., Welch M., Iqbal Z., Cohen-Barnhouse A., Morgan N.K., Boshoff J., Ruhnke I. Flock and individual hen variation in range usage is associated with multi-tier aviary usage in commercial free-range laying hens. Br Poultry Sci. 2019 doi: 10.1080/00071668.2019.1686123. [DOI] [PubMed] [Google Scholar]
- Singh M., Hernandez C.E., Hinch G., Cowieson A.J. Wanderers versus stay at home: who has the better guts? Anim Prod Sci. 2016;27:78–81. [Google Scholar]
- Stanley D., Denman S.E., Huges R.J., Geier M.S., Crowly T.M., Chen H., Haring V.R., Moore R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl Microbiol Biotechnol. 2012;96:1361–1369. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- Svihus B. Gastrointestinal tract development: implications for free-range and conventional production. Aust Poult Sci Symp. 2012:7–13. [Google Scholar]
- Swick R.A., Wu S., Zuo J., Rodgers N., Barekatain M.R., Choct M. Implications and development of a net energy system for broilers. Anim Prod Sci. 2013;53:1231–1237. [Google Scholar]
- Tiller H. Nutrition and animal welfare in egg production systems. 13th Eur Symp Poult Nutr. 2001;13:226–232. [Google Scholar]
- Wingfield J.C., Kitaysky A.S. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integr Comp Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- Wu S., Swick R.A., Noblet J., Rodgers N., Cadogan D., Choct M. Net energy prediction and energy efficiency of feed for broiler chickens. Poultry Sci. 2019;98:1222–1234. doi: 10.3382/ps/pey442. [DOI] [PubMed] [Google Scholar]
- Yahav S., Straschnow A., Plavnik I., Hurwitz S. Effects of diurnally cycling versus constant temperatures on chicken growth and food intake. 1996;37(1):43–54. doi: 10.1080/00071669608417835. [DOI] [PubMed] [Google Scholar]