Abstract
During examination of cases of chilblains in children and adolescents, we identified four patients who also showed skin lesions similar to erythema multiforme (EM). They had no other known triggers for EM. One of them had a positive PCR for SARS‐CoV‐2, while the other three were negative. Skin biopsies from two patients showed features not typical of EM, such as deep perivascular and perieccrine infiltrate and absence of necrosis of keratinocytes. Immunohistochemistry for SARS‐CoV/SARS‐CoV‐2 spike protein showed granular positivity in endothelial cells and epithelial cells of eccrine glands in both biopsies. All patients had an excellent outcome, and had minimal or no systemic symptoms. The coincidence of EM, a condition commonly related to viruses, and chilblains in the setting of COVID‐19, and the positivity for SARS‐CoV/SARS‐CoV‐2 spike protein by immunohistochemistry strongly suggest a link between EM‐like lesions and SARS‐CoV‐2.
Keywords: chilblains, COVID‐19, erythema multiforme, exanthem, pernio, SARS‐CoV‐2, skin, viral diseases
1. INTRODUCTION
Skin manifestations including urticarial, morbilliform, vesicular and petechial exanthems, and vasospastic manifestations such as livedo reticularis and acral ischemic lesions 1 , 2 , 3 , 4 , 5 have been reported in association with coronavirus disease 2019 (COVID‐19). In particular, chilblains on the feet and hands have being extensively documented worldwide during the peak incidence of the pandemic. 6 , 7 , 8 During our examination of 22 cases of chilblains in children and adolescents, 9 we identified four patients who also showed skin lesions similar to erythema multiforme. We report these cases and discuss their clinical and histopathologic features, as well as the possible relationship to COVID‐19.
2. CASE REPORTS
The four children (3 male and 1 female; age 11‐17 years) had been referred from the emergency department as part of a study to record acral skin lesions during the peak period of incidence of COVID‐19 in Madrid, Spain. 9 Clinical examination confirmed chilblains on the feet (four cases) and also on the hands (two cases). In addition, complete examination disclosed different skin lesions involving the hands, feet, forearms, elbows, arms, ankles, legs, thighs, and ears (Table 1). They consisted of target (three rings) and targetoid (two rings), confluent macules, papules and plaques, with different sizes, some with hemorrhage or a small central crust (Figures 1 and 2). Three patients complained of itch, and mild pain in the lesions was noted by one of them.
TABLE 1.
Summary of clinical features of 4 patients with erythema multiforme
| Case | Sex | Age | Site of involvement | Local symptoms | Systemic symptoms | Household contacts | Laboratory analyses | PCR | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 12 y | Elbows, knees, forearms, ankles, dorsal and lateral feet, hands, ears | Pruritus | None | None | Not done | − |
Biopsy taken Topical corticosteroids used Complete recovery |
| 2 | M | 17 y | Elbows, knees, dorsal feet, hands | Pruritus | Mild respiratory symptoms | Yes (suspected COVID‐19) | Coagulation and D‐dimer normal | − |
Biopsy taken Oral corticosteroids used Complete recovery |
| 3 | F | 11 y | Elbows, knees, thighs, arms, forearms, legs, ankles, dorsal feet, dorsal hands | None | Mild GI symptoms | None | Hemogram and coagulation normal | + |
No treatment Complete recovery |
| 4 | M | 15 y | Elbows, knees, forearms, ankles, dorsal feet, hands | Pruritus and pain | Mild respiratory symptoms | None | Hemogram, coagulation, and D‐dimer normal | − |
No treatment Complete recovery |
FIGURE 1.
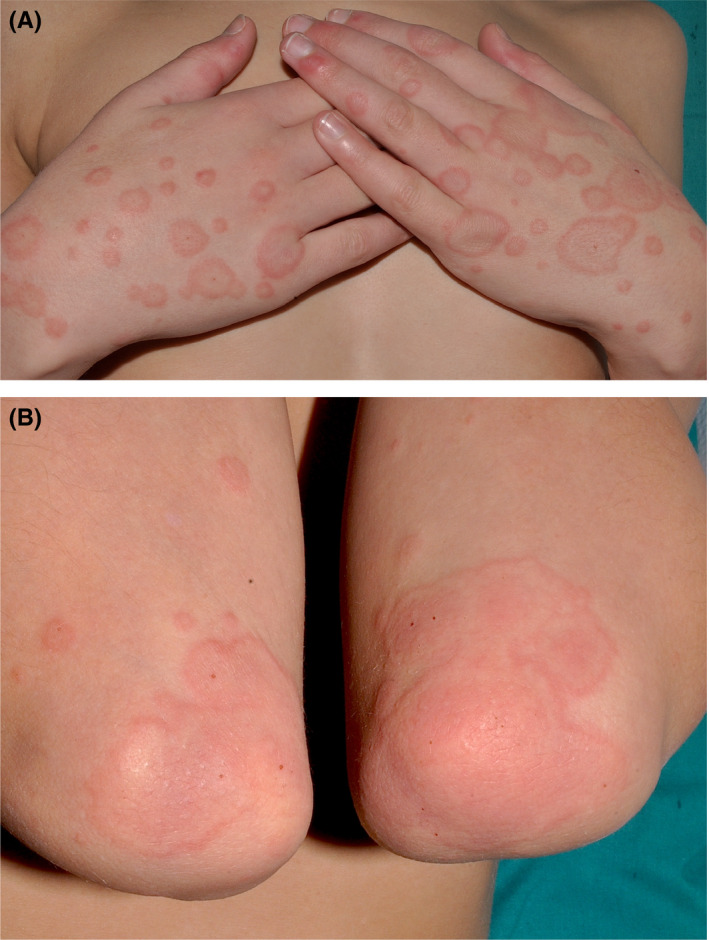
A and B, Case 1.Large, confluent targets and targetoid lesions on the hands and elbows, with some small hemorrhagic crusts
FIGURE 2.
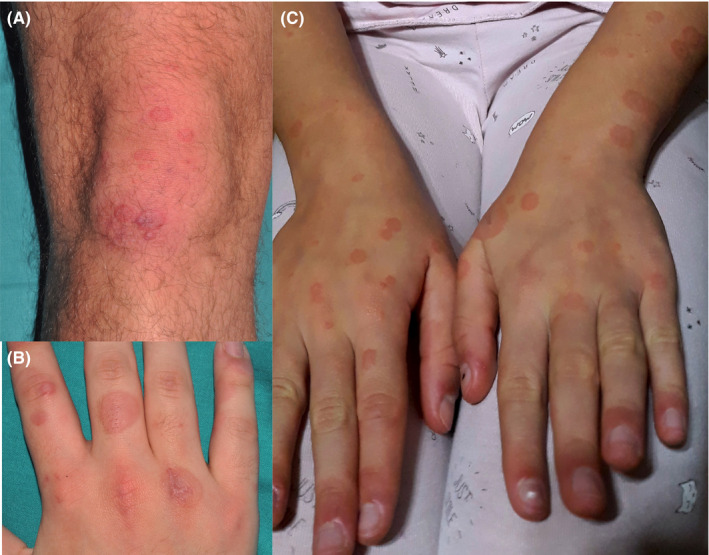
A and B, Case 2. Targetoid lesions on dorsum of hands and knees. C, Case 3. Targetoid lesions on the hands and forearms. Also, note pernio‐like erythema on the fingertips
None of the patients had a previous history of erythema multiforme. No recent lip sores, no new medications and no vaccinations within 30 days before the onset of the skin lesions were reported. One patient was on treatment with methylphenidate at the same dose for more than one year. SARS‐CoV‐2 PCR from oropharyngeal and nasopharyngeal swabs was performed in all the cases, and only one was positive. Routine hematological and biochemical analyses were normal, when done.
Skin biopsies were taken in two cases (Figure 3). Histopathology revealed a relatively normal epidermis lacking parakeratosis or hyperkeratosis. Spongiosis and mild vacuolar interface damage and exocytosis of lymphocytes were seen, being moderate in one case and mild in the other. Necrotic keratinocytes were absent in both samples. A superficial and deep perivascular and perieccrine lymphocytic infiltrate reaching the adipose tissue was noted, which was mild in one case and moderate in the other one. No eosinophils were seen in the infiltrate. Vascular ectasia and mild features of lymphocytic vasculitis were observed, but fibrinoid necrosis and thrombosis were absent.
FIGURE 3.
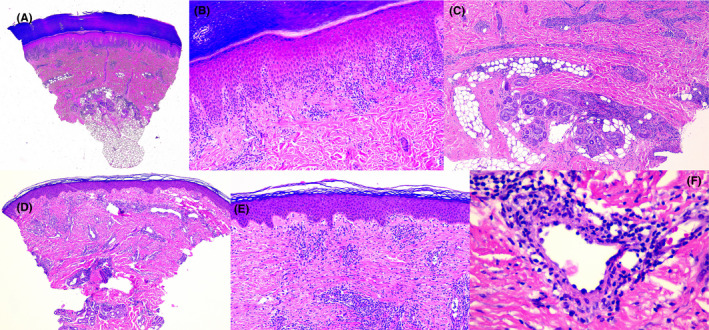
A‐C, Skin biopsy from the lateral foot in Case 1. A, Interface dermatitis with superficial and deep perivascular lymphocytic inflammation (H&E, original magnification 20×). B, Moderate exocytosis with vacuolar changes and spongiosis. No necrotic keratinocytes are seen. (H&E stain, original magnification 100×). C, Deep extension of the inflammatory infiltrate involving eccrine glands (H&E stain, original magnification 40×). D‐F, Skin biopsy from the knee in Case 2. A, Superficial and deep perivascular inflammation and vascular ectasia (H&E stain, original magnification 20×). B, Mild exocytosis, vacuolar changes, and spongiosis. No necrotic keratinocytes are seen. (H&E stain, original magnification 40×). C, A mid dermal vessel showing transmural lymphocytic infiltration and plump endothelial lining (H&E stain, original magnification 400×)
Immunohistochemistry showed similar results in both cases. The inflammatory infiltrate was mainly composed by CD3+ T cells, with a mix of CD4+ and CD8+ populations (CD4 predominant). Scattered B cells were present (CD20+ and CD79a+) as well as very few CD30+ cells. Immunohistochemical stain with the antibody against SARS‐CoV/SARS‐CoV‐2 spike protein (clone 1A9, GeneTex) showed granular positivity in endothelial cells and epithelial cells of eccrine glands in both cases (Figure 4).
FIGURE 4.
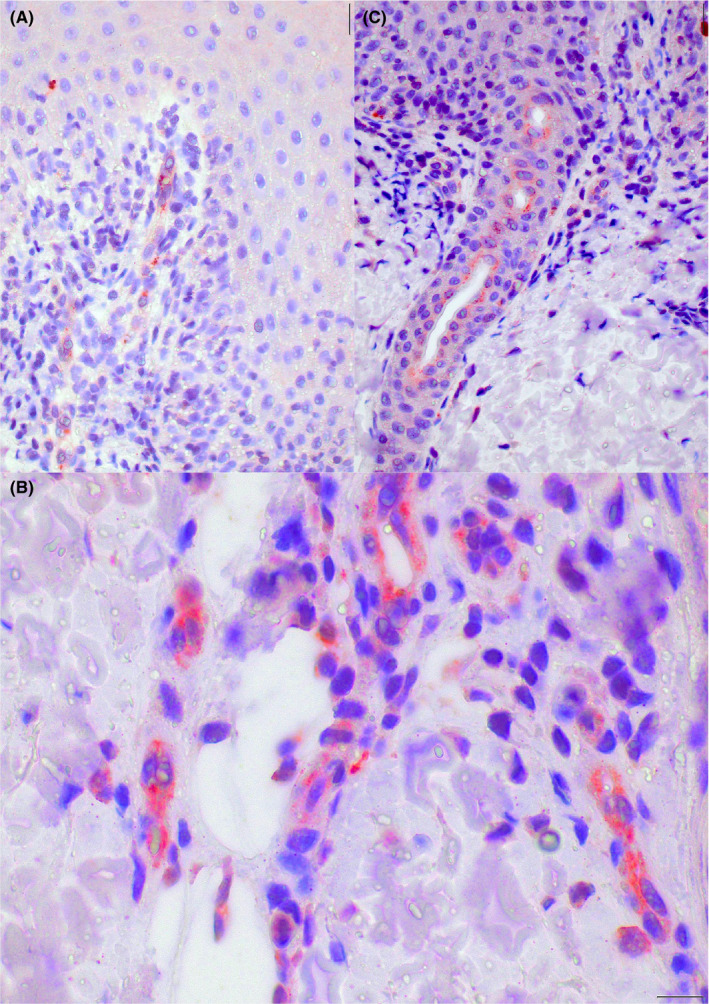
Case 1. Cytoplasmic granular positivity for SARS‐CoV/SARS‐CoV‐2 spike protein in endothelial cells of the upper dermis vessels (A and B), and epithelial cells of the acrosyringia (C) (original magnification A: 100×; B: 200×; C:400×)
One patient was given a short course of oral corticosteroids, and another patient received topical corticosteroids. All patients experienced complete remission of their skin lesions within one to three weeks and had no complications.
3. DISCUSSION
We present four patients with lesions clinically consistent with erythema multiforme (EM) and histopathological features not specific or typical for EM. These lesions were located on the most common cutaneous sites of involvement of classical EM. They appeared in patients with COVID‐19–related chilblains, similar to those extensively described in social networks in most countries affected by the pandemic, and also in medical journals. 6 , 7 , 8 , 9 The outbreak of these acral ischemic lesions, usually rare in children and adolescents, coincided with the peak incidence of COVID‐19, strongly suggesting a causal role for SARS‐CoV‐2. In these patients with chilblains, the rate of positivity for SARS‐Cov‐2 PCR has been consistently very low however, 8 , 9 a reason why some authors have argued against a viral causality.
The presence of EM lesions in adolescents with chilblains in the setting of COVID‐19 has been rarely reported, 10 but may have been overlooked, possibly because chilblains in case series were often reported via photographs and may have not been seen in an ideal clinical setting by dermatologists. One report describes “erythemato‐papular targetoid lesions on the hands and elbows after few days” 10 in 2 out of 11 children, and biopsies were described as “mild superficial perivascular dermatitis”. 10 In our series, 18% of children (4 of 22) with COVID‐19–related chilblains were also affected with EM‐like lesions.
Infections, usually viral, are the most common cause of EM in children and adults. 11 Among viruses, herpes simplex virus is most frequently found, followed by Epstein‐Barr virus, varicella, adenovirus, cytomegalovirus, and orf. 11 Drugs are the second most common cause of EM, and vaccines are an unusual cause. 11 None of our patients had a recent history or clinical signs of any of these disorders or exposures.
A causal link of EM with COVID‐19 in our patients is supported by the following features: (a) A positive SARS‐CoV‐2 PCR test in one patient; (b) a history of contact with a suspected COVID‐19 case in one patient and mild respiratory symptoms some days before the skin lesions appeared in three cases; (c) occurrence during the peak period of incidence of COVID‐19 in Madrid; (d) the presence of EM and chilblains in the sam‐ paitents, appearing during an outbreak in an epidemic setting; and (e) the positive immunohistochemistry for SARS‐CoV/SARS‐CoV‐2 spike protein in the endothelia and eccrine epithelia in the two cases biopsied.
Despite the clinical diagnosis, histopathology failed to demonstrate the typical features of EM. Both cases showed variable degrees of interface dermatitis, without necrotic keratinocytes, and a deep extension of the inflammatory infiltrate extending into the subcutis with vasculopatic changes. Necrotic keratinocytes, which are commonly seen in EM biopsies, were not identified, and spongiosis was mild to moderate in our patients. Similarly, the deep extension of the inflammatory process to involve the deep vascular plexus and eccrine glands is not a feature of EM. However, the overall histopathological features are similar to those found in chilblains, including those described during COVID‐19 pandemic. 7 , 9
All four patients had an excellent outcome, without complications or severe disease involvement, and lesions eventually disappeared within 1‐3 weeks of follow‐up. Treatment with oral corticosteroids was well tolerated in one of them.
In conclusion, EM‐like lesions may appear together with chilblains in adolescents in the setting of the COVID‐19 pandemic. This coincidence and the positivity for SARS‐CoV/SARS‐CoV‐2 spike protein by immunohistochemistry strongly suggest a link between EM‐like lesions and SARS‐CoV‐2.
ACKNOWLEDGMENT
The authors wish to thank Bárbara Quintela Bravo for her work with the immunohistochemistries in this study
Torrelo A, Andina D, Santonja C, et al. Erythema multiforme‐like lesions in children and COVID‐19. Pediatr Dermatol. 2020;37:442–446. 10.1111/pde.14246
IRB Approval: This study was approved by the Institutional Review Board, Ethics Committee, Hospital Infantil Universitario Niño Jesús, Madrid, Spain
REFERENCES
- 1. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5). 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 2. Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with Coronavirus Disease 2019 ? J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19–associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: Transient livedo reticularis. J Eur Acad Dermatol Venereol. 2020. 10.1016/j.jaad.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazzotta F, Troccili T. Acute acro‐ischemia in the child at the time of COVID‐19. Dermatol Pediatr. 2020;3‐5. [Google Scholar]
- 7. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6(6):489–492. 10.1016/j.jdcr.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piccolo V, Neri I, Filippeschi C, et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020. 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zoghaib S, Kechichian E, Souaid K, Soutou B, Helou J, Tomb R. Triggers, clinical manifestations, and management of pediatric erythema multiforme: a systematic review. J Am Acad Dermatol. 2019;81(3):813‐822. [DOI] [PubMed] [Google Scholar]


