To the Editor,
Finding an effective treatment against coronavirus disease‐2019 (COVID‐19) is urgently needed. 1 Humanized virus suppressing factor‐variant 13 (hzVSF‐v13) is a monoclonal IgG4 against vimentin, which is expressed on the surface of virus‐infected cells. hzVSF‐v13 was first isolated from mice infected with the encephalomyocarditis virus, and has a broad‐spectrum of antiviral activity and anti‐inflammatory effect on virus‐induced inflammation. 2 It has also shown activity against the human coronavirus OC43 in vitro and in vivo as well as a good safety profile in a phase I clinical trial. 3 Here, we report the compassionate use of hzVSF‐v13 in two patients with progressive COVID‐19 pneumonia despite standard treatment.
From 1 February to 10 March 2020, patients with laboratory‐confirmed COVID‐19 received hzVSF‐v13 as a compassionate treatment in the Seoul National University Hospital, if they fulfilled all the following criteria: (1) SpO2 ≤ 93% in ambient air or PaO2/FiO2 < 300 mm Hg, (2) rapid progression of radiological pneumonia despite antiviral treatment with supportive care, and (3) provided written informed consent. 4 The Korean Ministry of Food and Drug Safety approved the compassionate use of hzVSF‐v13, and the Institutional Review Board of Seoul National University Hospital approved this study (IRB No. 2004‐087‐1117).
According to manufacturer's guidance, 200 mg of hzVSF‐v13 (in normal saline 100 mL) was planned to be administered using 0.2‐μm in‐line filter at day 1 and 3. Additional doses on days 7 and 14 could be administered according to the attending physician's decision. The plasma of hzVSF‐v13‐treated patients was collected on treatment days 0, 1, 7, and 14 (±1 day). ELISA kit (Invitrogen, Life Technologies, Camarillo, CA) was used to examine the plasma levels of interleukin‐6 (IL‐6), tumor necrosis factor‐α, monocyte chemoattractant protein‐1 (MCP‐1), interferon‐γ, and IL‐2 following the manufacturer's instructions. Nasopharyngeal swab samples were collected three times a week and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) viral loads were quantified as previously described. 5
There were two patients who met the above criteria, and both agreed receiving compassionate hzVSF‐v13. They were 81‐ and 29‐year‐old men, with critical and severe COVID‐19 pneumonia, 6 respectively (Supporting Information Table). The first patient had no pre‐existing medical condition. Upon admission (12th day of illness), he had been receiving lopinavir and ritonavir. However, his condition had worsened, and he underwent mechanical ventilation and extracorporeal membrane oxygenation (21st and 23rd day of illness, respectively). Bacterial and fungal cultures were negative. We administered hzVSF‐v13 on the 21st, 23rd, and 27th days of illness (50, 200, and 200 mg, respectively; Figure 1). On the 9th day of treatment, fever subsided and the PaO2/FiO2 ratio improved. The viral load in the nasopharyngeal samples was undetectable on the 6th day. Laboratory tests also revealed a marked decline in C‐reactive protein, IL‐6, tumor necrosis factor‐α, and MCP‐1 levels in plasma from the 7th day of treatment.
Figure 1.
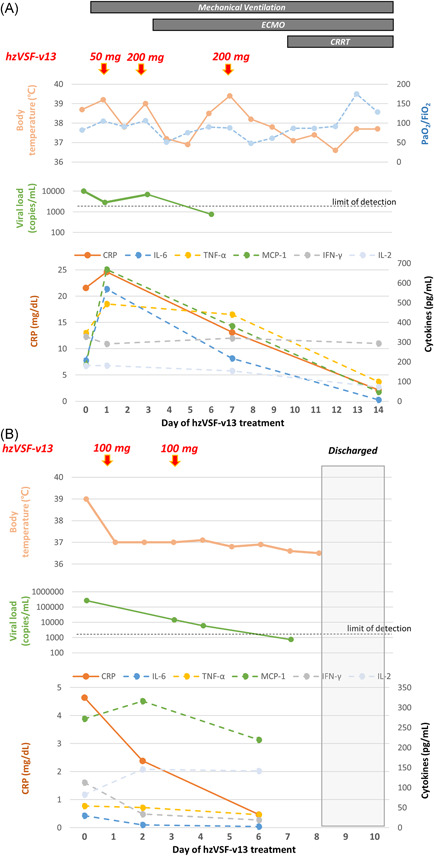
Clinical courses, quantification of SARS‐CoV‐2 viral loads, and inflammatory markers in two patients (panel A and B, respectively) treated with hzVSF‐v13 in this study. CRP, C‐reactive protein; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; IFN‐γ, interferon‐γ; MCP‐1, monocyte chemoattractant protein‐1; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TNF‐α, tumor necrosis factor‐α
The second patient had been receiving intermittent hemodialysis because of IgA nephropathy‐induced end‐stage renal disease. Despite the lopinavir and ritonavir therapy since the 7th day of illness, pulmonary infiltration had progressed and fever persisted. He was administered oxygen therapy on the 11th day and 100‐mg hzVSF‐v13 on the 11th and 13th day of illness (Figure 1). Fever subsided after the first dose and his CRP level declined. Pulmonary infiltration and oxygenation improved on the 2nd day of treatment. We detected no SARS‐CoV‐2 in the swab samples on the 4th day and noted a decrease in inflammatory cytokine levels on the 6th day. We deisolated the patients on the 38th and 18th days of illness, respectively. Neither patient experienced hzVSF‐v13‐elicited adverse events.
In this study, two patients with COVID‐19 pneumonia, who received compassionate hzVSF‐v13 therapy, recovered successfully. Inflammatory markers or viral loads declined after the administration of hzVSF‐v13, followed by an improvement in oxygenation profile. Notably, the 81‐year‐old man who required extracorporeal membrane oxygenation and continuous renal replacement treatment because of severe pneumonia and multiorgan failure also recovered.
As viral load and worsening of symptoms are not well‐correlated in COVID‐19, 7 , 8 anti‐inflammatory effect as well as antiviral activity might be crucial in treating COVID‐19. 8 We showed that hzVSF‐v13 treatment not only reduced the viral load but also decreased pro‐inflammatory cytokine levels. Therefore, hzVSF‐v13 may be a promising drug for treating COVID‐19.
We should interpret the results cautiously because of the small sample size and the single‐arm nature of our study. In addition, as hzVSF‐v13 was not administered in the early course of COVID‐19 in the present study, examining its sole therapeutic effect is difficult. We do not know if the patients' conditions or cytokine levels would have improved without hzVSF‐v13 treatment. Nonetheless, considering the lack of approved therapeutics, the efficacy of hzVSF‐v13 against COVID‐19 is worth further evaluation.
We report two patients with COVID‐19 pneumonia who recovered after the compassionate use of hzVSF‐v13. Our findings warrant placebo‐controlled clinical trials to evaluate the efficacy of hzVSF‐v13 against COVID‐19.
Supporting information
Supporting information
Contributor Information
Wan Beom Park, Email: wbpark1@snu.ac.kr.
Yoon‐Won Kim, Email: ywkim@immunemed.co.kr.
REFERENCES
- 1. Infectious Diseases Society of America . Guidelines on the treatment and management of patients with COVID‐19 infection. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed May 18, 2020. [DOI] [PMC free article] [PubMed]
- 2. Kim Y‐W, Kim YJ, Hong HJ, Park SJ, Kim MW, Park S, Inventors; ImmuneMed Inc., assignee. An antibody or peptide specifically binding to peptide derived from vimentin. Korea patent 10201600726972016.
- 3. Safety, tolerability, pharmacokinetic characteristics of hzVSF‐v13 in healthy male volunteers; 2018. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03653208
- 4. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han MS, Seong MW, Heo EY, et al. Sequential analysis of viral load in a neonate and her mother infected with SARS‐CoV‐2. Clin Infect Dis. 2020. 10.1093/cid/ciaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis. 2020;20(4):400‐402. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


