Abstract
Background
We aimed to determine whether inhibin A could be a reliable and accurate predictor of preterm birth, and discuss the possible pathogenic processes of inhibin A leading to preterm birth.
Methods
A retrospective cohort study was conducted on consecutive singleton pregnant women who underwent the second-trimester quad screen test at a gestational age of 15–20 weeks at Keelung Chang-Gung Memorial Hospital from March 2011 to May 2015. Data including maternal characteristics and pregnancy outcomes were collected from an electric medical record database. Data regarding pregnancy terminations before a gestational age of 24 weeks and regarding pregnancies that involved chromosomal or congenital anomalies were excluded from this analysis. One-way analysis of variance was used to compare second-trimester α-fetoprotein, human chorionic gonadotropin, unconjugated estriol, and inhibin A in women with preterm deliveries versus those with term deliveries.
Results
Although a total of 935 women with singleton pregnancies were enrolled, pregnancy outcome and complete maternal data were obtained from only 770 (82.3%)of them. In total, 687 (89.2%) women delivered at or after 37 weeks of gestation and 83 (10.8%) women delivered before 37 weeks of gestation. The results showed that the inhibin A level was significantly increased in the preterm labor group (p = 0.009). A cutoff inhibin A value above 2.25 was identified statistical significantly in the preterm labor group.
Conclusions
From our results, an inhibin A level above 2.25 multiples of the median in the quad screen test may be associated with preterm labor afterward. Closely monitoring for uterine contractions or cervical length measurement in the second trimester may be indicated in patients with unexplained elevated inhibin A levels.
Keywords: Inhibin A, Preterm labor, Second-trimester quad screen test
At a glance of commentary
Scientific background on the subject
In recent 20 years, a number of studies have demonstrated that the elevated levels of inhibin A in mid-trimester pregnant women are associated with the risk of preterm birth. However, there are no precise predictive values could be found to make clinical judgments.
What this study adds to the field
We observed the same phenomenon during clinical practice. And we identified a value of inhibin A above 2.25 may predict subsequent preterm labor is this study. We believe that these results may give important information for evaluation of the risk of preterm birth.
Preterm birth refers to a delivery that occurs before 37 weeks of gestation. It is the leading cause of infant morbidity and mortality, especially if it occurs before 34 weeks of gestation. The worldwide incidence of preterm birth ranges around 5%–18%, accounting for approximately 70% of neonatal deaths, 36% of infant deaths, and 25%–50% of cases of long-term neurologic impairment in children [1]. Approximately 50%–70% of preterm deliveries are spontaneous, and the current understanding of the mechanisms leading to preterm labor is limited to intra-amniotic infection, decidual senescence, and breakdown of maternal–fetal tolerance [2]. Less than 10% of women with a clinical diagnosis of preterm labor give birth within 7 days of presentation; however, the rate of preterm delivery has not decreased in the past 30 years [3], mainly because of failure to identify the high-risk group during routine prenatal care.
There are many risk factors for preterm birth, and several maternal characteristics and biomarkers have been shown to be associated with preterm labor. Hence, several attempts have been made to develop an accurate and efficient method to predict preterm birth; however, the reported results have been contradictory, making early detection of preterm labor rather difficult [4]. Several studies have indicated that the first- and second-trimester maternal serum markers are significantly associated with the development of adverse pregnancy outcomes, and abnormal levels of serum markers have been reported to be associated with gestational hypertension, pre-eclampsia, fetal loss, preterm delivery, intrauterine fetal growth restriction, and placental abruption [5], [6], [7], [8], [9]. Tul et al. reported that a high inhibin A level is associated with preterm delivery before 34 weeks [9]. Furthermore, Jelliffe-Pawlowski et al. observed that second-trimester inhibin A at the 95th percentile or above was a risk factor for pregnancies ending in preterm birth [10]. Several studies have indicated a relationship between inhibin A and preterm birth, but typically conclude that the predictive value of inhibin A in isolation is too limited to be used clinically; hence, further research in this regard is warranted [9], [10], [11], [12], [13].
In this study, we aimed to determine whether inhibin A could be a reliable and accurate predictor of preterm birth which ever debated in previous studies, and review literature to discuss the possible pathogenic processes of inhibin A leading to preterm birth.
Materials and methods
A retrospective cohort study was conducted on consecutive singleton pregnant women who underwent the second-trimester quad screen test at a gestational age of 15–20 weeks at Keelung Chang-Gung Memorial Hospital from March 2011 to May 2015. Data were collected from an electric medical record database and thoroughly reviewed by a single investigator. Maternal characteristics including age, ethnicity, parity, body weight and gestational age (by the last menstrual period and/or ultrasound) during the quad test, smoking status, use of in vitro fertilization, medical disease, and previous obstetrics history were collected. Neonatal outcomes including gestational age at delivery, delivery method, and body weight at birth were reviewed. Data regarding pregnancy terminations before 24 weeks of gestational age and pregnancies that involved chromosomal or congenital anomalies were excluded from this analysis.
Maternal blood samples were collected, centrifuged, stored at 4 °C, and sent to a central laboratory for analysis within 7 days of collection. For biomarker analyses, we evaluated data regarding alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), unconjugated estriol (uE3), and inhibin A in the quad screen test, which were converted to multiples of the median (MoMs) and were adjusted for gestational age, maternal body weight, and pre-existing diabetes. The biomarker levels were measured using commercially available kits and the AutoDELFIA analyzer (PerkinElmer Life Sciences, Waltham, MA, USA and Applied Biosystems, Brea, CA, USA).
Student's t-test was used to compare second-trimester AFP, hCG, uE3, and inhibin A in women with preterm deliveries versus those with term deliveries. All analyses were performed using Statistical Package for the Social Sciences (IBM SPSS Statistics 21.0), and the results were considered statistically significant when the p values were lower than 0.05.
Results
In this study, a total of 935 women with singleton pregnancies were enrolled; however, pregnancy outcomes and complete maternal data were obtained from only 770 (82.3%) of them. All data regarding pregnancies that resulted in miscarriages or terminations owing to fetuses with major defects and pregnancies that were complicated by iatrogenic delivery before 24 weeks were excluded. Women in whom some variables were missing were excluded. In total, 687 (89.2%) women delivered at or after 37 weeks and 83 (10.8%) women delivered before 37 weeks, either because of spontaneous onset of labor or preterm premature rupture of membranes. No difference was observed between the term group (gestational age ≥ 37 weeks) and preterm group (gestational age < 37 weeks) with regard to maternal age, parity, and maternal body weight. However, cesarean section rates were much higher in the preterm group (43.4%) than in the term group (28.4%) [Table 1].
Table 1.
Maternal characteristics and obstetric history in the screened population.
| GA≧37 weeks | GA<37 weeks | p value | |
|---|---|---|---|
| Sample numble | 687 | 83 | |
| Age | 30.3 | 30.6 | 0.474 |
| Weight | 57.8 | 59.7 | 0.146 |
| Parity | 1.23 | 1.44 | 0.09 |
| CS | 28.4% | 43.4% | 0.001 |
| NSD | 71.6% | 56.6% |
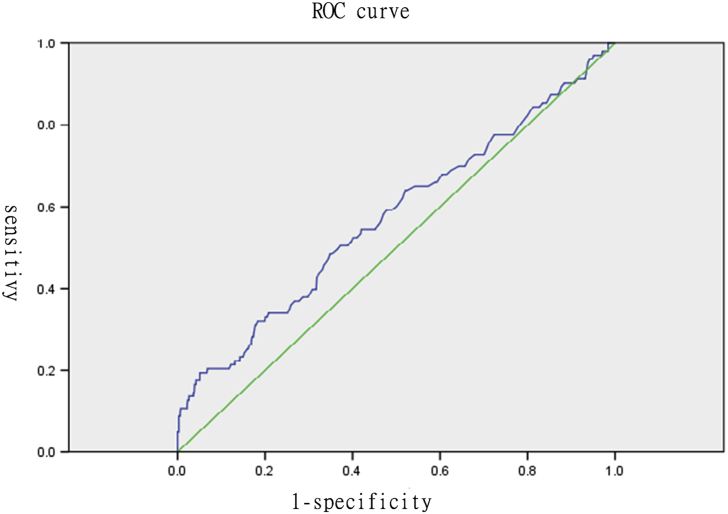
The distributions of all serum markers in these two groups are shown in Table 2. In examination of the statistical relationships of each marker with preterm labor, the results of one-way analysis of variance [Table 3] showed that AFP, hCG, and inhibin A were significantly difference in the preterm labor group (p < 0.05). Notably, women in the preterm group had relatively higher levels of inhibin A (mean ± SD, 1.39 ± 1.05 in the preterm group vs. 1.19 ± 0.57 in the term group) [Table 2], and the divergence of inhibin A was the most significant (F: 6.908) [Table 3]. We speculated that inhibin A helps predict preterm labor. Therefore, the predictive ability of inhibin A was assessed, and the cutoff points for defining “high” serum inhibin A levels to predict preterm labor were chosen using the Fisher exact test and receiver operating characteristic (ROC) curve [Fig. 1]. The area under the Curve of ROC (AUC) is 0.574 with upper limit 0.638 and lower limit: 0.510. We identified a value of inhibin A above 2.25 may predict subsequent preterm labor and the significance was approved according to Pearson chi-squared test (P: 0.04) and Fisher exact test (P:0.048) [Table 4].
Table 2.
MoM value range of each maternal serum marker in the quad screen test.
| Descriptives | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean |
Minimum | Maximum | |||
| Lower Bound | Upper Bound | ||||||||
| AFP | GA≧37 weeks | 687 | 1.1116 | 0.3639 | 0.0139 | 1.0843 | 1.1388 | 0.40 | 2.54 |
| GA<37 weeks | 83 | 1.2040 | 0.4964 | 0.0545 | 1.0956 | 1.3124 | 0.50 | 3.59 | |
| Total | 770 | 1.1215 | 0.3811 | 0.0137 | 1.0946 | 1.1485 | 0.40 | 3.59 | |
| uE3 | GA≧37 weeks | 687 | 1.0741 | 0.3060 | 0.0117 | 1.0512 | 1.0971 | 0.38 | 2.19 |
| GA<37 weeks | 83 | 1.0878 | 0.2990 | 0.0328 | 1.0225 | 1.1531 | 0.34 | 1.90 | |
| Total | 770 | 1.0756 | 0.3051 | 0.0110 | 1.0540 | 1.0972 | 0.34 | 2.19 | |
| hCG | GA≧37 weeks | 687 | 1.3211 | 0.6695 | 0.0255 | 1.2709 | 1.3712 | 0.20 | 4.67 |
| GA<37 weeks | 83 | 1.4901 | 1.1376 | 0.1249 | 1.2417 | 1.7385 | 0.16 | 8.02 | |
| Total | 770 | 1.3393 | 0.7353 | 0.0265 | 1.2873 | 1.3913 | 0.16 | 8.02 | |
| Inhibin A | GA≧37 weeks | 687 | 1.1969 | 0.5719 | 0.0218 | 1.1541 | 1.2398 | 0.37 | 6.13 |
| GA<37 weeks | 83 | 1.3924 | 1.0490 | 0.1151 | 1.1634 | 1.6215 | 0.48 | 6.82 | |
| Total | 770 | 1.2180 | 0.6424 | 0.0232 | 1.1726 | 1.2635 | 0.37 | 6.82 | |
Abbreviations: AFP: alpha-fetoprotein; CI: confidence interval; hCG: human chorionic gonadotropin; MoM: multiples of the median; uE3: unconjugated estriol; Std: standard.
Table 3.
The results of student t-test in the statistical relationships of each marker with preterm labor.
| t-test for Equality of Means |
||||||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | Std. error difference | 95% confidence interval of the difference |
t | df | Sig. (2-tailed) | |||
| lower | upper | |||||||
| AFP | Equal variances assumed | 0.09242 | 0.04419 | 0.00567 | 0.17916 | 2.091 | 768 | 0.037 |
| uE3 | Equal variances assumed | 0.01368 | 0.03547 | −0.05596 | 0.08332 | 0.386 | 768 | 0.700 |
| hCG | Equal variances assumed | 0.16906 | 0.08528 | 0.00165 | 0.33647 | 1.982 | 768 | 0.048 |
| Inhibin A | Equal variances assumed | 0.19547 | 0.07437 | 0.04947 | 0.34146 | 2.628 | 768 | 0.009 |
Abbreviations: df: the degrees of freedom in the source; Std.: standard; Sig: signification.
Fig. 1.
The predictive ability of inhibin A was assessed by receiver operating characteristic (ROC) curve. The area under the Curve of ROC is 0.574 with upper limit 0.638 and lower limit: 0.510.
Table 4.
Cross tabulations reveal the cutoff value of inhibin A for prediction of preterm labor.
| Cross tabulation | ||||
|---|---|---|---|---|
| GA≧37 weeks | GA<37 weeks | |||
| Inhibin A | ||||
| Inhibin A≧2.25 | ||||
| Count | 36 | 9 | 45 | |
| % within INHIBINA | 80.0% | 20.0% | 100.0% | |
| Adjusted Residual | −2.1 | 2.1 | ||
| Inhibin A<2.25 | ||||
| Count | 651 | 74 | 725 | |
| % within INHIBINA | 89.8% | 10.2% | 100.0% | |
| Adjusted Residual | 2.1 | −2.1 | ||
| Total | ||||
| Count | 687 | 83 | 770 | |
| % within INHIBINA | 89.2% | 10.8% | 100.0% | |
| Chi–Square tests | ||||
| Value | df | Signification | Exact signification | |
| Pearson Chi–Square | 4.225 | 1 | 0.040 | |
| Fisher's exact test | 0.048 | |||
| N of Valid Cases | 770 | |||
Abbreviation: df: the degrees of freedom in the source.
Discussion
The second-trimester quad screen test is a widely used screening test for Down syndrome and neural tube defects. This test comprises four components, namely maternal serum AFP, hCG, uE3, and inhibin A. These biomarkers are measured not only to detect Down syndrome but also help predict some adverse fetal outcome. The excessive or deficient release of some placental hormones in association with gestational diseases may reflect abnormal differentiation of the placenta, impaired fetal metabolism, or an adaptive response of the fetoplacental unit to adverse conditions [14]. For example, inhibin A is overexpressed in the second-trimester placental tissue in pregnancies affected by fetal Down syndrome. The mechanisms probably include increased protein translation or a lower rate of protein degradation, or increased gene expression of the subunits [15].
Inhibin, first isolated in 1923, is a heterodimer composed of alpha and beta subunits linked by a disulphide bridge, and is a member of the transforming growth factor beta (TGFb) superfamily. It exists in two forms, inhibin A and inhibin B; however, only inhibin A shows significant changes during pregnancy. The corpus luteum contributes to elevation of inhibin A levels in the first trimester, which peak at 8–10 weeks and decrease during the second trimester. The levels rise again in the third trimester and reach considerably high concentrations in term placenta, which helps in firmly supporting the placenta. Thus, the human placenta is a major site of production and secretion of inhibin A [15], [16], [17]. Measurement of abnormal elevation of inhibin A levels in second trimester for early detection of adverse pregnancy outcomes has been investigated, and it has been suggested that elevated serum inhibin A reflects trophoblast viability. The cellular mechanisms underlying increased maternal inhibin A levels are unclear, but several reports have supported that inhibin A is involved in the control of fetomaternal communication, which is required to maintain pregnancy. Decreased maternal serum inhibin has been reported in ectopic pregnancies, and increased probability of pregnancy loss has been associated with lower serum inhibin A levels after in vitro fertilization [14], [15], [16], [17], [18]. Some studies have reported the use of inhibin A as a predictor of intrauterine fetal growth restriction, although the results have been controversial [12], [13], [19], [20].
In our study, we observed that inhibin A levels were significantly increased in the preterm group. This finding was compatible with that of the FASTER trial in 2005, which evaluated each marker of the quad test alone and reported that preterm birth has a significant association with isolated elevation of inhibin A [13]. One-third of preterm infants are born to mothers with an intra-amniotic infection that is largely subclinical, and microorganisms and their products induce a cascade of chemokines, cytokines, prostaglandins, and proteases, leading to activation of parturition [2]. Cytokines, especially IL-β, have been reported to be associated with stimulation of inhibin A secretion [20]. We presume that elevated inhibin A levels indicate occult infection or inflammatory events in early pregnancy, triggering preterm labor subsequently. On the other hands, approximately 30% of women who undergo preterm labor show uteroplacental ischemia [2], and this may lead to diminished or intermittent perfusion of the intervillous space. The chronic ischemia–reperfusion injury of the placenta leads to insufficient oxygen supply or oxidative stress, which has been considered to trigger alteration in the level of inhibin A. Muttukrishna suggested that the transcription of the inhibin subunit genes is switched on under impaired placental growth, and therefore, inhibin A synthesis and excretion increase in the placenta as a compensatory mechanism to enable the progression of the pregnancy well into the second trimester [20].
In the last two decades, several studies have reported that an elevated level of inhibin A in maternal serum during the second trimester may contribute to the subsequent development of pre-eclampsia [21], [22], [23]. Preterm labor, low birth body weight, and pre-eclampsia were possibly correlated with the pathological conditions of placental development, and the release of trophoblastic factors from the placenta is altered because of a dysfunctional syncytiotrophoblast. Fitzgerald et al. found significant reduction of cytotrophoblast proliferation in pregnancies complicated by extreme preterm delivery. The cytotrophoblast progenitors subsequently increased differentiation and fusion into syncytiotrophoblast which released hCG and/or inhibin into maternal blood. The elevated second-trimester maternal serum levels of inhibin A may result from premature accelerated differentiation of the villous cytotrophoblasts [24]. Further, we propose a hypothesis to explain the elevated levels of inhibin A in the preterm labor group. Most of the biomarkers are derived from the mother and the placenta (or fetus). Hence, if a marker decreases in placental tissues owing to placental insufficiency, it may increase in maternal blood owing to the compensatory effect of maternal sources, which may help to maintain the pregnancy. However, it is still difficult to distinguish confirm certain biomarker in predicting adverse pregnant outcome because of complex cross-interaction between mother and fetus. If a factor is only derived from the mother and not present or released from the placenta, it could be a good marker which simply response to an inflammatory or metabolic condition of the mother during pregnancy. The huge variety of reasons and uncertain maternal or placental sources which influence variability the calculation of any biomarker detected in maternal blood [25].
In several recent studies have investigated serum biomarkers as predictors of preterm delivery. Shin et al. observed that insulin-like growth factor binding protein 3 (IGFBP-3) is an independent predictor of preterm delivery in asymptomatic women [26], and Pummara et al. showed that a PAPP-A level ≤10th percentile was significantly associated with an increased risk of idiopathic preterm birth [27]. Although, as per current guidelines, prevention of preterm birth in patients with abnormal serum biomarkers is not recommended for progesterone prophylaxis, a transvaginal ultrasound may be performed to measure if the patients have a short cervical length. Early detection of preterm labor enables close surveillance of pregnant women at risk and the planning of an appropriate management strategy. Vaginal progesterone in women at risk of preterm labor, cervical cerclage in high-risk women with a shortened cervix, corticosteroids to improve the outcomes of premature neonates, and tocolytic agents that allow time for the administration of antenatal corticosteroids and transfer to a tertiary care facility have been suggested for prevention and management of preterm labor [28]. Despite the remarkable advances in the care of premature neonates, the development of preventive interventions for preterm labor is still a major target of obstetric research.
Conclusion
Although the causes of preterm labor are not well understood, the interactions between maternal blood and placenta may alter specific markers in maternal blood. It is essential to identify reliable and accurate predictors of obstetric risk to enable early intervention. Early prediction and treatment of preterm birth would greatly reduce mortality, morbidity, and the associated costs. In this study, we present an inhibin A level above 2.25 MoM in the quad screen test may be considered as an index for detecting preterm labor before the onset of symptoms. Although the physiological functions and mechanisms of inhibin A during normal and abnormal pregnancies are not well understood, we suggest that close monitoring for uterine contraction or cervical length measurement in the second trimester may be indicated in patients with unexplained elevated inhibin A levels. A larger, creative prospective study may provide more detailed information about the role of inhibin A and various other biomarkers in preterm labor, encourage the development of a low-cost, noninvasive screening tool to detect at-risk pregnancies, and possibly contribute to preventing future preterm labor.
Conflicts of interest
Authors have declared that no competing interests.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics Practice bulletin No. 171: management of preterm labor. Obstet Gynecol. 2016;128:e155–e164. doi: 10.1097/AOG.0000000000001711. [DOI] [PubMed] [Google Scholar]
- 2.Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg F., Gravino G., Schembri-Wismayer P., Calleja-Agius J. Prediction of preterm birth. Minerva Ginecol. 2013;65:345–360. [PubMed] [Google Scholar]
- 5.Dugoff L. First-and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052–1061. doi: 10.1097/AOG.0b013e3181da93da. [DOI] [PubMed] [Google Scholar]
- 6.An J.J., Ji H.Y., You J.Y., Woo S.Y., Choi S.J., Oh S.Y. Introduction of a nomogram for predicting adverse pregnancy outcomes based on maternal serum markers in the quad screen test. Arch Gynecol Obstet. 2015;292:589–594. doi: 10.1007/s00404-015-3685-2. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe A., Langlois S., Macfarlane J., Vallance H., Joseph K.S. Prediction of obstetrical risk using maternal serum markers and clinical risk factors. Prenat Diagn. 2014;34:172–179. doi: 10.1002/pd.4281. [DOI] [PubMed] [Google Scholar]
- 8.Lain S.J., Algert C.S., Tasevski V., Morris J.M., Roberts C.L. Record linkage to obtain birth outcomes for the evaluation of screening biomarkers in pregnancy: a feasibility study. BMC Med Res Methodol. 2009;9:48. doi: 10.1186/1471-2288-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tul N., Pusenjak S., Osredkar J., Spencer K., Novak-Antolic Z. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG, PAPP-A and inhibin-A. Prenat Diagn. 2003;23:990–996. doi: 10.1002/pd.735. [DOI] [PubMed] [Google Scholar]
- 10.Jelliffe-Pawlowski L.L., Baer R.J., Blumenfeld Y.J., Ryckman K.K., O'Brodovich H.M., Gould J.B. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG. 2015;122:1484–1493. doi: 10.1111/1471-0528.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosner J.Y., Fox N.S., Saltzman D., Klauser C.K., Rebarber A., Gupta S. Abnormal biochemical analytes used for aneuploidy screening and adverse pregnancy outcomes in twin gestations. Am J Perinatol. 2015;32:1331–1335. doi: 10.1055/s-0035-1564428. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon A., Wilson R.D., Audibert F., Allen V.M., Blight C., Brock J.A. Society of Obstetricians and Gynaecologists of Canada Genetics Committee. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30:918–949. doi: 10.1016/S1701-2163(16)32973-5. [DOI] [PubMed] [Google Scholar]
- 13.Dugoff L., Hobbins J.C., Malone F.D., Vidaver J., Sullivan L., Canick J.A. FASTER trial research consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260–267. doi: 10.1097/01.AOG.0000172419.37410.eb. [DOI] [PubMed] [Google Scholar]
- 14.Reis F.M., D'Antona D., Petraglia F. Predictive value of hormone measurements in maternal and fetal complications of pregnancy. Endocr Rev. 2002;23:230–257. doi: 10.1210/edrv.23.2.0459. [DOI] [PubMed] [Google Scholar]
- 15.Florio P., Cobellis L., Luisi S., Ciarmela P., Severi F.M., Bocchi C. Changes in inhibins and activin secretion in healthy and pathological pregnancies. Mol Cell Endocrinol. 2001;180:123–130. doi: 10.1016/s0303-7207(01)00503-2. [DOI] [PubMed] [Google Scholar]
- 16.Florio P., Luisi S., Ciarmela P., Severi F.M., Bocchi C., Petraglia F. Inhibins and activins in pregnancy. Mol Cell Endocrinol. 2004;225:93–100. doi: 10.1016/j.mce.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Luisi S., Florio P., Reis F.M., Petraglia F. Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy. Hum Reprod Update. 2005;11:123–135. doi: 10.1093/humupd/dmh057. [DOI] [PubMed] [Google Scholar]
- 18.Makanji Y., Zhu J., Mishra R., Holmquist C., Wong W.P., Schwartz N.B. Inhibin at 90: from discovery to clinical application, a historical review. Endocr Rev. 2014;35:747–794. doi: 10.1210/er.2014-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David A.L., Jauniaux E. Ultrasound and endocrinological markers of first trimester placentation and subsequent fetal size. Placenta. 2016;40:29–33. doi: 10.1016/j.placenta.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Muttukrishna S. Role of inhibin in normal and high-risk pregnancy. Semin Reprod Med. 2004;22:227–234. doi: 10.1055/s-2004-831898. [DOI] [PubMed] [Google Scholar]
- 21.Aquilina J., Thompson O., Thilaganathan B., Harrington K. Improved early prediction of pre-eclampsia by combining second-trimester maternal serum inhibin-A and uterine artery Doppler. Ultrasound Obstet Gynecol. 2001;17:477–484. doi: 10.1046/j.1469-0705.2001.00382.x. [DOI] [PubMed] [Google Scholar]
- 22.Allen R.E., Rogozinska E., Cleverly K., Aquilina J., Thangaratinam S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:194–201. doi: 10.1016/j.ejogrb.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y.T., Shen M.H., Jin A.Y., Li H., Zhu R. Maternal circulating levels of transforming growth factor-β superfamily and its soluble receptors in hypertensive disorders of pregnancy. Int J Gynaecol Obstet. 2017;137:246–252. doi: 10.1002/ijgo.12142. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald B., Levytska K., Kingdom J., Walker M., Baczyk D., Keating S. Villous trophoblast abnormalities in extremely preterm deliveries with elevated second trimester maternal serum hCG or inhibin-A. Placenta. 2011;32:339–345. doi: 10.1016/j.placenta.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Huppertz B. Maternal-fetal interactions, predictive markers for preeclampsia, and programming. J Reprod Immunol. 2015;108:26–32. doi: 10.1016/j.jri.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Shin J.E., Shin J.C., Kim S.J., Lee Y., Park I.Y., Lee S. Early midtrimester serum insulin-like factors and cervical length to predict preterm delivery. Taiwan J Obstet Gynecol. 2016;55:45–49. doi: 10.1016/j.tjog.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Pummara P., Tongsong T., Wanapirak C., Sirichotiyakul S., Luewan S. Association of first-trimester pregnancy-associated plasma protein A levels and idiopathic preterm delivery: a population-based screening study. Taiwan J Obstet Gynecol. 2016;55:72–75. doi: 10.1016/j.tjog.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Rundell K., Panchal B. Preterm labor: prevention and management. Am Fam Physician. 2017;95:366–372. [PubMed] [Google Scholar]