Abstract
Homeostatic regulation of cardiomyocytes plays a crucial role in maintaining the normal physiological activity of cardiac tissue. Severe cardiotoxicity results in cardiac diseases including but not limited to arrhythmia, myocardial infarction and myocardial hypertrophy. Drug-induced cardiotoxicity limits or forbids further use of the implicated drugs. Such drugs that are currently available in the clinic include anti-tumor drugs (doxorubicin, cisplatin, trastuzumab, etc.), antidiabetic drugs (rosiglitazone and pioglitazone), and an antiviral drug (zidovudine). This review focused on cardiomyocyte death forms and related mechanisms underlying clinical drug-induced cardiotoxicity, including apoptosis, autophagy, necrosis, necroptosis, pryoptosis, and ferroptosis. The key proteins involved in cardiomyocyte death signaling were discussed and evaluated, aiming to provide a theoretical basis and target for the prevention and treatment of drug-induced cardiotoxicity in the clinical practice.
Keywords: cardiotoxicity, cardiomyocytes, cell death, apoptosis, autophagy, necrosis
Introduction
Cardiotoxicity commonly refers to toxicity that has a detrimental impact on the heart, which might finally lead to myocardiopathy such as arrhythmia, myocardial infarction and myocardial hypertrophy. These inevitable side effects, especially of anticancer drugs, are usually the main causes of treatment termination and drug development failure. In addition, modern cancer treatments recommend a combination of multiple agents, almost always leading to synergistic side-effects (Ewer and Ewer, 2015). In the past few decades, more than 10% of clinical drugs were forced out of the market due to cardiovascular side effects, which still hindered the drug development and seriously affected the improvement of patient health. Many studies have revealed that drug-induced myocardial damage may be a stepwise process accompanied by the increase of cardiac biomarkers and structural myocardial deformation, finally resulting in left ventricular ejection fraction (LVEF) decrease (Pistillucci et al., 2015; Patel and Cornell, 2019). Currently, the widely accepted definition of cardiotoxicity is the decline in LVEF of at least 10% to less than 55% (Nicol et al., 2019). Clinical data show that cardiomyocyte death or damage concomitantly takes place with the progression of cardiotoxicity, indicating that drug-induced cardiomyocyte death may be the main cause of cardiotoxicity.
Overview of Cell Death Forms
It is widely accepted that cell death, proliferation and differentiation are essential throughout the pathological and physiological processes. Although more than ten types of cell death have been discovered to date, the most common forms of cell death in drug-induced cardiotoxicity are apoptosis, autophagy and necrosis (Galluzzi et al., 2018). In addition, recently discovered cell death forms, such as necroptosis, pyroptosis and ferroptosis, are also involved in drug-induced cardiotoxicity.
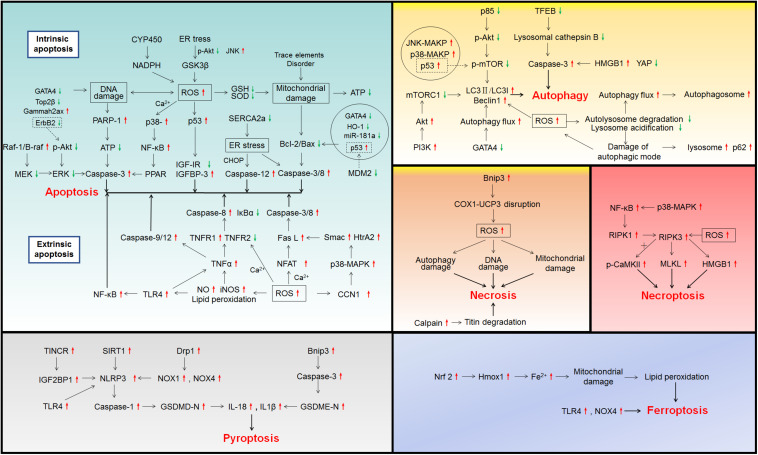
Apoptosis
Morphologically, apoptosis is the most widely studied cell death form, exhibiting signs of cell shrinkage, increased cytoplasmic density, decreased mitochondrial membrane potential (MMP) disappearance and changes in permeability. Eventually, intact apoptotic bodies are formed to be efficiently absorbed and degraded by adjacent cells. Based on the underlying mechanisms, apoptosis is divided into intrinsic and extrinsic apoptosis. Intrinsic apoptosis is caused by microenvironment disorders such as DNA damage, excessive oxidative stress, mitotic disaster, loss of growth factor signaling and endoplasmic reticulum (ER) stress (Brumatti et al., 2010; Wu and Bratton, 2013; Czabotar et al., 2014; Roos et al., 2016). B cell lymphoma-2 (Bcl-2) family pro-apoptotic members, such as Bax, Bak, and BH3-only protein, primarily regulate the intrinsic cell apoptosis via their influence on mitochondria. Bcl-2 stimulates mitochondria translocation of Bax/Bak and causes mitochondrial membrane permeabilization, which eventually leads to the release of cytochrome C in the cytoplasm, where the apoptosome forms and caspases cascade reactions arise (Hutt, 2015). Unlike intrinsic apoptosis, the extrinsic apoptosis is mainly initiated by two kinds of plasma membrane receptors including Fas cell surface death receptor (Fas) and tumor necrosis factor receptor (TNF) super family member (TNFR1, TNFRSF10A, and TNFRSF10B) along with their respective homologous ligands. Death-inducing signaling complex (DISC), composed of death ligands and corresponding receptors on the cell membrane, is a receptor proximal protein complex that helps connect the death receptor signaling with caspases cascade reactions. The pro-apoptotic proteins caspase-8 and caspase-10 are recruited and cleaved by upstream caspase enzyme to initiate apoptosis (Barnhart et al., 2003; Yang, 2015).
Autophagy
As a pro-survival mechanism, autophagy occurs in destroying and recovering unwanted or damaged cellular components, playing an essential role in maintaining intracellular metabolic homeostasis (Yang et al., 2017). A series of evidences suggest that the autophagy-activating kinase 1 (ULK-1) initiates autophagy by phosphorylating Beclin1 and activating the vacuolar protein sorting 34 (VPS34) complex (Klionsky et al., 2016). As a critical signaling protein of autophagy, the mammalian target of rapamycin (mTOR) can be activated by nutritional deficiency, growth factor and receptor tyrosine kinase, which subsequently forms mammalian target of rapamycin complex 1 (mTORC1) with several other proteins. The mTORC1 further binds to the ULK-1 complex and blocks ULK-1-mediated Beclin 1 phosphorylation, thus inhibiting autophagy initiation (Jung et al., 2010). In contrast, the adenosine 5-monophosphate activated protein kinase (AMPK) mediates autophagy by decreasing mTOR-related autophagy suppression and phosphorylating the ULK-1 complex at Ser317 and Ser777 (Kim et al., 2011).
Necrosis
In the past few decades, necrosis is typically described as a form of passive and irreversible cell death that is always associated with pathology, usually accompanied by morphological characteristics such as increased membrane permeability, disintegration of organelles and cell swelling. As a traditional cell death form, necrosis occurs usually after the exposure to extreme physical or chemical insults, and therefore is regarded as an accident and unregulated cell death form. With in-depth study, more regulated cell death forms are proposed, including necroptosis, pryoptosis and ferroptosis, which share similar morphological characteristics with necrosis.
Other Regulated Cell Death Forms: Necroptosis, Pryoptosis, and Ferroptosis
In contrast to necrosis, necroptosis is regulated by specific transduction mechanism. Death receptor TNFR1 plays a key role in the development of necroptosis (Kaiser et al., 2013). Activation of TNFR1 can stimulate RIPK1 to further recruit RIPK3 that leads to necrosomes formation (Grootjans et al., 2017). In addition, sequential activation of RIPK3/MLKL is also crucial in necroptosis signaling (Song and Wang, 2013). Phosphorylated MLKL can destroy the plasma membrane and organelles to release inflammatory factors, and elicit an immune response, indicating the occurrence of necroptosis (Galluzzi et al., 2018). It is worth mentioning that caspase-8 plays a suppressing role in necroptosis since it inactivates RIPK1 and RIPK3 (Belmonte et al., 2015; Tummers and Green, 2017).
Pyroptosis, firstly proposed by Cooksonis, is widely recognized as inflammatory and regulated cell death form that usually occurs in defense of exogenous pathogens such as virus, bacteria and fungi (Cookson and Brennan, 2001; Jorgensen and Miao, 2015). Activation of caspases including caspase-1, caspase-3, caspase-4 and caspase-11 is necessary for initiating the pyroptosis, which further specifically cleaves GSDMD or GSDME to generate holes in the membrane and release interleukin-1beta (IL-1β) and IL-18, inducing pyroptosis (Shi et al., 2015; Man et al., 2017). Recent studies have identified a potential relationship between pyroptosis and myocardial injury (Chen et al., 2018).
Unlike pyroptosis, ferroptosis is firstly discovered in carcinoma cells and characterized by the accumulation of iron and lipid reactive oxygen species (ROS), which could deplete anti-oxidases and cause mitochondrial damages, leading to cell death (Sumneang et al., 2020). Moreover, Chen et al. (2019) found that the inhibition of Toll like receptor 4 (TLR4) and triphosphopyridine nucleotide oxidase 4 (NOX4) significantly alleviated ferroptosis. Glutathione peroxidase 4 (GPx4) can prevent erastin and RSL3-induced ferroptosis via suppressing lipid peroxidation (Imai et al., 2017).
Cardiomyocyte Death in Drug-Induced Cardiotoxicity
Multiple evidences have suggested that there is a strong correlation between drug-induced cardiomyocyte death and cardiotoxicity. Here, we will summarize and discuss cardiomyocyte death induced by the drugs listed in Table 1 and their underlying cell death mechanisms shown in Figure 1.
TABLE 1.
Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity.
| Drug type | Drugs | Cell death type | Cell type/animal | Mechanism | References |
| Anticancer drug | Doxorubicin | Apoptosis | C57BL/6J mice | Cytochrome P450-NADPH-ROS↑-DNA damage | Deng et al., 2007 |
| Adult cardiac myocytes, HL-1 cardiac muscle cells | GATA4↓-DNA damage | Kim et al., 2003 | |||
| Cardiomyocytes; C57BL/6J mice | ROS↑, RNS↑-PARP-1-ATP↓ | Mukhopadhyay et al., 2009 | |||
| Sprague Dawley (SD) rats | Fe↑-ROS↑-Bcl-2/Bax↓-caspase-3↑, Cytochrome C↑ | Childs et al., 2002 | |||
| Cardiomyocytes; Mice | Top2β-DNA damage-ROS↑ | Zhang et al., 2012 | |||
| Pluripotent stem cells-derived cardiomyocytes | TNFR1↑, Fas↑, DR4↑, DR5↑, TRAIL↑ | Zhao and Zhang, 2017 | |||
| Adult rat cardiomyocytes, Rat H9c2 cardiac cells | ROS↑-Ca2+↑-NFAT↑- Fas L↑- caspase-3/8↑ | Kalivendi et al., 2005 | |||
| Neonatal rat cardiomyocytes, cardiac fibroblasts; C57/B6 mice | CFs-Fas L exosome release- cardiomyocyte | Zhang et al., 2019 | |||
| Primary cardiomyocytes | Fas L↑-caspase-8↑ | Yamaoka et al., 2000 | |||
| Rat cardiomyoblast H9c2 cells; C57BL/6 mice | ROS↑-CCN1↑-p38-MAPK↑-Smac↑, HtrA2↑-Fas L↑ | Hsu and Mo, 2016 | |||
| H9c2 cells | TNFα↑-TNFR1↑, TNFR2↓-caspase-8↑, IκBα↓ | Chiosi et al., 2007 | |||
| Male Wistar rats | ROS↑-NO↑, iNOS↑, Lipid peroxidation-TNFα↑, IL-1β↑, IL-6↑ | Akolkar et al., 2017 | |||
| SD rats | Ca2+↑-TNFα↑-caspase-9/12↑ | Agustini et al., 2016 | |||
| Adult Wistar rats | ROS↑-mitochondrial damage | Giulivi et al., 1995 | |||
| Neonatal cardiac myocytes | Mn-SOD↓-MMP↓-Bcl-2↓, Bax↑, Cytochrome C↑ | Chae et al., 2005 | |||
| Kunming mice | Mn-SOD↓-MMP↓, MPT-caspase↑ | Li W. J. et al., 2018 | |||
| NRCMs; C57BL/6 mice | miR-146a↑-ErbB4↓ | Horie et al., 2010 | |||
| Primary cardiomyocytes, H9c2 cells | miR-181a↓-Bcl-2↓ | Zhao et al., 2018 | |||
| H9c2 cells | HO-1↓-Bax↑, Cytochrome C↑ | Bernuzzi et al., 2009 | |||
| Male wild-type Balb/c mice | p53↑-Bax↑, Cytochrome C↑ | Zhang et al., 2011 | |||
| H9c2 cells | ROS↑, p53↑-IGF-IR↓, IGFBP-3↑ | Fabbi et al., 2015 | |||
| Autophagy | H9c2 cells; C57BL/6J mice | ROS↑-LC3II/LC3I↑, Beclin1↑ | Zhang et al., 2015 | ||
| Adult rat cardiomyocytes | p53↑, p38-MAKP↑, JNK-MAKP↑ | Ludke et al., 2017 | |||
| Kunming mice | p85↓-phosphorylated AKT↓-phosphorylated mTOR↓ | Yu et al., 2017 | |||
| MHC-CB7 mice | p53↑-phosphorylated mTOR↓ | Zhu et al., 2009 | |||
| Adult rat ventricular myocytes (ARVMs); Male Wistar rats | ROS↑-lysosome acidification↓-autophagy flux↑, autophagosomes↑ | Dimitrakis et al., 2012 | |||
| ARVMs, NRCMs, H9 human embryonic stem Cells induced cardiomyocytes; C57BL/6 mice | lysosome acidification↓, autolysosome degradation↓-damage of autophagic mode | Li et al., 2016 | |||
| NRCMs, adult rat cardiomyocytes, adult mouse cardiomyocytes, H9c2 rat embryonic cardiomyoblasts; C57BL/6J mice, SD rats | TFEB↓-lysosomal cathepsin B↓-caspase-3↑ | Bartlett et al., 2016 | |||
| NRCMs; Wistar rats | HMGB1↑, YAP↓-caspase-3↑ | Luo et al., 2018 | |||
| NRCMs | GATA4↓-autophagy flux↑-Bcl-2↓, Beclin 1↑ | Kobayashi et al., 2010 | |||
| Necrosis | Cardiomyocytes; C57BL/6J mice | ROS↑, RNS↑ | Mukhopadhyay et al., 2009 | ||
| H9c2 cells | nuclear swelling, DNA damage, mitochondrial dysfunction | Rharass et al., 2016 | |||
| C57BL/6J mice | ROS↑-autophagy damage | Li et al., 2014 | |||
| NRCMs, ARVMs | calpains↑-titin degradation | Lim et al., 2004 | |||
| Postnatal rat cardiac myocytes | BNIP3↑-COX1-UCP3 disruption-ROS↑- mitochondrial dysfunction | Dhingra et al., 2014 | |||
| Necroptosis | Mice | ROS↑-RIPK3 + phosphorylated-CaMKII-MTPT opening | Zhang T. et al., 2016 | ||
| Neonatal mouse ventricular myocytes; C57BL/6 mice | p38-MAPK↑-NF-κB↑-RIP1↑-RIP3↑-MLKL↑ | Yu et al., 2020 | |||
| Pyroptosis | H9c2 cells; C57BL/6J mice | TLR4↑-NLRP3↑-caspase-1↑-IL1β↑, IL-18↑ | Singla et al., 2019; Tavakoli et al., 2019 | ||
| HL-1 cardiomyocytes; C57BL/6J mice | BNIP3↑-caspase-3↑-GSDME↑ | Zheng et al., 2020 | |||
| Neonatal rat ventricular cardiomyocytes (NRVCs); H9c2 cells; C57BL/6J mice | Drp1↑-NOX1↑, NOX4↑-NLRP3↑-caspase-1↑ | Zeng et al., 2020 | |||
| Primary neonatal rat cardiomyocytes; H9c2 cells; Wistar rats | TINCR-lncRNA↑-IGF2BP1↑-NLRP3↑-caspase-3↑-GSDMD-IL1β↑, IL-18↑ | Meng et al., 2019 | |||
| H9c2 cells; Rats | SIRT1↑-NLRP3↑-caspase-1↑-IL1β↑, IL-18↑ | Sun et al., 2020; Zhai et al., 2020 | |||
| Ferroptosis | mice | Nrf2↑-Hmox1↑-Fe↑-mitochondrial damage-lipid peroxidation | Fang et al., 2019 | ||
| Male SD rats | TLR4↑, NOX4↑ | Chen et al., 2019 | |||
| Cisplatin | Apoptosis | Cardiomyocytes; C57BL/6 mice | ROS↑-mitochondrial dysfunction, ER stress-caspase-3↑ | Ma et al., 2010 | |
| Male Albino rats | mitochondrial DNA injury, nuclear DNA infraction | El-Awady el et al., 2011 | |||
| Cyclophosphamide | Apoptosis | Male Wistar rats | sarcoplasmic reticulum dilatation, mitochondrial disruption, nuclear membrane invagination | Lushnikova et al., 2008 | |
| H9c2; Wistar albino rats | acrolein-ROS↑, RNS↑ | Nagi et al., 2011; Kurauchi et al., 2017 | |||
| Male Wistar albino rats | mitochondrial dysfunction-ATP↓-caspase-3↑ | Refaie et al., 2020 | |||
| Male Wistar rats | ROS↑-TLR4↑-NF-κB↑ | El-Agamy et al., 2017 | |||
| Female Wistar albino rats | DNA damage-Bcl2↑, caspase↑ | Avci et al., 2017 | |||
| 5 -Fluorouracil | Apoptosis | ARVMs | ROS↑, GSH↓-lipid peroxidation, MMP-caspase↑ | Eskandari et al., 2015 | |
| Autophagy | Human umbilical vein endothelial cells, Human colorectal cancer cells Human cardiac myocytes | autophagosome↑ | Focaccetti et al., 2015 | ||
| ARVMs | lysosomal membrane leakiness- autophagy damage | Eskandari et al., 2015 | |||
| Arsenic trioxide | Apoptosis | Cardiac myocytes; Wistar rats | ROS↑, Ca2+↑ | Raghu and Cherian, 2009 | |
| H9c2 cells; Wistar rats | ROS↑-GSH↓, GPx↓, GST↓, SOD↓-lipid peroxidation, MMP↓ | Varghese et al., 2017 | |||
| H9c2 cells | mitochondrial damage-ATP↓-caspase-3↑ | Vineetha et al., 2015 | |||
| Male Hy-line chickens | trace elements disorder-mitochondrial damage- Bax caspase-3/8 | Li et al., 2019 | |||
| ARVMs; Male SD rats | SERCA2a↓-ER stress-CHOP↑, caspase-12↑, GRP78↑ | Zhang J.Y. et al., 2016 | |||
| Chickens | TNFα↑, NF-κB↑, COX-2↑, iNOS↑ | Li et al., 2017 | |||
| NRVCs; Wistar albino rats | ROS↑, Ca2+↑-p38↑, JNK MAPK↑-NF-κB↑, IKK↑- PARP, caspase-3↑ | Ghosh et al., 2009; Fan et al., 2013 | |||
| Human pluripotent stem cells induced cardiomyocytes | gammah2ax↑-DNA damage | Bao et al., 2019 | |||
| Autophagy | Culture HL-1 murine atrial cardiomyocytes | Parkin-mitophagy | Watanabe et al., 2014 | ||
| Hy-line chickens, carp | PI3K↑-Akt↑-mTORC1↓ | Li S. et al., 2018; Zhao et al., 2019 | |||
| Trastuzumab | Apoptosis | NRVMs, ARVMs | Bax↑, Bcl-xS↑, Bcl-xL↓-MMP↓-ATP↓-caspase↑ | Grazette et al., 2004 | |
| Primary cardiomyocytes | ErbB2↓-DNA damage | Rohrbach et al., 2005 | |||
| ARVMs | neuregulin-1/ErbB2-phosphorylated Akt ↓ | Sawyer et al., 2002 | |||
| C3H/HeJ mice | TLR4 -TNFα↑ | Yousif and Al-Amran, 2011 | |||
| NRVCs | MDM2↓- p53↑ | Singh et al., 2011 | |||
| Human primary cardiomyocytes | Beclin1↓, Atg 5-12/14↓-EebB1-Y845/ErbB2-Y1248 -ERK/mTOR/ULK-1-ROS↑, mitochondrial dysfunction | Mohan et al., 2016 | |||
| Sunitinib | Apoptosis | H9c2; Male C57BL/6NRj mice | ROS↑-mitochondrial damage | Bouitbir et al., 2019 | |
| SD rats | miR-133A↑-phosphorylation of Ask1/MKK7/JNK↓ | Cooper et al., 2018 | |||
| Autophagy | H9c2 cells | autophagy flux↑ | Zhao et al., 2010 | ||
| Imatinib | Apoptosis | Primary cardiomyocytes; Mice | GATA4↓-Bcl-2↓, Bcl-xL↓ | Maharsy et al., 2014 | |
| H9c2 cells | Sab-JNK-ROS↑, mitochondrial damage-caspase- 3/7/9↑ | Chambers et al., 2017 | |||
| Autophagy | Neonatal cardiomyocytes | autophagy block-lysosome↑, p62↑ | Hu et al., 2012 | ||
| Nilotinib | Apoptosis | H9c2 cells | ROS↑, ATF4↑, CHOP↑-MMP↓, caspase-3↑ | Lekes et al., 2016 | |
| H9c2 cells | ER stress-JNK↑, phosphorylated Akt↓-phosphorylated GSK3β↓-Nox4/ROS↑ | Yang et al., 2018 | |||
| Sorafenib | Apoptosis | Zebrafish | Raf-1/B-raf↓-MEK↓-ERK↓ | Cheng et al., 2011 | |
| Ponatinib | Apoptosis | NRCMs; Zebrafish | Phosphorylated Akt↓, ERK1/2↓-caspase-3↑ | Singh et al., 2019 | |
| Dasatinib | Necroptosis | CCC-HEH-2 human embryonic cardiac tissues | RIP1↑, RIP3↑-HMGB1↑ | Xu et al., 2018 | |
| Mitoxantrone | Apoptosis | H9c2 cells | ROS↑-Ca2+↑-MMP↓-ATP↓-caspase-3↑ | Rossato et al., 2013 | |
| Neonatal cardiomyocytes H9c2 cells | Top2β↓-DNA damage | Damiani et al., 2018 | |||
| Antidiabetic drug | Rosiglitazone | Apoptosis | H9c2 cells | NAPHD↑, iNOS↑, SOD↓, GR↓ | Mishra et al., 2014 |
| C57BL/6 mice | ROS↑-mitochondrial dysfunction | He et al., 2014 | |||
| Pioglitazone | Apoptosis | Wistar rats | Sphingomyelinase↑, ceramidase↑ | Baranowski et al., 2007 | |
| Primary cardiomyocytes | Bax↑, phosphorylated p53↑, phosphorylated vegfr-2↓, Akt↓, mTOR↓ | Zhong et al., 2018 | |||
| Antiviral drug | Zidovudine | Apoptosis | Rats | ROS↑, peroxynitrite↑-DNA breaks-NAD +-ATP↓ | Szabados et al., 1999 |
| Primary human cardiomyocytes | ROS↑-mitochondrial disruptions-caspase-3/7↑ | Gao et al., 2011 | |||
| Mice | Fas/Fas L↑-caspase-3↑ | Purevjav et al., 2007 | |||
| Autophagy | C2C12 myocyte cells | autophagy inhibition-MMP, ROS↑ | Lin et al., 2019 | ||
| Necrosis | Primary human cardiomyocytes | Zidovudine-PARP↑ | Gao et al., 2011 | ||
| Teratogen | Cyclophosphamide | Apoptosis | Primigravida Swiss Webster mice | DNA fragmentation degradation-caspase-3↑ | Mirkes and Little, 1998 |
| Swiss-Webster mice | p38-MAPK↑-caspases cascade reactions | Mirkes et al., 2000 |
FIGURE 1.
Signaling pathways involved in drug-induced cardiotoxicity.
Anticancer Drugs
Doxorubicin (DOX)
Apoptosis
Enhanced production of ROS is recognized as the classic mechanism of DOX induced cardiomyocyte death. ROS consists of both free radicals and non-free radicals derived from oxygen, including superoxide anions (O2–), hydrogen peroxide (H2O2), hydroxyl radicals (OH–), ozone (O3) and singlet oxygen (1O2) (Zorov et al., 2014). DOX can be reduced to semiquinone by the endothelial nitric oxide synthase (eNOS) and triphosphopyridine nucleotide (NADPH) oxidase, which in turn leads to production of O2–, a major free radical that can produce other ROS, such as H2O2 and hydroxyl radicals (OH⋅) (Vasquez-Vivar et al., 1997). This is the main pathway by which DOX treatment generates ROS. Moreover, Deng et al. (2007) found that reactions between DOX and NADPH could produce superoxide in the absence of any enzyme activity, suggesting ROS production may be caused by the chemical interaction of DOX and NADPH. The generated ROS further induced DNA damage, especially DNA single-strand breaks. Previous studies showed that DOX treatment suppressed the DNA binding activity of GATA binding protein 4 (GATA4), an oxidant-sensitive transcription factor that plays an important role in transducing nuclear events (Kim et al., 2003). In vitro, DNA breaks activated nuclear poly ADP-ribose polymerase 1 (PARP-1) to induce the synthesis of poly-ADP-ribose and cause ATP consumption by subsequent glycohydrolase reactions and ATP conversion, leading to the collapse of heart energy metabolism (Mukhopadhyay et al., 2009). Scaffold protein Sirt6 was proved to be protective against DOX-induced DNA damage (Brito et al., 2016). In addition to DNA damage, DOX-induced intracellular ion disorder also contributes to ROS production.
A previous study demonstrated that under aerobic conditions, DOX could automatically combine with iron to form DOX-iron complex, which increased the contents of OH– by self-reduction, contributing to subsequent lipid peroxidation through membrane interactions (Malisza and Hasinoff, 1995). Another study also discovered that the levels of iron and ROS were up-regulated in DOX-treated cardiomyocytes, which finally induced the mitochondrial apoptosis through caspase-3 activation and cytochrome C release (Childs et al., 2002). The participation of intracellular iron in the degradation of hypoxia-inducible factors (HIF) has been demonstrated (Peyssonnaux et al., 2008). Latter research indicated that iron/HIF signaling mediated the cardio-protective effect of dexrazoxane, the unique authorized protectant for DOX-induced cardiotoxicity (Spagnuolo et al., 2011). Paradoxically, DOX-induced ROS increased the synthesis of ferritin and mediated the protective effect of DOX against iron-induced cardiotoxicity (Corna et al., 2004).
In addition, topoisomerase 2 beta (Top2β) was found to involve in ROS formation during DOX treatment (Vejpongsa and Yeh, 2014). Topoisomerase 2 (Top2), consisting of Top2α and Top2β, played a crucial role in DNA replication, transcription, and repair (Wang, 2002). Top2 was considered a target for the anticancer effect of anthracyclines (Zhu et al., 2016). Top2β interaction with DOX caused DNA double-strand breaks and further triggered transcriptome changes in cardiomyocytes (Zhang et al., 2012). The DOX-Top2β combination may inhibit the transcription of peroxisome proliferator-activated receptor gamma coactivator-1 (PGC1) including PGC1α and PGC1β, which play critical roles in mitochondrial biogenesis as antioxidant (Finkel, 2006; Finck and Kelly, 2007). Moreover, mice with cardiomyocyte-specific Top2β conditional knockout (Top2β–/–) presented less DNA damage and mitochondrial dysfunction as well as oxidative phosphorylation after DOX exposure (Zhang et al., 2012).
Doxorubicin-induced oxidative stress can also stimulate death reporters to combine with corresponding cognate ligands, thereby inducing assembly of the DISC complex, which in turn caused caspase cascades activations and substrates cleavages. A recent study showed that the four death reporters (Fas, TNFR1, DR4, and DR5) were significant increased at the mRNA and protein levels after DOX treatment in induced pluripotent stem cells (iPS) -derived cardiomyocytes (Zhao and Zhang, 2017). One study suggested that ROS-induced up-regulation of cytosolic calcium concentration further elevated the expression of Fas Ligand (Fas L) by stimulating the nuclear factor of activated T-cells (NFAT) signaling (Kalivendi et al., 2005). Moreover, the calcium and calmodulin can conversely bind with eNOS electrons to increase superoxide formation, exacerbating cardiotoxicity (Vasquez-Vivar et al., 1998). Additionally, cardiac fibroblasts exacerbated cardiomyocyte apoptosis by releasing exosomes carrying Fas L in a paracrine manner during DOX treatment. Rosmarinic acid was shown to decrease Fas L secretion by suppressing the level of NFAT activation and metalloproteinase 7 (MMP7) expressions in cardiac fibroblasts, and served a protective role (Zhang et al., 2019). Preclinical experiments demonstrated a decrease in cardiomyocyte death of rats treated with anti-Fas L antibody (Nakamura et al., 2000). Moreover, caspase-8 inhibition blocked Fas L-induced apoptosis, indicating that downstream signaling of apoptosis was mediated by Fas L (Yamaoka et al., 2000). The matricellular protein CCN1 triggered by DOX reacted with integrin α6β1 to promote the activation of p38 mitogen-activated protein kinase (p38-MAPK), which stimulated the release of second mitochondrial activator of caspase (SMAC) and high-temperature requirement protein A2 (HtrA2), synergizing with Fas L to induce cardiomyocytes apoptosis (Hsu and Mo, 2016). As an important receptor in extrinsic apoptosis, TNFR1 was also involved in DOX-related cardiomyocyte death. DOX changed the level of TNFα in H9c2 cells, leading to an increase in TNFR1 expression and a decrease in TNFR2 expression, accompanied by the activation of caspase-8 and suppression of IκBα (Chiosi et al., 2007). It is noteworthy that vitamin C, a well-known reductant, can evidently decline the levels of TNFα, IL-1β and IL-6 in DOX-treated mice, indicating that oxidation/nitrosation stress may be one of the targets of cardiac protection during DOX treatment (Akolkar et al., 2017). In addition, the intracellular calcium homeostasis protective agent mangiferin was also verified to be able to relieve the up-regulation of TNFα and caspase-9 induced by DOX and stimulates the calcium regulatory gene, preventing myocarditis and apoptosis (Agustini et al., 2016).
Another plausible mechanism is that DOX-induced oxidative stress breaks the oxido-reduction balance of mitochondria in cardiomyocytes. DOX treatment promotes ROS overproduction, which remains inside the mitochondrial membrane and induces mitochondrial dysfunction (Giulivi et al., 1995). DOX caused a down-regulation of antioxidant enzymes such as copper, manganese and zinc superoxide dismutases (SODs), glutathione peroxidase (GSH-Px) and catalase (Costa et al., 2013). This imbalance between oxidation and antioxidation aggravates mitochondrial damage. Therefore, overexpression of antioxidant enzymes can reduce DOX-induced cardiotoxicity. In vitro, γ-ray pre-irradiation increased manganese superoxide dismutase (Mn-SOD) levels in neonatal rat ventricular myocytes (NRCMs), which up-regulated MMP, Bcl-2 expressions, and decreased the Bax expression and cytochrome C release (Childs et al., 2002; Chae et al., 2005). Nevertheless, polysaccharide elevated MMP and restrained mitochondrial permeability transition (MPT) by activating manganese superoxide dismutase (Mn-SOD) and suppressing the subsequent caspases cascade reactions (Li W. J. et al., 2018).
Recent studies demonstrated that DOX could indirectly target some receptors or anti-apoptosis factors by regulating miRNAs. DOX increased the level of miR-146a and induced the targeted inhibition of human epidermal growth factor receptor-4 (ErbB4), which caused cardiomyocyte apoptosis and acute cardiotoxicity (Horie et al., 2010). The miR-181a directly targeted the Bcl-2 transcript and negatively regulated Bcl-2 expression, which mediates the protective effect of propofol against DOX-induced cardiotoxicity in vitro and in vivo (Zhao et al., 2018). Moreover, miR-29b was found to target 3’ untranslated region of Bax and restrained Bax expression, hence alleviating DOX-induced cardiomyocyte apoptosis (Jing et al., 2018).
Several studies showed that varying DOX dosages caused apoptosis through different pathways. A study reported that treatment with a high concentration of DOX (2 μM) tended to promote ROS accumulation, while a lower concentration (0.25 M) was more likely to suppress the expression of haem oxygenase 1 (HO-1). HO-1 down-regulation induced cardiomyocyte apoptosis by activating caspase-3 and the release of mitochondrial cytochrome C (Bernuzzi et al., 2009). Another study found that a high concentration of DOX (1 μM) tended to cause DNA damage, PARP-1 dissociation and grievous apoptosis, and a low concentration of DOX (0.5 μM) could activate the p53-related mitochondrial apoptosis pathway (Cunha-Oliveira et al., 2018). Furthermore, DOX dose-dependently increased p53 expression in H9c2 cells, which inhibits type 1 insulin-like growth factor receptor (IGF-1R) transcription and induces IGF binding protein-3 (IGFBP-3) transcription, resulting in resistance to IGF-1 and contributing to apoptosis (Fabbi et al., 2015). More in-depth study indicated that the regulation of DOX on p53 may involve Sirtuin 1 (SIRT1) -mediated deacetylation of p53 (Zhang et al., 2011).
Autophagy
Autophagy is commonly considered as a conservative and beneficial regulatory process that maintains intracellular homeostasis, which is initially activated to resist DOX-induced cardiotoxicity. Oxidative stress is considered the main inducement for autophagy. As reported, during DOX treatment, ROS increased the ratio of LC3II/LC3I and the level of Beclin 1, both being the bio-markers of autophagy (Zhang et al., 2015). In addition, Dox up-regulated the levels of pro-autophagy factors (p53, p38-MAPK, and JNK-MAPK), and down-regulated the p85 expression, the catalytic subunit of phosphoinosmde-3-kinase (PI3K) as well as Akt phosphorylation (Ludke et al., 2017; Yu et al., 2017).
Even though the autophagy process is indeed initiated by DOX to serve a protective role, it somehow fails to finish the process since overwhelming oxidative stress blocks the degradation of lysosomes and even causes autophagic cell death, which in fact turns the original protective effect into damage. Under these circumstances, the normal protein degradation of cardiomyocytes was disrupted, and the subsequent increase in ubiquitinated proteins resulted in the accumulation of autophagy flux and autophagosomes (Dimitrakis et al., 2012). Meanwhile, DOX suppressed lysosome acidification and autolysosome degradation, which blocked the autophagic flux and augmented the damage (Li et al., 2016). Moreover, DOX-induced up-regulation of histone deacetylase 6 (HDAC6) decreased α-tubulin acetylation level, giving rise to mitochondrial dysfunction and autophagy flux damage (Song et al., 2018). Lysosome dysfunction was found to involve in the depletion of transcription factor EB (TFEB). DOX can suppress the expression of TFEB and induce the impairment of lysosomal cathepsin B, which subsequently inhibited lysosomal autophagy, increasing the levels of ROS and caspase-3 cleavage (Bartlett et al., 2016).
In addition to ROS-related autophagy, DOX also regulates autophagy-related factors and cause autophagic cell death. High mobility group box 1 (HMGB1) plays a vital role in the process of autophagy. DOX increased HMGB1expression, while silencing HMGB1 could reverse cardiomyocyte damage by attenuating autophagy (Luo et al., 2018). In addition, inhibition of the transcription factor GATA4 was observed in DOX-treated cardiomyocytes, and GATA4 induces the expression of Bcl2, which can interact with Beclin 1 to silence autophagy, decreases the cardiotoxicity (Kobayashi et al., 2010). Moreover, rats treated with 3-methyladenine, a specific inhibitor of autophagy, showed fewer autophagic vacuoles and mitochondrial MPT, but higher levels of Na+-K+ ATPase activity and MMP as compared with DOX treatment alone (Lu et al., 2009).
It has been reported that starvation or caloric restriction prior to DOX insult can suppress cardiotoxicity. Caloric restriction attenuated DOX-induced ATP exhaustion and enhances the activity of AMPK, which eventually corrected the harmful autophagy caused by DOX, demonstrating a protective role (Chen et al., 2011). On the contrary, prior starvation mitigated acute DOX-induced cardiotoxicity via further augmenting autophagy (Kawaguchi et al., 2012). In addition, Astragalus polysaccharide and resveratrol can restore autophagy in mice and H9c2 cells through the AMPK/mTOR signaling pathway, alleviating cardiotoxicity (Gu et al., 2016; Cao et al., 2017).
Necrosis
Unlike apoptosis and autophagy, emerging evidence has indicated that cardiomyocyte necrosis is triggered by a high dosage or prolonged exposure to DOX treatment. Dose-dependently elevated by DOX, the accumulation of ROS and peroxynitrite increase the rate of necrosis in cardiomyocyte death (Mukhopadhyay et al., 2009; Fulbright et al., 2015). The commonly used dosages of DOX are ≤20 mg/kg in vivo and 1 μM in vitro. A single intraperitoneal injection of DOX at 25 mg/kg in mice could immediately cause necrotic death and cardiac insufficiency (Li et al., 2014), and 2 μM DOX can directly induce cardiomyocyte necrosis in vitro (Bernuzzi et al., 2009). Moreover, when the cardiomyocytes are exposed to DOX for a long period, initial apoptosis develops into necrosis as the cells preferentially exhibits early DNA impairment and nuclear swelling (Rharass et al., 2016). As discussed above, DOX can destroy the function of lysosomes and disrupt normal autophagy as a result of oxidative stress. Consequently, the delayed autophagy in cardiomyocytes causes more severe apoptotic secondary necrosis (Dimitrakis et al., 2012; Li et al., 2014). These studies further confirm the notion that necrosis arose with extended exposure to DOX treatment.
Moreover, DOX initiates necrotic cell death by regulating necrosis-related intracellular factors. The degradation of titin, a myofilament protein associated with myocardial damage, was induced by DOX, and finally induced cardiomyocyte necrotic death (Lim et al., 2004). In addition, BH3-only protein BNIP3 was activated by DOX to destroy the combination of respiratory chain complex IV subunit 1 (COX1) and uncoupling protein 3 (UCP3), which disrupted respiratory efficiency, eventually leading to necrotic cell death (Dhingra et al., 2014).
Necroptosis
It has been demonstrated that RIPK3 is activated by DOX to bind with phosphorylated-CaMKII, causing the opening of mitochondrial permeability transition pore (MTPT), and resulting in necroptosis (Zhang T. et al., 2016). More importantly, dexrazoxane alleviated Dox-induced inflammation and cardiomyocyte necroptosis through inhibiting p38-MAPK/nuclear factor kappa-B (NF-κB) signal (Yu et al., 2020).
Pyroptosis
The increased secretion of IL-1β and IL-18, activation of TLR4, NLRP3 inflammasome and caspases were found in DOX-treated H9c2 cells, suggesting the occurrence of pyroptosis (Singla et al., 2019). BNIP3, the upstream regulator of cardiomyocyte pyroptosis, can activate caspase-3 and lead to subsequent GSDME cleavage (Zheng et al., 2020). DRP1/NOX signaling was activated to cause mitochondrial damage, which involved in DOX-induced pyrotosis (Zeng et al., 2020). Moreover, up-regulated lncRNA TINCR recruited IGF2BP1 to enhance the NLRP3 expression that mediated Dox-induced pyrotosis (Meng et al., 2019). However, embryonic stem cells-derived exosomes and Heat-shock Protein 22 can reverse the Dox-induced cardiomyocytes pyrotosis via inhibiting TLR4/NLRP3/caspase-1 signaling (Tavakoli et al., 2019; Lan et al., 2020). Moreover, suppression of ROS was also reported to be able to alleviate Dox-induced cardiomyocyte pyrotosis, whose mechanism involved the inhibition of sirtuin 1/NLRP3 signaling pathway (Sun et al., 2020; Zhai et al., 2020).
Ferroptosis
Doxorubicin-induced accumulation of ROS and lipid peroxidation can lead to cardiomyocyte ferroptosis (Koleini et al., 2019). Activation of TLR 4 and NOX 4 has also been proven to promote DOX-induced cardiomyocyte ferroptosis (Chen et al., 2019). Administering DOX to mice induced cardiomyopathy with a rapid, systemic accumulation of nonheme iron via heme degradation by NF-E2-related factor 2 (Nrf2)-mediated up-regulation of heme oxygenase-1 (HMOX1), indicating the cardio-protective role of targeting ferroptosis for cardiomyopathy prevention (Fang et al., 2019).
Cisplatin
Cisplatin is a chemotherapeutic agent for a vast spectrum of cancers. However, its acute and cumulative cardiotoxicity partially limits anti-tumor treatment and clinical applications (Ma et al., 2010). Cisplatin-treated cardiomyocytes showed mitochondrial abnormalities such as mitochondrial membrane depolarization, inflammatory responses and increased ER stress, which finally stimulated the activity of caspase-3 and induced apoptosis (Chowdhury et al., 2016). In addition, emerging evidences demonstrated a close connection between oxidative stress and cisplatin-induced cardiomyocyte apoptosis. El-Awady el et al. (2011) discovered that cisplatin improved lipid peroxidation, decreased GSH content and suppressed SOD activity, implying oxidative stress induced by cisplatin. Moreover, mitochondrial DNA injury and nuclear DNA damage were observed. Antioxidant natural products such as tutin (vitamin P1), zingerone and cyanidin can inhibit cisplatin-induced inflammatory infiltration, DNA damage, and mitochondrial dysfunction, indicating the key role of oxidative stress in cisplatin-induced cardiomyocyte apoptosis (Qian et al., 2018; Soliman et al., 2018; Topal et al., 2018).
Cyclophosphamide
Cyclophosphamide is commonly applied in the treatment of malignant tumors such as leukemia and lymphoma, and it is also adopted to treat systemic lupus erythaematosus and polymyositis as an immunosuppressor. Because of the dose-dependent manner, cyclophosphamide-induced cardiotoxicity basically coincides with high-dose treatment (Nishikawa et al., 2015; Wadia, 2015). Acrolein, the active metabolite of cyclophosphamide, was confirmed to be mainly responsible for cardiomyocyte death (Conklin et al., 2015; Nishikawa et al., 2015; Kurauchi et al., 2017). The cardiomyocyte injuries caused by cyclophosphamide treatment included sarcoplasmic reticulum dilatation, mitochondrial disruption and nuclear membrane invagination (Lushnikova et al., 2008). Further studies attributed these injuries to oxidative stress, clarifying that acrolein caused oxidative and nitrite stress through the suppression of intracellular GSH and SOD and increase of MDA (Nagi et al., 2011; Kurauchi et al., 2017; Omole et al., 2018). Corresponding lipid peroxidation initiated mitochondrial function damage, which further led to a collapse in APT production and the activation of caspase-3, resulting in apoptosis (Nagi et al., 2011; Refaie et al., 2020). In addition, cyclophosphamide was verified to stimulate TLR4, through which it initiated the TLR4/NF-κB signaling to trigger an inflammatory reaction, and eventually apoptosis (El-Agamy et al., 2017). Furthermore, DNA damage was observed in cyclophosphamide-treated rats, which were accompanied with activation of caspase-3 and inhibition of Bcl-2 expression (Avci et al., 2017). Antioxidant drugs such as silymarin and curcumin can inhibit cyclophosphamide-induced cardiotoxicity via decreasing the fragments of mitochondrial DNA and nuclear DNA, suggesting that excessive ROS might be responsible for cyclophosphamide-induced DNA injuries.
With the exception of the evidence discussed above, cyclophosphamide acted as a teratogen to injure cardiomyocytes. Cyclophosphamide affected cardiomyocytes developing via DNA fragmentation degradation, caspase-3 activation and PARP cleavage (Mirkes and Little, 1998). Moreover, cyclophosphamide was found to activate the apoptotic pathways, culminating in abnormality of the heart via the p38-MAPK signaling (Mirkes et al., 2000).
Fluorouracil and Capecitabin
5-Fluorouracil, a pyrimidine antimetabolite, is used widely in clinical practice as an anti-tumor treatment for cancers such as intestinal cancer and liver cancer. Capecitabin, a tumor-targeting drug that takes effect after the intracellular transformation into 5-fluorouracil, was regarded as having similar cardiotoxicity. Multiple studies confirmed the cardiotoxicity induced by 5-fluorouracil, which was attributed to the coronary arteries injury (Clavel et al., 1988; Herrmann et al., 2016). Further study suggested that the cardiomyocyte apoptosis might also play an important role in 5-fluorouracil-induced cardiotoxicity (Tsibiribi et al., 2006). 5-Fluorouracil stimulated intracellular oxidative stress by O2– generation, which eventually activated the caspases cascade reactions, leading to apoptosis (Lamberti et al., 2012). To further determine the underlying mechanism, Eskandari et al. (2015) discovered that 5-fluorouracil-induced ROS increase was accompanied by the depletion of GSH, a ROS scavenger. Next, the generated ROS mediated lipid peroxidation on mitochondria to decrease MMP, causing mitochondrial dysfunction and caspase-3 activation. Fluoroacetate, the metabolite of 5-fluorouracil, can restrain aconitase to block the tricarboxylic acid cycle, resulting in a mitochondrial energy metabolism crisis (Lischke et al., 2015). Moreover, accumulation of autophagosomes and lysosomal membrane leakiness were observed in 5-fluorouracil-treated human cardiomyocytes, indicating the involvement of autophagic cell death in 5-fluorouracil-induced cardiotoxicity (Eskandari et al., 2015; Focaccetti et al., 2015).
Arsenic Trioxide
Arsenic trioxide represents a breakthrough in the field of acute promyelocytic leukemia therapeutic, but its cardiotoxicity remains an unresolved problem. In the past few decades, numerous studies have proposed that cardiomyocyte apoptosis is induced by ROS, and mitochondrial damage might be the main reason for arsenic trioxide-induced cardiotoxicity (Zhao et al., 2008; Raghu and Cherian, 2009; Vineetha et al., 2015). Arsenic trioxide can increase ROS level and calcium concentration, which was accompanied with cardiomyocyte apoptosis (Raghu and Cherian, 2009). Moreover, ROS generation depleted intracellular antioxidants such as GSH, GSH-Px, glutathione s-transferase (GST) and SOD, which caused lipid peroxidation and decreased MMP (Varghese et al., 2017). In addition, mitochondrial damage such as MTP and mitochondrial swelling was also observed under arsenic trioxide exposure, following by a decrease in oxygen consumption as well as ATP production, resulting in caspase-3 activation and apoptosis (Vineetha et al., 2015). Recently, Li et al. (2019) found that arsenic trioxide interfered with the dynamic balance of trace elements in chicken cardiomyocytes to break mitochondrial cristae and mitochondrial vacuoles, increasing the expressions of Bax and caspase-3/8. In addition to ROS and mitochondrial damage, calcium imbalance is also involved in arsenic trioxide-induced cardiotoxicity. Arsenic trioxide suppressed the activity of sarcoplasmic reticulum Ca2+-ATPase2a, by which cytoplasmic calcium was taken back to the sarcoplasmic reticulum (Zhang J.Y. et al., 2016). Consequently, the imbalance of calcium homeostasis and ER stress activated C/EBP-homologous protein (CHOP), caspase-12 and GRP78, leading to apoptosis.
By increasing the levels of the inflammatory cytokines TNFα, NF-κB, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), arsenic trioxides promoted the inflammatory reactions and brought ultrastructural damage to cardiomyocytes (Li et al., 2017). These inflammatory responses partly contributed to heavy metal-related cardiotoxicity (Lakkur et al., 2015). Additionally, it has been determined that arsenic trioxides up-regulates the content of phosphorylated p38 and JNK by oxidative stress stimulation and calcium overload, which further induces NF-κB phosphorylation, caspase-3 activation and PARP cleavage (Ghosh et al., 2009; Fan et al., 2013). Suppression of ROS apparently inhibited the activation of JNK, extracellular regulated protein kinases (ERK), and p38, which eventually reversed cardiomyocyte apoptosis (Miao et al., 2013; Zhang et al., 2017). One recent study reported that arsenic trioxides might induce cardiomyocyte apoptosis through DNA damage, since it dose-dependently increased the content of γH2AX, a sensitive biomarker for DNA breaks (Bao et al., 2019). Moreover, activation of Parkin, an E3 ubiquitin ligase, can inhibit arsenic trioxide-induced cardiotoxicity via the maintenance of mitochondrial as well as cellular homeostasis (Watanabe et al., 2014). It is well known that Parkin-induced ubiquitination of mitochondrial substrates finally leads to mitophagy. Therefore, mitophagy displays a positive role in maintaining cardiac homeostasis during arsenic trioxide exposure. However, a subsequent study demonstrated that arsenic trioxide-induced oxidative stress led to the formation of autophagosomes through PI3K/Akt/mTOR signaling, which resulted in myocardial damage (Li S. et al., 2018; Zhao et al., 2019). Moreover, cardiomyocyte necrosis was also observed under arsenic trioxide exposure (Raghu and Cherian, 2009; Vineetha et al., 2015), but the underlying mechanisms remained unclear.
Trastuzumab
A novel and widely used monoclonal antibody drug, Trastuzumab, is also reported to cause cardiotoxicity, such as cardiac insufficiency and heart failure. Under physiological condition, neuregulin-1 interacted with epidermal growth factor receptor-2 (ErbB2) to allow for the formation of ErbB4/ErbB2 heterodimer, which blocked cell death through an Akt-dependent signaling in cardiomyocytes (Sawyer et al., 2002; Lemmens et al., 2007). Functioning as an inhibitor of ErbB2 receptor, trastuzumab interrupted ErbB4/ErbB2 heterodimerization, thereby leading to apoptosis (Holbro and Hynes, 2004; Rohrbach et al., 2005). Interaction between Trastuzumab and ErbB2 triggered the downstream signal transduction pathways, such as increased levels of Bax and Bcl-xS, decreased Bcl-xL level and caspases cascade activations. Furthermore, the decreased MMP caused by Bcl-xL suppression and subsequent mitochondrial energy catastrophe also contributed to trastuzumab-induced apoptosis (Grazette et al., 2004). Singh et al. (2011) proposed that the cardiotoxicity induced by trastuzumab might stem from its negative regulation of murine double minute 2 (MDM2) and p53. In addition, trastuzumab-induced cardiomyocyte apoptosis was found to be related to inflammatory infiltration (Coppola et al., 2016), and the TLR4-mediated chemokine expressions of TNFα, MCP-1 and ICAM-1 contributed to inflammatory responses induced by trastuzumab (Yousif and Al-Amran, 2011).
A recent study reported that trastuzumab suppressed autophagy, resulting in mitochondrial dysfunction and ROS accumulation (Mohan et al., 2017). Trastuzumab insult inhibited the expressions of Beclin 1, autophagy related gene (Atg) 5-12 and Atg14. Moreover, Trastuzumab stimulated EebB1-Y845 and ErbB2-Y1248 and activated the ERK/mTOR/ULK-1 signaling to supress autophagy (Mohan et al., 2016).
Currently, drug combination therapies with trastuzumab are quite popular among cancer treatment plans. However, the combination therapies show more serious cardiotoxicity compared with single drug remedies. Trastuzumab combining with DOX exacerbated the exhaustion of antioxidant enzymes as well as damage to the mitochondrial structure (Xu et al., 2004). Trastuzumab-induced ErbB2 inhibition further suppressed c-Abl and Arg, which plays an antioxidant role by activating GSH-Px and catalase, thereby increasing DOX-induced cytotoxicity (Belmonte et al., 2015). Additionally, inhibition of the neuregulin-ErbB signaling by trastuzumab restrained the phosphorylation of ERK1/2 and Akt, and eventually exacerbated DOX-induced cardiomyocyte apoptosis and myocardial fiber injury (Sawyer et al., 2002). The underlying mechanism might also include the increased level of iNOS, which contributed to oxidative stress and inflammatory cell infiltration (Milano et al., 2020). Moreover, DOX-trastuzumab combination synergistically repressed Top2β, and eventually resulted in DNA double strand breaks and the ROS overproduction (Jiang et al., 2018). N-Acetyl Cysteine Amide, a ROS scavenger, can attenuate the DOX and trastuzumab-induced cardiac dysfunction (Goyal et al., 2016). Furthermore, paclitaxel-trastuzumab combination was reported to have a worsening effect on cardiomyocyte function, which might result from the inhibition of phosphorylated-ERK1/2. However, no cell death was observed during treatment in addition to myofibrillar structure changes (Pentassuglia et al., 2007).
Tyrosine-Kinase Inhibitors (Sunitinib, Imatinib, Nilotinib, Sorafenib, Ponatinib and Dasatinib)
Tyrosine-kinase inhibitors such as sunitinib, imatinib, nilotinib, sorafenib, ponatinib, and dasatinib are widely applied for chronic myelogenous leukemia and solid tumors, however, an increasing number of studies have reported cardiotoxicity associated with tyrosine-kinase inhibitors. Clinical research observed aberrantly shaped, swollen mitochondria in cardiomyocytes of patients who developed congestive heart after sunitinib treatment (Chu et al., 2007). Bouitbir et al. (2019) demonstrated that the oxidative stress caused by sunitinib was mainly responsible for the mitochondrial damage and final apoptosis of cardiomyocytes. By suppressing mitochondrial electron transport chain enzyme complexes, sunitinib induced ROS accumulation, which further decreased MMP and destroyed the mitochondrial structure to initiate caspases cascade reactions. In addition, sunitinib was confirmed to increase the expression of miR-133A, and suppress the apoptosis signal-regulating kinase 1 (ASK1)/mitogen activated kinase kinase 7 (MKK7)/JNK signaling to induce apoptosis and myocardial damage (Cooper et al., 2018). The elevation of autophagy flux was observed in sunitinib-treated H9c2 cells, (Zhao et al., 2010), and inhibition of autophagy was demonstrated to attenuate sunitinib-induced cardiotoxicity (Kimura et al., 2017), indicating involvement of autophagy in the cytotoxicity.
Mitochondrial damage was also observed in imatinib-treated cardiomyocytes. Imatinib down-regulates the expression of Bcl-2 and Bcl-xL by suppressing the content of the transcription factor GATA4, resulting in apoptosis. Moreover, aging was verified to be a risk factor during imatinib treatment because of its positive impact on the oxidative stress (Maharsy et al., 2014). In addition, Chambers et al. (2017) showed that imatinib induced oxidative stress and mitochondrial dysfunction via mediating JNK-related mitochondrial signaling and activating caspase-3/7/9. Autophagic death was also reported to be associated with imatinib-induced cardiotoxicity (Hu et al., 2012). Imatinib blocked the autophagic process as indicated by an increased level of lysosomes, which was consistent with the accumulation of p62, a protein degraded by autophagic clearance.
As a second-generation Bcr-Abl inhibitor, nilotinib mediated apoptosis mainly through the accumulation of ROS and ER stress (Doherty et al., 2013). The increase of ROS and ER stress biomarkers (ATF4 and CHOP), was observed in nilotinib-treated H9c2 cells, which subsequently decreased MMP and activated caspase-3, markers of apoptosis (Lekes et al., 2016). Recently, Yang et al. (2018) made further efforts to demonstrate that nilotinib induced ER stress to activate JNK and restrain Akt phosphorylation, which in turn suppressed glycogen synthase kinase-3 beta (GSK3β) phosphorylation and activated NOX4/ROS signaling, resulting in apoptosis.
Few studies reported potential apoptosis mechanisms associated with sorafenib, ponatinib and dasatinib. Cheng et al. (2011) demonstrated that sorafenib induced cardiomyocyte apoptosis and cardiotoxicity by inhibiting the Raf/MEK/ERK signaling. In addition, cardiomyocytes necrosis was observed during high-dose sorafenib treatment with unclear mechanism (Duran et al., 2014). The most recent study reported that ponatinib inhibited phosphorylation of Akt and ERK1/2, which contributed to the activation of pro-apoptotic caspase-3 (Singh et al., 2019). Xu et al. (2018) found that dasatinib dose-dependently up-regulated intracellular HMGB1 to induce cardiomyocyte necroptosis.
Mitoxantrone
With similar structure to DOX, mitoxantrone was thought to be an alternative to DOX with less cardiotoxicity (Herman et al., 1997). However, several studies have also reported mitoxantrone-induced cardiotoxicity. Mitoxantrone induced cardiomyocyte apoptosis associated with oxidative stress and mitochondrial dysfunction. With a time-dependent increase in the content of ROS, mitoxantrone promoted the accumulation of calcium and decreased the MMP, which further caused mitochondrial energy deficiency and activated caspase-3 (Rossato et al., 2013). In addition, DNA strand breaks were observed in apoptotic H9c2 cells treated with mitoxantrone, which was probably mediated by a Top2β-dependent signaling (Wu et al., 2013; Damiani et al., 2018).
Antidiabetic Drugs (Rosiglitazone and Pioglitazone)
Rosiglitazone and pioglitazone, as thiazolidinedione antidiabetic agents, were regarded as protective agents in diabetic cardiomyopathy and ischaemia-reperfusion injury (Cao et al., 2007; Baraka and Abdelgawad, 2010). However, with further expansion of their clinical applications, rosiglitazone and pioglitazone are also found to cause serious side effects such as myocardial hypertrophy and heart failure. It has been reported that rosiglitazone exerts both protective and detrimental effects in rats treated by ischaemia reperfusion (Palee et al., 2013). Although rosiglitazone was known as a PPARγ agonist, it caused cardiotoxicity via oxidative stress-induced mitochondrial dysfunction independent of PPARγ. By increasing the levels of NAPHD and iNOS, rosiglitazone induced oxidative stress accompanied by the exhaustion of antioxidant enzymes such as SOD and glutathione reductase, resulting in cardiotoxicity apoptosis (Mishra et al., 2014). In addition, the oxidative effect caused mitochondrial dysfunction, followed by cardiomyocyte energy deficiency (He et al., 2014). For pioglitazone, a study once reported that pioglitazone up-regulated the levels of sphingomyelinase and ceramidase, a mediator of cardiomyocyte apoptosis (Baranowski et al., 2007). Moreover, by activating Bax and phosphorylated p53 as well as suppressing phosphorylated Akt and mTOR, pioglitazone could induce apoptosis in a VEGFR-2 dependent manner (Zhong et al., 2018).
Antiviral Drug (Zidovudine)
Zidovudine, like other nucleoside reverse transcriptase inhibitors, is widely used for human immunodeficiency virus type 1 (HIV-1) infection. However, side effects such as hypertension and cardiomyopathy limit its long-term application. Zidovudine stimulated the accumulation of ROS and peroxynitrite, which in turn gives rise to single-strand DNA breaks, and eventually results in mitochondrial energy depletion in a NAD+-dependent manner (Szabados et al., 1999). Zidovudine induced the transport of protein kinase C δ (PKCδ) from the cytosol to the membrane, which promoted the activation of NADPH oxidases (Papparella et al., 2007). Meanwhile, mitochondrial damage is observed after zidovudine treatment (Fiala et al., 2004). Mitochondrial ROS caused by zidovudine played a significant role in mitochondrial disruption-induced apoptosis by activating caspase-3/7 (Ry et al., 2011). Fas/Fas L was also involved in zidovudine-induced cardiomyocytes apoptosis (Purevjav et al., 2007). Besides the above effects, zidovudine was shown to inhibit autophagosome maturation and decrease autophagic flux, leading to mitochondrial membrane polarization and ROS accumulation (Lin et al., 2019). Zidovudine-induced cardiomyocyte necrosis involved PARP activation (Gao et al., 2011).
Perspectives and Conclusion
Cardiotoxicity is a major concern when evaluating whether drugs can be put on the market during preclinical research and is an important reason for post-approval drug withdrawal. Even for widely used drugs, cardiotoxicity limits their clinical applications. Fortunately, the mechanisms of cardiotoxicity have gradually come to light in recent years. ROS serves a main driver in drug-induced cardiotoxicity, and thereby many antioxidants have undergone preclinical development or clinically research for cardiotoxicity. For example, dexrazoxane, an iron chelating agent against iron-mediated oxidative stress, is the cardio-protective medicine approved by FDA in July 1995 for preventing anthracycline-induced cardiotoxicity, and now has been widely applied in the clinical practice (Padegimas et al., 2020). In addition, numerous natural antioxidants serve as adjuvant therapies to reduce drug-induced cardiotoxicity, such as berberine, epigallocatechin-3-gallate, and resveratrol (Coelho et al., 2017; Yu et al., 2018).
Recently, immune checkpoint inhibitors including anti-PD-1, anti-PD-L1 and CTLA-4 blockade have attracted a substantial amount of attention, which may revolutionize the treatment of cancer. Ever since the first case of cardiotoxicity induced by ipilimumab (CTLA-4 blockade) was reported in 2013 (Voskens et al., 2013), more and more case reports have indicated immune checkpoint inhibitor-induced cardiotoxicity. Worse still is the underlying mechanism remains unknown, possibly due to the lack of suitable animal models. Immune inflammation and ROS accumulation may play key roles in the immune checkpoint inhibitor-induced cardiotoxicity, which needs to be confirmed by future studies.
To achieve a better treatment effect, combinations of anticancer drugs have been widely applied in the clinical practice, but unfortunately lead to greater cardiotoxicity than with individual drug. Much attention has been paid to trastuzumab combined with DOX for treating women with ErbB2-positive breast cancer. Addition of trastuzumab to adjuvant DOX chemotherapy increases the incidence of cardiotoxicity, and few studies have been conducted to explore the underlying mechanisms, with iNOS or Top2β-mediated oxidative stress being the only acceptable mechanism (Jiang et al., 2018; Milano et al., 2020). To sum up, the mechanisms of drug-induced cardiomyocyte death are not absolutely independent, with the crosstalk and overlap of signaling pathways perplexing and complicating the cardiotoxicity. Therefore, further in-depth mechanisms deserve urgent investigation to avoid synergistic cardiotoxicity.
In this review, we summarized and discussed six cardiomyocyte death forms associated with drug-induced cardiotoxicity, including apoptosis, autophagy, necrosis, necroptosis, pyroptosis and ferroptosis. However, most of studies focused on the apoptosis, and whether the coexistence of multiple cardiomyocyte death forms was a common phenomenon of drug-induced cardiotoxicity remains to be explored. A recent study found that caspase-8 was the molecular switch that controls apoptosis, necroptosis and pyroptosis, and prevented tissue damage during embryonic development and adulthood (Fritsch et al., 2019). Therefore, it may be an interesting and important research topic to explore the contribution of each form and conversion of different forms in drug-induced cardiomyocyte death.
Author Contributions
WM and SW wrote the manuscript. BZ and WL revised the manuscript. All authors read and approved the final version of the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants of the National Natural Scientific Foundation of China (Nos. 81703518 and 81973406), the Hunan Provincial Natural Scientific Foundation (No. 2019JJ50849), and the Scientific Research Project of Hunan Provincial Health and Family Planning Commission (No. B20180253).
References
- Agustini F. D., Arozal W., Louisa M., Siswanto S., Soetikno V., Nafrialdi N., et al. (2016). Cardioprotection mechanism of mangiferin on doxorubicin-induced rats: focus on intracellular calcium regulation. Pharm. Biol. 54 1289–1297. 10.3109/13880209.2015.1073750 [DOI] [PubMed] [Google Scholar]
- Akolkar G., Da Silva, Dias D., Ayyappan P., Bagchi A. K., Jassal D. S., et al. (2017). Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 313 H795–H809. [DOI] [PubMed] [Google Scholar]
- Avci H., Epikmen E. T., Ipek E., Tunca R., Birincioglu S. S., Aksit H., et al. (2017). Protective effects of silymarin and curcumin on cyclophosphamide-induced cardiotoxicity. Exp. Toxicol. Pathol. 69 317–327. 10.1016/j.etp.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Bao Z., Han Z., Zhang B., Yu Y., Xu Z., Ma W., et al. (2019). Arsenic trioxide blocked proliferation and cardiomyocyte differentiation of human induced pluripotent stem cells: implication in cardiac developmental toxicity. Toxicol. Lett. 309 51–58. 10.1016/j.toxlet.2019.03.008 [DOI] [PubMed] [Google Scholar]
- Baraka A., Abdelgawad H. (2010). Targeting apoptosis in the heart of streptozotocin-induced diabetic rats. J. Cardiovasc. Pharmacol. Ther. 15 175–181. 10.1177/1074248409356557 [DOI] [PubMed] [Google Scholar]
- Baranowski M., Blachnio A., Zabielski P., Gorski J. (2007). Pioglitazone induces de novo ceramide synthesis in the rat heart. Prostaglandins Other Lipid Mediat. 83 99–111. 10.1016/j.prostaglandins.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Barnhart B. C., Alappat E. C., Peter M. E. (2003). The CD95 type I/type II model. Semin. Immunol. 15 185–193. 10.1016/s1044-5323(03)00031-9 [DOI] [PubMed] [Google Scholar]
- Bartlett J. J., Trivedi P. C., Yeung P., Kienesberger P. C., Pulinilkunnil T. (2016). Doxorubicin impairs cardiomyocyte viability by suppressing transcription factor EB expression and disrupting autophagy. Biochem. J. 473 3769–3789. 10.1042/bcj20160385 [DOI] [PubMed] [Google Scholar]
- Belmonte F., Das S., Sysa-Shah P., Sivakumaran V., Stanley B., Guo X., et al. (2015). ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 309 H1271–H1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernuzzi F., Recalcati S., Alberghini A., Cairo G. (2009). Reactive oxygen species-independent apoptosis in doxorubicin-treated H9c2 cardiomyocytes: role for heme oxygenase-1 down-modulation. Chem. Biol. Interact 177 12–20. 10.1016/j.cbi.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Bouitbir J., Alshaikhali A., Panajatovic M. V., Abegg V. F., Paech F., Krahenbuhl S. (2019). Mitochondrial oxidative stress plays a critical role in the cardiotoxicity of sunitinib: running title: sunitinib and oxidative stress in hearts. Toxicology 426:152281. 10.1016/j.tox.2019.152281 [DOI] [PubMed] [Google Scholar]
- Brito V. B., Nascimento L. V., Nunes R. B., Moura D. J., Lago P. D., Saffi J. (2016). Exercise during pregnancy decreases doxorubicin-induced cardiotoxic effects on neonatal hearts. Toxicology 36 46–57. 10.1016/j.tox.2016.08.017 [DOI] [PubMed] [Google Scholar]
- Brumatti G., Salmanidis M., Ekert P. G. (2010). Crossing paths: interactions between the cell death machinery and growth factor survival signals. Cell Mol. Life Sci. 67 1619–1630. 10.1007/s00018-010-0288-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Shen T., Huang X., Lin Y., Chen B., Pang J., et al. (2017). Astragalus polysaccharide restores autophagic flux and improves cardiomyocyte function in doxorubicin-induced cardiotoxicity. Oncotarget 8 4837–4848. 10.18632/oncotarget.13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Ye P., Long C., Chen K., Li X., Wang H. (2007). Effect of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on ischemia-reperfusion injury in rats. Pharmacology 79 184–192. 10.1159/000100870 [DOI] [PubMed] [Google Scholar]
- Chae H. J., Kim H. R., Lee W. G., Kwak Y. K., Kim W. H. (2005). Radiation protects adriamycin-induced apoptosis. Immunopharmacol. Immunotoxicol. 27 211–232. 10.1081/iph-200067715 [DOI] [PubMed] [Google Scholar]
- Chambers T. P., Santiesteban L., Gomez D., Chambers J. W. (2017). Sab mediates mitochondrial dysfunction involved in imatinib mesylate-induced cardiotoxicity. Toxicology 382 24–35. 10.1016/j.tox.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Chen G., Chelu M. G., Dobrev D., Li N. (2018). Cardiomyocyte inflammasome signaling in cardiomyopathies and atrial fibrillation: mechanisms and potential therapeutic implications. Front. Physiol. 9:1115. 10.3389/fphys.2018.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Xu X., Kobayashi S., Timm D., Jepperson T., Liang Q. (2011). Caloric restriction mimetic 2-deoxyglucose antagonizes doxorubicin-induced cardiomyocyte death by multiple mechanisms. J. Biol. Chem. 286 21993–22006. 10.1074/jbc.m111.225805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Xu S., Zhao C., Liu B. (2019). Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 516 37–43. 10.1016/j.bbrc.2019.06.015 [DOI] [PubMed] [Google Scholar]
- Cheng H., Kari G., Dicker A. P., Rodeck U., Koch W. J., Force T. (2011). A novel preclinical strategy for identifying cardiotoxic kinase inhibitors and mechanisms of cardiotoxicity. Circ. Res. 109 1401–1409. 10.1161/circresaha.111.255695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs A. C., Phaneuf S. L., Dirks A. J., Phillips T., Leeuwenburgh C. (2002). Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 62 4592–4598. [PubMed] [Google Scholar]
- Chiosi E., Spina A., Sorrentino A., Romano M., Sorvillo L., Senatore G., et al. (2007). Change in TNF-alpha receptor expression is a relevant event in doxorubicin-induced H9c2 cardiomyocyte cell death. J. Interferon Cytokine Res. 27 589–597. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Sinha K., Banerjee S., Sil P. C. (2016). Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors 42 647–664. 10.1002/biof.1301 [DOI] [PubMed] [Google Scholar]
- Chu T. F., Rupnick M. A., Kerkela R., Dallabrida S. M., Zurakowski D., Nguyen L., et al. (2007). Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel M., Simeone P., Grivet B. (1988). [Cardiac toxicity of 5-fluorouracil. review of the literature, 5 new cases]. Presse Med. 17 1675–1678. [PubMed] [Google Scholar]
- Coelho A. R., Martins T. R., Couto R., Deus C., Pereira C. V., Simoes R. F., et al. (2017). Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim. Biophys. Acta Mol. Basis Dis. 1863 2904–2923. 10.1016/j.bbadis.2017.07.030 [DOI] [PubMed] [Google Scholar]
- Conklin D. J., Haberzettl P., Jagatheesan G., Baba S., Merchant M. L., Prough R. A., et al. (2015). Glutathione S-transferase P protects against cyclophosphamide-induced cardiotoxicity in mice. Toxicol. Appl. Pharmacol. 285 136–148. 10.1016/j.taap.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson B. T., Brennan M. A. (2001). Pro-inflammatory programmed cell death. Trends Microbiol. 9 113–114. 10.1016/s0966-842x(00)01936-3 [DOI] [PubMed] [Google Scholar]
- Cooper S. L., Sandhu H., Hussain A., Mee C., Maddock H. (2018). Involvement of mitogen activated kinase kinase 7 intracellular signalling pathway in Sunitinib-induced cardiotoxicity. Toxicology 394 72–83. 10.1016/j.tox.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Coppola C., Riccio G., Barbieri A., Monti M. G., Piscopo G., Rea D., et al. (2016). Antineoplastic-related cardiotoxicity, morphofunctional aspects in a murine model: contribution of the new tool 2D-speckle tracking. Onco Targets Ther. 9 6785–6794. 10.2147/ott.s106528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corna G., Santambrogio P., Minotti G., Cairo G. (2004). Doxorubicin paradoxically protects cardiomyocytes against iron-mediated toxicity: role of reactive oxygen species and ferritin. J. Biol. Chem. 279 13738–13745. 10.1074/jbc.m310106200 [DOI] [PubMed] [Google Scholar]
- Costa V. M., Carvalho F., Duarte J. A., Bastos Mde L., Remiao F. (2013). The heart as a target for xenobiotic toxicity: the cardiac susceptibility to oxidative stress. Chem. Res. Toxicol. 26 1285–1311. 10.1021/tx400130v [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T., Ferreira L. L., Coelho A. R., Deus C. M., Oliveira P. J. (2018). Doxorubicin triggers bioenergetic failure and p53 activation in mouse stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol. 348 1–13. 10.1016/j.taap.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Czabotar P. E., Lessene G., Strasser A., Adams J. M. (2014). Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15 49–63. 10.1038/nrm3722 [DOI] [PubMed] [Google Scholar]
- Damiani R. M., Moura D. J., Viau C. M., Brito V., Moras A. M., Henriques J. P., et al. (2018). Influence of PARP-1 inhibition in the cardiotoxicity of the topoisomerase 2 inhibitors doxorubicin and mitoxantrone. Toxicol. In Vitro 52 203–213. 10.1016/j.tiv.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Deng S., Kruger A., Kleschyov A. L., Kalinowski L., Daiber A., Wojnowski L. (2007). Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic. Biol. Med. 42 466–473. 10.1016/j.freeradbiomed.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Dhingra R., Margulets V., Chowdhury S. R., Thliveris J., Jassal D., Fernyhough P., et al. (2014). Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc. Natl. Acad. Sci. U.S.A. 111 E5537–E5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrakis P., Romay-Ogando M. I., Timolati F., Suter T. M., Zuppinger C. (2012). Effects of doxorubicin cancer therapy on autophagy and the ubiquitin-proteasome system in long-term cultured adult rat cardiomyocytes. Cell Tissue Res. 350 361–372. 10.1007/s00441-012-1475-8 [DOI] [PubMed] [Google Scholar]
- Doherty K. R., Wappel R. L., Talbert D. R., Trusk P. B., Moran D. M., Kramer J. W., et al. (2013). Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 272 245–255. 10.1016/j.taap.2013.04.027 [DOI] [PubMed] [Google Scholar]
- Duran J. M., Makarewich C. A., Trappanese D., Gross P., Husain S., Dunn J., et al. (2014). Sorafenib cardiotoxicity increases mortality after myocardial infarction. Circ. Res. 114 1700–1712. 10.1161/circresaha.114.303200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agamy D. S., Elkablawy M. A., Abo-Haded H. M. (2017). Modulation of cyclophosphamide-induced cardiotoxicity by methyl palmitate. Cancer Chemother. Pharmacol. 79 399–409. 10.1007/s00280-016-3233-1 [DOI] [PubMed] [Google Scholar]
- El-Awady el S. E., Moustafa Y. M., Abo-Elmatty D. M., Radwan A. (2011). Cisplatin-induced cardiotoxicity: mechanisms and cardioprotective strategies. Eur. J. Pharmacol 650 335–341. 10.1016/j.ejphar.2010.09.085 [DOI] [PubMed] [Google Scholar]
- Eskandari M. R., Moghaddam F., Shahraki J., Pourahmad J. (2015). A comparison of cardiomyocyte cytotoxic mechanisms for 5-fluorouracil and its pro-drug capecitabine. Xenobiotica 45 79–87. 10.3109/00498254.2014.942809 [DOI] [PubMed] [Google Scholar]
- Ewer M. S., Ewer S. M. (2015). Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 12 547–558. 10.1038/nrcardio.2015.65 [DOI] [PubMed] [Google Scholar]
- Fabbi P., Spallarossa P., Garibaldi S., Barisione C., Mura M., Altieri P., et al. (2015). Doxorubicin impairs the insulin-like growth factor-1 system and causes insulin-like growth factor-1 resistance in cardiomyocytes. PLoS One 10:e0124643. 10.1371/journal.pone.0124643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Wang C., Zhang Y., Hang P., Liu Y., Pan Z., et al. (2013). Genistein ameliorates adverse cardiac effects induced by arsenic trioxide through preventing cardiomyocytes apoptosis. Cell Physiol. Biochem. 31 80–91. 10.1159/000343351 [DOI] [PubMed] [Google Scholar]
- Fang X., Wang H., Han D., Xie E., Yang X., Wei J., et al. (2019). Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 116 2672–2680. 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M., Murphy T., Macdougall J., Yang W., Luque A., Iruela-Arispe L., et al. (2004). HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc. Toxicol. 4 327–337. [DOI] [PubMed] [Google Scholar]
- Finck B. N., Kelly D. P. (2007). Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115 2540–2548. 10.1161/circulationaha.107.670588 [DOI] [PubMed] [Google Scholar]
- Finkel T. (2006). Cell biology: a clean energy programme. Nature 444 151–152. 10.1038/444151a [DOI] [PubMed] [Google Scholar]
- Focaccetti C., Bruno A., Magnani E., Bartolini D., Principi E., Dallaglio K., et al. (2015). Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One 10:e0115686. 10.1371/journal.pone.0115686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch M., Gunther S. D., Schwarzer R., Albert M. C., Schorn F., Werthenbach J. P., et al. (2019). Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575 683–687. 10.1038/s41586-019-1770-6 [DOI] [PubMed] [Google Scholar]
- Fulbright J. M., Egas-Bejar D. E., Huh W. W., Chandra J. (2015). Analysis of redox and apoptotic effects of anthracyclines to delineate a cardioprotective strategy. Cancer Chemother. Pharmacol. 76 1297–1307. 10.1007/s00280-015-2879-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Aaronson S. A., Abrams J. M., Adam D., Agostinis P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death. Differ. 25 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R. Y., Mukhopadhyay P., Mohanraj R., Wang H., Horvath B., Yin S., et al. (2011). Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 4 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J., Das J., Manna P., Sil P. C. (2009). Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol. Appl. Pharmacol. 240 73–87. 10.1016/j.taap.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Giulivi C., Boveris A., Cadenas E. (1995). Hydroxyl radical generation during mitochondrial electron transfer and the formation of 8-hydroxydesoxyguanosine in mitochondrial DNA. Arch. Biochem. Biophys. 316 909–916. 10.1006/abbi.1995.1122 [DOI] [PubMed] [Google Scholar]
- Goyal V., Bews H., Cheung D., Premecz S., Mandal S., Shaikh B., et al. (2016). The Cardioprotective role of N-Acetyl cysteine amide in the prevention of doxorubicin and trastuzumab-mediated cardiac dysfunction. Can. J. Cardiol. 32 1513–1519. 10.1016/j.cjca.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Grazette L. P., Boecker W., Matsui T., Semigran M., Force T. L., Hajjar R. J., et al. (2004). Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J. Am. Coll. Cardiol. 44 2231–2238. 10.1016/j.jacc.2004.08.066 [DOI] [PubMed] [Google Scholar]
- Grootjans S., Vanden Berghe T., Vandenabeele P. (2017). Initiation and execution mechanisms of necroptosis: an overview. Cell Death. Differ. 24 1184–1195. 10.1038/cdd.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Hu W., Song Z. P., Chen Y. G., Zhang D. D., Wang C. Q. (2016). Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. Int. Immunopharmacol. 32 1–7. 10.1016/j.intimp.2016.01.002 [DOI] [PubMed] [Google Scholar]
- He H., Tao H., Xiong H., Duan S. Z., Mcgowan F. X., Mortensen R. M., et al. (2014). Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor gamma-independent mitochondrial oxidative stress in mouse hearts. Toxicol. Sci. 138 468–481. 10.1093/toxsci/kfu015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. H., Zhang J., Hasinoff B. B., Clark J. R., Ferrans V. J. (1997). Comparison of the structural changes induced by doxorubicin and mitoxantrone in the heart, kidney and intestine and characterization of the Fe(III)-mitoxantrone complex. J. Mol. Cell Cardiol. 29 2415–2430. 10.1006/jmcc.1997.0477 [DOI] [PubMed] [Google Scholar]
- Herrmann J., Yang E. H., Iliescu C. A., Cilingiroglu M., Charitakis K., Hakeem A., et al. (2016). Vascular toxicities of cancer therapies: the old and the new–an evolving avenue. Circulation 133 1272–1289. 10.1161/circulationaha.115.018347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro T., Hynes N. E. (2004). ErbB receptors: directing key signaling networks throughout life. Annu. Rev. Pharmacol. Toxicol. 44 195–217. 10.1146/annurev.pharmtox.44.101802.121440 [DOI] [PubMed] [Google Scholar]
- Horie T., Ono K., Nishi H., Nagao K., Kinoshita M., Watanabe S., et al. (2010). Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 87 656–664. 10.1093/cvr/cvq148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. L., Mo F. E. (2016). Matricellular protein CCN1 mediates doxorubicin-induced cardiomyopathy in mice. Oncotarget 7 36698–36710. 10.18632/oncotarget.9162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Lu S., Mcalpine I., Jamieson J. D., Lee D. U., Marroquin L. D., et al. (2012). Mechanistic investigation of imatinib-induced cardiac toxicity and the involvement of c-Abl kinase. Toxicol. Sci. 129 188–199. 10.1093/toxsci/kfs192 [DOI] [PubMed] [Google Scholar]
- Hutt K. J. (2015). The role of BH3-only proteins in apoptosis within the ovary. Reproduction 149 R81–R89. [DOI] [PubMed] [Google Scholar]
- Imai H., Matsuoka M., Kumagai T., Sakamoto T., Koumura T. (2017). Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol. Immunol. 403 143–170. 10.1007/82_2016_508 [DOI] [PubMed] [Google Scholar]
- Jiang J., Mohan N., Endo Y., Shen Y., Wu W. J. (2018). Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget 9 6095–6108. 10.18632/oncotarget.23543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Yang J., Jiang L., Chen J., Wang H. (2018). MicroRNA-29b regulates the mitochondria-dependent apoptotic pathway by targeting bax in doxorubicin cardiotoxicity. Cell Physiol. Biochem. 48 692–704. 10.1159/000491896 [DOI] [PubMed] [Google Scholar]
- Jorgensen I., Miao E. A. (2015). Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 265 130–142. 10.1111/imr.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. H., Ro S. H., Cao J., Otto N. M., Kim D. H. (2010). mTOR regulation of autophagy. FEBS Lett. 584 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. J., Sridharan H., Huang C., Mandal P., Upton J. W., Gough P. J., et al. (2013). Toll-like receptor 3-mediated necrosis via TRIF. RIP3, and MLKL. J. Biol. Chem. 288 31268–31279. 10.1074/jbc.m113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivendi S. V., Konorev E. A., Cunningham S., Vanamala S. K., Kaji E. H., Joseph J., et al. (2005). Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem. J. 389 527–539. 10.1042/bj20050285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Takemura G., Kanamori H., Takeyama T., Watanabe T., Morishita K., et al. (2012). Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 96 456–465. 10.1093/cvr/cvs282 [DOI] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B., Guan K.-L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Ma A. G., Kitta K., Fitch S. N., Ikeda T., Ihara Y., et al. (2003). Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol. Pharmacol. 63 368–377. 10.1124/mol.63.2.368 [DOI] [PubMed] [Google Scholar]
- Kimura T., Uesugi M., Takase K., Miyamoto N., Sawada K. (2017). Hsp90 inhibitor geldanamycin attenuates the cytotoxicity of sunitinib in cardiomyocytes via inhibition of the autophagy pathway. Toxicol. Appl. Pharmacol 329 282–292. 10.1016/j.taap.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Abdelmohsen K., Abe A., Abedin M. J., Abeliovich H., Acevedo Arozena A., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Volden P., Timm D., Mao K., Xu X., Liang Q. (2010). Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J. Biol. Chem. 285 793–804. 10.1074/jbc.m109.070037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleini N., Nickel B. E., Edel A. L., Fandrich R. R., Ravandi A., Kardami E. (2019). Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact 303 35–39. 10.1016/j.cbi.2019.01.032 [DOI] [PubMed] [Google Scholar]
- Kurauchi K., Nishikawa T., Miyahara E., Okamoto Y., Kawano Y. (2017). Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res Notes 10:406. 10.1186/s13104-017-2726-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkur S., Judd S., Bostick R. M., Mcclellan W., Flanders W. D., Stevens V. L., et al. (2015). Oxidative stress, inflammation, and markers of cardiovascular health. Atherosclerosis 243 38–43. 10.1016/j.atherosclerosis.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti M., Porto S., Marra M., Zappavigna S., Grimaldi A., Feola D., et al. (2012). 5-Fluorouracil induces apoptosis in rat cardiocytes through intracellular oxidative stress. J. Exp. Clin. Cancer Res. 31:60. 10.1186/1756-9966-31-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Wang Y., Huang K., Zeng Q. (2020). Heat shock protein 22 attenuates doxorubicin-induced cardiotoxicity via regulating inflammation and apoptosis. Front. Pharmacol. 11:257. 10.3389/fphar.2020.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekes D., Szadvari I., Krizanova O., Lopusna K., Rezuchova I., Novakova M., et al. (2016). Nilotinib induces ER stress and cell death in H9c2 cells. Physiol. Res. 65 S505–S514. [DOI] [PubMed] [Google Scholar]
- Lemmens K., Doggen K., De Keulenaer G. W. (2007). Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 116 954–960. 10.1161/circulationaha.107.690487 [DOI] [PubMed] [Google Scholar]
- Li D. L., Wang Z. V., Ding G., Tan W., Luo X., Criollo A., et al. (2016). Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation 133 1668–1687. 10.1161/circulationaha.115.017443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang W., Niu T., Wang H., Li B., Shao L., et al. (2014). Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid Med. Cell Longev 2014:748524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhao H., Wang Y., Shao Y., Liu J., Xing M. (2019). Arsenic-induced cardiotoxicity correlates with mitochondrial damage and trace elements imbalance in broiler chickens. Poult. Sci. 98 734–744. 10.3382/ps/pey469 [DOI] [PubMed] [Google Scholar]
- Li S., Zhao H., Wang Y., Shao Y., Wang B., Wang Y., et al. (2018). Regulation of autophagy factors by oxidative stress and cardiac enzymes imbalance during arsenic or/and copper induced cardiotoxicity in Gallus gallus. Ecotoxicol. Environ. Saf. 148 125–134. 10.1016/j.ecoenv.2017.10.018 [DOI] [PubMed] [Google Scholar]
- Li W. J., Zhang X. Y., Wu R. T., Song Y. H., Xie M. Y. (2018). Ganoderma atrum polysaccharide improves doxorubicin-induced cardiotoxicity in mice by regulation of apoptotic pathway in mitochondria. Carbohydr. Polym. 202 581–590. 10.1016/j.carbpol.2018.08.144 [DOI] [PubMed] [Google Scholar]
- Li S. W., Sun X., He Y., Guo Y., Zhao H. J., Hou Z. J., et al. (2017). Assessment of arsenic trioxide in the heart of Gallus gallus: alterations of oxidative damage parameters, inflammatory cytokines, and cardiac enzymes. Environ. Sci. Pollut. Res. Int. 24 5781–5790. 10.1007/s11356-016-8223-7 [DOI] [PubMed] [Google Scholar]
- Lim C. C., Zuppinger C., Guo X., Kuster G. M., Helmes M., Eppenberger H. M., et al. (2004). Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J. Biol. Chem. 279 8290–8299. 10.1074/jbc.m308033200 [DOI] [PubMed] [Google Scholar]
- Lin H., Stankov M. V., Hegermann J., Budida R., Panayotova-Dimitrova D., Schmidt R. E., et al. (2019). Zidovudine-mediated autophagy inhibition enhances mitochondrial toxicity in muscle cells. Antimicrob. Agents Chemother 63:e01443-18. 10.1128/AAC.01443-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke J., Lang C., Sawodny O., Feuer R. (2015). Impairment of energy metabolism in cardiomyocytes caused by 5-FU catabolites can be compensated by administration of amino acids. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015 5363–5366. [DOI] [PubMed] [Google Scholar]
- Lu L., Wu W., Yan J., Li X., Yu H., Yu X. (2009). Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int. J. Cardiol. 134 82–90. 10.1016/j.ijcard.2008.01.043 [DOI] [PubMed] [Google Scholar]
- Ludke A., Akolkar G., Ayyappan P., Sharma A. K., Singal P. K. (2017). Time course of changes in oxidative stress and stress-induced proteins in cardiomyocytes exposed to doxorubicin and prevention by vitamin C. PLoS One 12:e0179452. 10.1371/journal.pone.0179452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Zhu Y., Chen M., Yan H., Yang B., Yang X., et al. (2018). HMGB1 contributes to adriamycin-induced cardiotoxicity via up-regulating autophagy. Toxicol. Lett. 292 115–122. 10.1016/j.toxlet.2018.04.034 [DOI] [PubMed] [Google Scholar]
- Lushnikova E. L., Nepomnyashchikh L. M., Sviridov E. A., Klinnikova M. G. (2008). Ultrastructural signs of cyclophosphamide-induced damage to cardiomyocytes. Bull. Exp. Biol. Med. 146 366–371. 10.1007/s10517-008-0287-z [DOI] [PubMed] [Google Scholar]
- Ma H., Jones K. R., Guo R., Xu P., Shen Y., Ren J. (2010). Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin. Exp. Pharmacol. Physiol. 37 460–465. 10.1111/j.1440-1681.2009.05323.x [DOI] [PubMed] [Google Scholar]
- Maharsy W., Aries A., Mansour O., Komati H., Nemer M. (2014). Ageing is a risk factor in imatinib mesylate cardiotoxicity. Eur. J. Heart Fail. 16 367–376. 10.1002/ejhf.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza K. L., Hasinoff B. B. (1995). Production of hydroxyl radical by iron(III)-anthraquinone complexes through self-reduction and through reductive activation by the xanthine oxidase/hypoxanthine system. Arch. Biochem. Biophys. 321 51–60. 10.1006/abbi.1995.1367 [DOI] [PubMed] [Google Scholar]
- Man S. M., Karki R., Kanneganti T. D. (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277 61–75. 10.1111/imr.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Lin H., Zhang J., Lin N., Sun Z., Gao F., et al. (2019). Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3. J. Mol. Cell Cardiol. 136 15–26. 10.1016/j.yjmcc.2019.08.009 [DOI] [PubMed] [Google Scholar]
- Miao X., Tang Z., Wang Y., Su G., Sun W., Wei W., et al. (2013). Metallothionein prevention of arsenic trioxide-induced cardiac cell death is associated with its inhibition of mitogen-activated protein kinases activation in vitro and in vivo. Toxicol. Lett. 220 277–285. 10.1016/j.toxlet.2013.04.025 [DOI] [PubMed] [Google Scholar]
- Milano G., Biemmi V., Lazzarini E., Balbi C., Ciullo A., Bolis S., et al. (2020). Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc. Res. 116 383–392. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E., Little S. A. (1998). Teratogen-induced cell death in postimplantation mouse embryos: differential tissue sensitivity and hallmarks of apoptosis. Cell Death. Differ. 5 592–600. 10.1038/sj.cdd.4400390 [DOI] [PubMed] [Google Scholar]
- Mirkes P. E., Wilson K. L., Cornel L. M. (2000). Teratogen-induced activation of ERK, JNK, and p38 MAP kinases in early postimplantation murine embryos. Teratology 62 14–25. [DOI] [PubMed] [Google Scholar]
- Mishra P., Singh S. V., Verma A. K., Srivastava P., Sultana S., Rath S. K. (2014). Rosiglitazone induces cardiotoxicity by accelerated apoptosis. Cardiovasc. Toxicol. 14 99–119. 10.1007/s12012-013-9234-y [DOI] [PubMed] [Google Scholar]
- Mohan N., Jiang J., Wu W. J. (2017). Implications of autophagy and oxidative stress in trastuzumab-mediated cardiac toxicities. Austin. Pharmacol. Pharm. 2:1005. [PMC free article] [PubMed] [Google Scholar]
- Mohan N., Shen Y., Endo Y., Elzarrad M. K., Wu W. J. (2016). Trastuzumab, but not pertuzumab, dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol. Cancer Ther. 15 1321–1331. 10.1158/1535-7163.mct-15-0741 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Rajesh M., Batkai S., Kashiwaya Y., Hasko G., Liaudet L., et al. (2009). Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am. J. Physiol. Heart Circ. Physiol. 296 H1466–H1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi M. N., Al-Shabanah O. A., Hafez M. M., Sayed-Ahmed M. M. (2011). Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 25 135–142. 10.1002/jbt.20369 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ueda Y., Juan Y., Katsuda S., Takahashi H., Koh E. (2000). Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: in vivo study. Circulation 102 572–578. 10.1161/01.cir.102.5.572 [DOI] [PubMed] [Google Scholar]
- Nicol M., Baudet M., Cohen-Solal A. (2019). Subclinical left ventricular dysfunction during chemotherapy. Cardiac Fail. Rev. 5 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T., Miyahara E., Kurauchi K., Watanabe E., Ikawa K., Asaba K., et al. (2015). Mechanisms of fatal cardiotoxicity following high-dose cyclophosphamide therapy and a method for its prevention. PLoS One 10:e0131394. 10.1371/journal.pone.0131394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omole J. G., Ayoka O. A., Alabi Q. K., Adefisayo M. A., Asafa M. A., Olubunmi B. O., et al. (2018). Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J. Evid. Based Integr. Med. 23:2156587218757649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padegimas A., Clasen S., Ky B. (2020). Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc. Med. 30 22–28. 10.1016/j.tcm.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palee S., Weerateerangkul P., Chinda K., Chattipakorn S. C., Chattipakorn N. (2013). Mechanisms responsible for beneficial and adverse effects of rosiglitazone in a rat model of acute cardiac ischaemia-reperfusion. Exp. Physiol. 98 1028–1037. 10.1113/expphysiol.2012.070433 [DOI] [PubMed] [Google Scholar]
- Papparella I., Ceolotto G., Berto L., Cavalli M., Bova S., Cargnelli G., et al. (2007). Vitamin C prevents zidovudine-induced NAD(P)H oxidase activation and hypertension in the rat. Cardiovasc. Res. 73 432–438. 10.1016/j.cardiores.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Patel V. G., Cornell R. F. (2019). Cardiovascular complications associated with multiple myeloma therapies: incidence, pathophysiology, and management. Curr. Oncol. Rep. 21:29. [DOI] [PubMed] [Google Scholar]
- Pentassuglia L., Timolati F., Seifriz F., Abudukadier K., Suter T. M., Zuppinger C. (2007). Inhibition of ErbB2/neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytes. Exp. Cell Res. 313 1588–1601. 10.1016/j.yexcr.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C., Nizet V., Johnson R. S. (2008). Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle 7 28–32. 10.4161/cc.7.1.5145 [DOI] [PubMed] [Google Scholar]
- Pistillucci G., Ciorra A. A., Sciacca V., Raponi M., Rossi R., Veltri E. (2015). Troponin I and B-type natriuretic peptide (BNP) as biomarkers for the prediction of cardiotoxicity in patients with breast cancer treated with adjuvant anthracyclines and trastuzumab. Clin. Terapeutica 166 E67–E71. [DOI] [PubMed] [Google Scholar]
- Purevjav E., Nelson D. P., Varela J. J., Jimenez S., Kearney D. L., Sanchez X. V., et al. (2007). Myocardial fas ligand expression increases susceptibility to AZT-induced cardiomyopathy. Cardiovasc. Toxicol. 7 255–263. 10.1007/s12012-007-9004-9 [DOI] [PubMed] [Google Scholar]
- Qian P., Yan L. J., Li Y. Q., Yang H. T., Duan H. Y., Wu J. T., et al. (2018). Cyanidin ameliorates cisplatin-induced cardiotoxicity via inhibition of ROS-mediated apoptosis. Exp. Ther. Med. 15 1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu K. G., Cherian O. L. (2009). Characterization of cytotoxicity induced by arsenic trioxide (a potent anti-APL drug) in rat cardiac myocytes. J. Trace Elem. Med. Biol. 23 61–68. 10.1016/j.jtemb.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Refaie M. M. M., Shehata S., El-Hussieny M., Abdelraheem W. M., Bayoumi A. M. A. (2020). Role of ATP-sensitive potassium channel (KATP) and eNOS in mediating the protective effect of nicorandil in cyclophosphamide-induced cardiotoxicity. Cardiovasc. Toxicol. 20 71–81. 10.1007/s12012-019-09535-8 [DOI] [PubMed] [Google Scholar]
- Rharass T., Gbankoto A., Canal C., Kursunluoglu G., Bijoux A., Panakova D., et al. (2016). Oxidative stress does not play a primary role in the toxicity induced with clinical doses of doxorubicin in myocardial H9c2 cells. Mol. Cell. Biochem. 413 199–215. 10.1007/s11010-016-2653-x [DOI] [PubMed] [Google Scholar]
- Rohrbach S., Muller-Werdan U., Werdan K., Koch S., Gellerich N. F., Holtz J. (2005). Apoptosis-modulating interaction of the neuregulin/erbB pathway with anthracyclines in regulating Bcl-xS and Bcl-xL in cardiomyocytes. J. Mol. Cell Cardiol. 38 485–493. 10.1016/j.yjmcc.2004.12.013 [DOI] [PubMed] [Google Scholar]
- Roos W. P., Thomas A. D., Kaina B. (2016). DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 16 20–33. 10.1038/nrc.2015.2 [DOI] [PubMed] [Google Scholar]
- Rossato L. G., Costa V. M., Vilas-Boas V., De Lourdes Bastos M., Rolo A., Palmeira C., et al. (2013). Therapeutic concentrations of mitoxantrone elicit energetic imbalance in H9c2 cells as an earlier event. Cardiovasc. Toxicol. 13 413–425. 10.1007/s12012-013-9224-0 [DOI] [PubMed] [Google Scholar]
- Ry G., Mukhopadhyay P., Mohanraj R., Wang H., Horváth B., Yin S. (2011). Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 4 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer D. B., Zuppinger C., Miller T. A., Eppenberger H. M., Suter T. M. (2002). Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 105 1551–1554. 10.1161/01.cir.0000013839.41224.1c [DOI] [PubMed] [Google Scholar]
- Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526 660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Singh A. P., Glennon M. S., Umbarkar P., Gupte M., Galindo C. L., Zhang Q., et al. (2019). Ponatinib-induced cardiotoxicity: delineating the signalling mechanisms and potential rescue strategies. Cardiovasc. Res. 115 966–977. 10.1093/cvr/cvz006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. K., Shukla P. C., Quan A., Lovren F., Pan Y., Wolfstadt J. I., et al. (2011). Herceptin, a recombinant humanized anti-ERBB2 monoclonal antibody, induces cardiomyocyte death. Biochem. Biophys. Res. Commun. 411 421–426. 10.1016/j.bbrc.2011.06.169 [DOI] [PubMed] [Google Scholar]
- Singla D. K., Johnson T. A., Tavakoli Dargani Z. (2019). Exosome treatment enhances anti-inflammatory M2 macrophages and reduces inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy. Cells 8:1224. 10.3390/cells8101224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A. F., Anees L. M., Ibrahim D. M. (2018). Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedebergs Arch. Pharmacol. 391 819–832. 10.1007/s00210-018-1506-4 [DOI] [PubMed] [Google Scholar]
- Song B. W., Wang L. (2013). [Necroptosis: a programmed cell necrosis]. Sheng Li Ke Xue Jin Zhan 44 281–286. [PubMed] [Google Scholar]
- Song R., Yang Y., Lei H., Wang G., Huang Y., Xue W., et al. (2018). HDAC6 inhibition protects cardiomyocytes against doxorubicin-induced acute damage by improving alpha-tubulin acetylation. J. Mol. Cell Cardiol. 124 58–69. 10.1016/j.yjmcc.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Spagnuolo R. D., Recalcati S., Tacchini L., Cairo G. (2011). Role of hypoxia-inducible factors in the dexrazoxane-mediated protection of cardiomyocytes from doxorubicin-induced toxicity. Br. J. Pharmacol. 163 299–312. 10.1111/j.1476-5381.2011.01208.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sumneang N., Siri-Angkul N., Kumfu S., Chattipakorn S. C., Chattipakorn N. (2020). The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch. Biochem. Biophys. 680:108241. 10.1016/j.abb.2019.108241 [DOI] [PubMed] [Google Scholar]
- Sun Z., Lu W., Lin N., Lin H., Zhang J., Ni T., et al. (2020). Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem. Pharmacol. 175:113888. 10.1016/j.bcp.2020.113888 [DOI] [PubMed] [Google Scholar]
- Szabados E., Fischer G. M., Toth K., Csete B., Nemeti B., Trombitas K., et al. (1999). Role of reactive oxygen species and poly-ADP-ribose polymerase in the development of AZT-induced cardiomyopathy in rat. Free Radic. Biol. Med. 26 309–317. 10.1016/s0891-5849(98)00199-3 [DOI] [PubMed] [Google Scholar]
- Tavakoli R., Dargani Z., Singla D. K. (2019). Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am. J. Physiol. Heart Circ. Physiol. 317 H460–H471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal I., Ozbek Bilgin A., Keskin Cimen F., Kurt N., Suleyman Z., Bilgin Y., et al. (2018). The effect of rutin on cisplatin-induced oxidative cardiac damage in rats. Anatol. J. Cardiol. 20 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibiribi P., Bui-Xuan C., Bui-Xuan B., Lombard-Bohas C., Duperret S., Belkhiria M., et al. (2006). Cardiac lesions induced by 5-fluorouracil in the rabbit. Hum. Exp. Toxicol. 25 305–309. 10.1191/0960327106ht628oa [DOI] [PubMed] [Google Scholar]
- Tummers B., Green D. R. (2017). Caspase-8: regulating life and death. Immunol. Rev. 277 76–89. 10.1111/imr.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese M. V., Abhilash M., Paul M. V., Alex M., Nair R. H. (2017). Omega-3 fatty acid protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Cardiovasc. Toxicol. 17 109–119. 10.1007/s12012-016-9361-3 [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J., Kalyanaraman B., Martasek P., Hogg N., Masters B. S., Karoui H., et al. (1998). Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 95 9220–9225. 10.1073/pnas.95.16.9220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J., Martasek P., Hogg N., Masters B. S., Pritchard K. A., Kalyanaraman B. (1997). Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 36 11293–11297. 10.1021/bi971475e [DOI] [PubMed] [Google Scholar]
- Vejpongsa P., Yeh E. T. (2014). Topoisomerase 2beta: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin. Pharmacol. Ther. 95 45–52. 10.1038/clpt.2013.201 [DOI] [PubMed] [Google Scholar]
- Vineetha V. P., Soumya R. S., Raghu K. G. (2015). Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur. J. Pharmacol. 754 162–172. 10.1016/j.ejphar.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Voskens C. J., Goldinger S. M., Loquai C., Robert C., Kaehler K. C., Berking C., et al. (2013). The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 8:e53745. 10.1371/journal.pone.0053745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia S. (2015). Acute cyclophosphamide hemorrhagic myopericarditis: dilemma case report. Literature review and proposed diagnostic criteria. J. Clin. Diagn Res. 9 Oe01–Oe03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. (2002). Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3 430–440. 10.1038/nrm831 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Funakoshi T., Unuma K., Aki T., Uemura K. (2014). Activation of the ubiquitin-proteasome system against arsenic trioxide cardiotoxicity involves ubiquitin ligase Parkin for mitochondrial homeostasis. Toxicology 322 43–50. 10.1016/j.tox.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Wu C.-C., Bratton S. B. (2013). Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 19 546–558. 10.1089/ars.2012.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Li Y. C., Wang Y. R., Li T. K., Chan N. L. (2013). On the structural basis and design guidelines for type II topoisomerase-targeting anticancer drugs. Nucleic Acids Res. 41 10630–10640. 10.1093/nar/gkt828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., He B., Wang Y. J., Fu Q. (2004). [Effect of herceptin combined with Doxorubicin on rat cardiotoxicity]. Ai Zheng 23 367–371. [PubMed] [Google Scholar]
- Xu Z., Jin Y., Yan H., Gao Z., Xu B., Yang B., et al. (2018). High-mobility group box 1 protein-mediated necroptosis contributes to dasatinib-induced cardiotoxicity. Toxicol. Lett. 296 39–47. 10.1016/j.toxlet.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Yamaoka M., Yamaguchi S., Suzuki T., Okuyama M., Nitobe J., Nakamura N., et al. (2000). Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J. Mol. Cell Cardiol. 32 881–889. 10.1006/jmcc.2000.1132 [DOI] [PubMed] [Google Scholar]
- Yang J. K. (2015). Death effecter domain for the assembly of death-inducing signaling complex. Apoptosis 20 235–239. 10.1007/s10495-014-1060-6 [DOI] [PubMed] [Google Scholar]
- Yang Q., Wen L., Meng Z., Chen Y. (2018). Blockage of endoplasmic reticulum stress attenuates nilotinib-induced cardiotoxicity by inhibition of the Akt-GSK3beta-Nox4 signaling. Eur. J. Pharmacol 822 85–94. 10.1016/j.ejphar.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Yang S., Liu J., Qu C., Sun J., Zhang B. Q., Sun Y. R., et al. (2017). [Potassium channels and autophagy]. Sheng Li Xue Bao 69 509–514. [PubMed] [Google Scholar]
- Yousif N. G., Al-Amran F. G. (2011). Novel Toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) induced cardiac injury in mice. BMC Cardiovasc. Disord. 11:62 10.1186/1471-2261-11-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Wang C., Kong Q., Wu X., Lu J. J., Chen X. (2018). Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine 40 125–139. 10.1016/j.phymed.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Yu W., Sun H., Zha W., Cui W., Xu L., Min Q., et al. (2017). apigenin attenuates adriamycin-induced cardiomyocyte apoptosis via the PI3K/AKT/mTOR pathway. Evid Based Complement. Alternat. Med. 2017:2590676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Ruan Y., Huang X., Dou L., Lan M., Cui J., et al. (2020). Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem. Biophys. Res. Commun. 523 140–146. 10.1016/j.bbrc.2019.12.027 [DOI] [PubMed] [Google Scholar]
- Zeng C., Duan F., Hu J., Luo B., Huang B., Lou X., et al. (2020). NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 101523. 10.1016/j.redox.2020.101523 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J., Tao L., Zhang S., Gao H., Zhang Y., Sun J., et al. (2020). Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother. Res. 34 649–659. 10.1002/ptr.6557 [DOI] [PubMed] [Google Scholar]
- Zhang C., Feng Y., Qu S., Wei X., Zhu H., Luo Q., et al. (2011). Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 90 538–545. 10.1093/cvr/cvr022 [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Sun G. B., Luo Y., Wang M., Wang W., Du Y. Y., et al. (2017). Salvianolic acid A protects H9c2 Cells from arsenic trioxide-induced injury via inhibition of the MAPK signaling pathway. Cell Physiol. Biochem. 41 1957–1969. 10.1159/000472409 [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Sun G. B., Wang M., Liao P., Du Y. Y., Yang K., et al. (2016). Arsenic trioxide triggered calcium homeostasis imbalance and induced endoplasmic reticulum stress-mediated apoptosis in adult rat ventricular myocytes. Toxicol. Res. 5 682–688. 10.1039/c5tx00463b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhang Y., Cui M., Jin L., Wang Y., Lv F., et al. (2016). CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 22 175–182. 10.1038/nm.4017 [DOI] [PubMed] [Google Scholar]
- Zhang S., Liu X., Bawa-Khalfe T., Lu L. S., Lyu Y. L., Liu L. F., et al. (2012). Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18 1639–1642. 10.1038/nm.2919 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhu J. X., Ma Z. G., Wu H. M., Xu S. C., Song P., et al. (2019). Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int. J. Biol. Sci. 15 556–567. 10.7150/ijbs.29907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y., Meng C., Zhang X. M., Yuan C. H., Wen M. D., Chen Z., et al. (2015). Ophiopogonin D attenuates doxorubicin-induced autophagic cell death by relieving mitochondrial damage in vitro and in vivo. J. Pharmacol. Exp. Ther. 352 166–174. 10.1124/jpet.114.219261 [DOI] [PubMed] [Google Scholar]
- Zhao H., Wang Y., Liu J., Guo M., Fei D., Yu H., et al. (2019). The cardiotoxicity of the common carp (Cyprinus carpio) exposed to environmentally relevant concentrations of arsenic and subsequently relieved by zinc supplementation. Environ. Pollut. 253 741–748. 10.1016/j.envpol.2019.07.065 [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhang X., Zheng Y., Li Y., Wang X., Hu N., et al. (2018). Propofol protects rat cardiomyocytes from anthracycline-induced apoptosis by regulating MicroRNA-181a In vitro and in vivo. Oxid Med. Cell Longev 2018:2109216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhang B. (2017). Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 7:44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Y., Li G. Y., Liu Y., Chai L. M., Chen J. X., Zhang Y., et al. (2008). Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br. J. Pharmacol. 154 105–113. 10.1038/bjp.2008.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xue T., Yang X., Zhu H., Ding X., Lou L., et al. (2010). Autophagy plays an important role in sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicol. Appl. Pharmacol. 248 20–27. 10.1016/j.taap.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhong T., Ma Y., Wan X., Qin A., Yao B., et al. (2020). Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 242:117186. 10.1016/j.lfs.2019.117186 [DOI] [PubMed] [Google Scholar]
- Zhong W., Jin W., Xu S., Wu Y., Luo S., Liang M., et al. (2018). Pioglitazone induces cardiomyocyte apoptosis and inhibits cardiomyocyte hypertrophy Via VEGFR-2 signaling pathway. Arq. Bras. Cardiol. 111 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Sarkar S., Scott L., Danelisen I., Trush M. A., Jia Z., et al. (2016). Doxorubicin redox biology: redox cycling, topoisomerase inhibition, and oxidative stress. React. Oxyg Species 1 189–198. 10.20455/ros.2016.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Soonpaa M. H., Chen H., Shen W., Payne R. M., Liechty E. A., et al. (2009). Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 119 99–106. 10.1161/circulationaha.108.799700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D. B., Juhaszova M., Sollott S. J. (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94 909–950. 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]