Identification of blue‐green cytoplasmic inclusions in neutrophils and/or monocytes on peripheral blood smears is a rare, and likely underreported, finding described in few case reports and small case series studies in critically ill patients with acute liver dysfunction and lactic acidosis. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 As these inclusions are thought to herald poor prognosis and death shortly after identification, they have been referred to as ‘green crystals of death’ or ‘critical green inclusions’.
The coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has led to hundreds of thousands of deaths worldwide as of May 2020. Though many patients have mild symptoms, a subset develop severe pneumonia, acute respiratory distress syndrome (ARDS), multiorgan failure, and death. Over one‐third of patients with COVID‐19 have elevated serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST), however, it is unclear whether liver dysfunction is directly caused by viral infection, sequelae of sepsis, or a complication of other comorbidities. 12 , 13
To better understand and manage COVID‐19, it is imperative that biological indicators associated with adverse outcomes be identified. One such indicator that has been described in approximately 80% of critically ill patients with COVID‐19 is lymphocytopenia. 14 As a result, emerging COVID‐19‐related studies emphasize lymphocyte counts, but do not readily provide information on blood smears. One group described COVID‐19‐related leukocyte morphologic changes that could provide further insights into the inflammatory process associated with the disease; however it remains unknown whether there are distinct morphologic changes that can aid in identifying patients at risk of poor outcomes. 15 Given the liver dysfunction and leukocyte morphologic changes associated with SARS‐CoV‐2 infection, blue‐green inclusions may be underreported in this population and may correlate with short‐term mortality.
Here we describe six critically ill patients diagnosed with SARS‐CoV‐2 infection during the COVID‐19 pandemic in New York City who all died within days of these peculiar blood smear findings. To our knowledge, these represent the first reported cases describing blue‐green leukocyte inclusions in patients with COVID‐19.
Within 21 days, six patients who tested positive for SARS‐CoV‐2 by real‐time polymerase chain reaction (PCR) from a nasopharyngeal swab specimen at presentation were found to have blue‐green leukocyte inclusions on routine peripheral blood smears. Common comorbidities included hypertension and hyperlipidemia, while two (Cases 1 and 2) had end stage renal disease secondary to diabetes mellitus. All six presented with fever and dyspnoea, while Cases 2 and 3 also presented with acute liver disease. Case 2 had a recent admission for methicillin‐resistant Staphylococcus aureus bacteraemia from presumed prosthetic valve endocarditis that was treated with vancomycin and rifampin. She was readmitted for acute liver failure and hepatic encephalopathy from presumed drug‐induced liver injury, secondary to rifampin. Case 3 had jaundice at presentation and was found to have cholangitis and a liver abscess, requiring biliary sphincterotomy, stent, and drain placement six days prior to inclusion detection. Five of six showed bilateral multifocal pneumonia on imaging studies. All six patients developed acute kidney injury, requiring renal replacement therapy in Cases 2–4. All six patients required intubation for acute hypoxic respiratory failure, and developed leukocytosis, and markedly elevated D‐dimer, though blood cultures were negative in all cases.
Blue‐green inclusions were identified up to 20 days after initial COVID‐19 testing and correlated with significant acute elevations in transaminases, lactic acid and lactate dehydrogenase (Table I). Inclusions in Case 1 were identified at the time of manual differential review and reported, while inclusions in Cases 2–6 were not identified by automated differential assessment but found retrospectively when correlating cases with acute transaminase elevations. In Case 1, inclusions were present in 5% of neutrophils and 1% of monocytes, while inclusions were rare (<1%) in the remaining cases. Inclusions varied from blue to dark green and varied considerably in size and shape (Fig 1). In Case 2, inclusions were present in monocytes only. In Cases 3–6, inclusions were present only in neutrophils. Toxic changes in neutrophils and circulating nucleated red blood cells, metamyelocytes, and myelocytes were seen in all cases.
Table I.
Patient demographics and laboratory data at detection of blue‐green cytoplasmic inclusions.
| Case | Age | Sex | Inclusiontype | Days from COVID‐19 diagnosis to inclusions | Days from inclusion to death | Neutrophils | Lymphocytes | Monocytes | Lactic acid | AST | ALT | LDH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | N/Mo | 13 | 1 | 38·9 | 0·8 | 0·9 | 20 | >6000 | 3634 | 933 |
| 2 | 89 | F | Mo | 0 | 3 | 14·6 | 1·8 | 1·5 | 4·9 | 1081 | 1203 | 1252 |
| 3 | 46 | M | N | 6 | 10 | 45·1 | 0·5 | 2·7 | 5·8 | >6000 | 1699 | 3245 |
| 4 | 46 | M | N | 9 | 1 | 9·8 | 2·5 | 1·1 | 5·37 | >6000 | 2077 | 1007 |
| 5 | 58 | M | N | 20 | 1 | 10·6 | 0·6 | 0·3 | 20 | 3583 | 1471 | 913 |
| 6 | 69 | M | N | 16 | 1 | 30·5 | 2·5 | 1·1 | 1·97 | 3397 | 2369 | 2014 |
F, female; M, male; N, inclusions in neutrophils; N/Mo, inclusions in neutrophils and monocytes; Mo, inclusions in monocytes; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase. Reference intervals: neutrophils (1·8–7·0 × 109/l); lymphocytes (1·0–4·8 × 109/l); monocytes (0·2–0·9 × 109/l); lactic acid (0·5–1·6 mmol/l); AST (<34 U/l); ALT (10–49 U/l); LDH (118–230 U/l).
Fig 1.
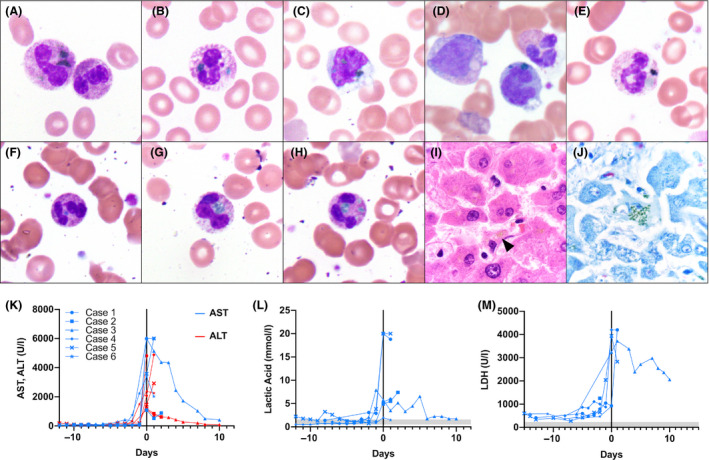
Blue‐green cytoplasmic inclusions in peripheral blood (Wright–Giemsa) at initial detection, post mortem liver stains, and laboratory data trends. Case 1: (A) neutrophil with green inclusions and toxic changes; (B) neutrophil with blue and green inclusions; (C) monocyte with green inclusions. Case 2: (D) monocyte with blue‐green inclusions with adjacent metamyelocyte and neutrophil. Case 3: (E) neutrophil with dark green inclusions. Case 4: (F) neutrophil with blue‐green inclusion and toxic changes. Case 5: (G) neutrophil with green inclusion. Case 6: (H) neutrophil with green inclusions and toxic changes; (I) haematoxylin and eosin stained post mortem hepatocytes with yellow‐brown intracellular pigment (arrowhead); (J) Giemsa‐stained post mortem hepatocytes with green, granular intracytoplasmic staining (all images photographed at 1000× magnification). (K) Aspartate aminotransferase (AST) (ref. <34 U/l), alanine aminotransferase (ALT) (ref. 10–49 U/l); (L) lactic acid (ref. 0·5–1·6 mmol/l); (M) lactate dehydrogenase (LDH) (ref. 118–230 U/l), trends relative to inclusion detection (day 0). Shaded areas represent reference intervals.
Five of six patients had lymphocytopenia early in their disease course, but normalized following inclusion detection. Four patients died within 48 hours of inclusion identification, while two died within 10 days. Blood smears from five additional SARS‐CoV‐2 positive patients, who survived, were reviewed at the time of acute elevations in AST (range 553–6000 U/l) and did not show evidence of blue‐green inclusions.
Autopsies were performed in Cases 4–6, revealing markedly heavy lungs with histologic evidence of diffuse alveolar damage with hyaline membranes. Histologic sections from all three livers showed centrilobular necrosis, consistent with ischaemic shock. Scattered yellow‐brown pigment consistent with lipofuscin was identified on hematoxylin and eosin (H&E)‐stained sections. Hepatic lipofuscin in all three cases showed green, granular, cytoplasmic staining within hepatocytes with Giemsa stains, and were morphologically similar to the leukocyte inclusions (Fig 1). These findings are consistent with previously reported autopsy findings, suggesting that inclusions may be derived from lipid‐rich lipofuscin released from necrotic hepatocytes. 3 , 6 , 8
Most prior reports describe blue‐green leukocyte inclusions in critically ill patients with liver injury associated with acute elevations in AST in 93%, ALT in 87%, and lactate in 77% at inclusion detection, and short‐term mortality in 60% (median: three days; range 1–599 days). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11
All six of our cases presented with inclusions within a 21‐day interval. Short‐term mortality was 100% (median: one day; range 1–10 days) and associated with significant acute elevations in transaminases, lactic acid, and LDH in all cases at the time of inclusion detection, 0–20 days after initial COVID‐19 testing (Table I). Laboratories with ‘increased awareness’ for blue‐green inclusion detection have shown detection rates of 1·1 cases/month. 8 This suggests blue‐green inclusions in hospitalized patients with COVID‐19 may be more frequent compared to the occurrence in other critically ill patients, given the relatively short interval of this study.
Reporting the presence of blue‐green inclusions, particularly in those associated with markedly elevated transaminases and lactic acid, should be considered to possibly aid in identifying patients at greater risk of short‐term mortality in the setting of COVID‐19. However, more studies are necessary to determine the incidence of these inclusions in this population.
Conflicts of interest
The authors declare that they have no conflicts of interest related to this article.
Author contributions
MDC designed the study, collected the specimens, analyzed the data, and wrote the manuscript. LVV and SRB designed and supervised the study, and edited the manuscript. FNE collected the specimens and data. WST, MM, AB and SPS performed the autopsies and collected data. ZZ and HSY reviewed the data and edited the manuscript.
References
- 1. Courville EL, Crisman S, Linden MA, Yohe S. Green neutrophilic inclusions are frequently associated with liver injury and may portend short‐term mortality in critically Ill patients. Lab Med. 2017;48:18–23. [DOI] [PubMed] [Google Scholar]
- 2. Gorup T, Cohen AT, Sybenga AB, Rappaport ES. Significance of green granules in neutrophils and monocytes. Proc (Bayl Univ Med Cent). 2018;31:94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haberichter KL, Crisan D. Green Neutrophilic inclusions and acute hepatic failure: clinical significance and brief review of the literature. Ann Clin Lab Sci. 2017;47:58–61. [PubMed] [Google Scholar]
- 4. Harris VN, Malysz J, Smith MD. Green neutrophilic inclusions in liver disease. J Clin Pathol. 2009;62:853–4. [DOI] [PubMed] [Google Scholar]
- 5. Hodgkins SR, Jones J. A Case of blue‐green neutrophil inclusions. ASCLS Today. 2019;32:431. [Google Scholar]
- 6. Hodgson TO, Ruskova A, Shugg CJ, McCallum VJ, Morison IM. Green neutrophil and monocyte inclusions ‐ time to acknowledge and report. Br J Haematol. 2015;170:229–35. [DOI] [PubMed] [Google Scholar]
- 7. Jazaerly T, Gabali AM. Green neutrophilic inclusions could be a sign of impending death!. Blood. 2014;123:614. [DOI] [PubMed] [Google Scholar]
- 8. Patel N, Hoffman CM, Goldman BI, Bentley K, Burack WR, Evans AG. Green Inclusions in neutrophils and monocytes are an indicator of acute liver injury and high mortality. Acta Haematol. 2017;138:85–90. [DOI] [PubMed] [Google Scholar]
- 9. Sin E, Korus AJ, Patterson A, Psevdos G. An unanticipated finding on peripheral smear: Blue‐green crystals of impeding death? Am J Hematol. 2019;94:733–4. [DOI] [PubMed] [Google Scholar]
- 10. Soos MP, Heideman C, Shumway C, Cho M, Woolf A, Kumar C. Blue‐green neutrophilic inclusion bodies in the critically ill patient. Clin Case Rep. 2019;7:1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vicente‐Steijn R, Tomé A, Maduell F, Xipell M, Castro P, Molina A, et al. Green inclusions in neutrophils: A critical finding that must be reported. Int J Lab Hematol. 2020;42:e101–e104. [DOI] [PubMed] [Google Scholar]
- 12. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zini G, Bellesi S, Ramundo F, d'Onofrio G. Morphological anomalies of circulating blood cells in COVID‐19. Am J Hematol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


