Introduction
The COVID‐19 pandemic is impacting all urological cancer services. On 19 March 2020, the BAUS provided pragmatic guidance for prostate cancer diagnostic services (Table 1) [1].
Table 1.
BAUS guidance on the management of the prostate cancer diagnostic pathway during the COVID‐19 pandemic [1].
|
COVID‐19 policy recommendation 1: PSA >20 µg/mL (Category 1): Patients with a PSA >20 ng/mLwith identified metastatic disease to initiate hormone therapy. |
|
COVID‐19 policy recommendation 2: PSA <20 µg/mL, high PSAD (Category 2): Treat as normal pathway until services restricted. Patients with PSA <20 µg/mL, but a high PSAD >0.15 µg/L/cc, with prostate volume determined on transabdominal ultrasonography or by estimated DRE, because of the presumed limited availability of MRI, should be offered a perineal prostate biopsy (potentially limited core numbers) if available in the outpatient setting. TRUS biopsy should be avoided if possible. |
|
COVID‐19 policy recommendation 3: PSA <20 µg/mL, low PSAD (Category 3): Where the PSAD (PSA/prostate volume) is low (<0.15 µg/L/cc), all patients should be reassured, discharged and have a repeat PSA test in the primary care setting in 6 months, with re‐referral if the repeat PSA test in 6 months provides a PSAD >0.15 µg/L/cc. This PSA value should be listed in hospital correspondence. |
PSAD, PSA density.
On 16 March, Guy’s and St Thomas’ NHS Foundation Trust declared a major incident status and, in advance of the BAUS guidance, we suspended our transperineal (TP) biopsy service; however, a backlog of referrals remained. In February 2020, 101 MRIs were reviewed: 31 patients were discharged for PSA observation; 61 were listed for primary TP biopsy and nine were listed for active surveillance TP biopsy. Patients with evidence of metastatic bone disease were started on hormonal therapy immediately, without biopsy. By 30 March, 49 patients remained on the TP biopsy waiting list, so were stratified by priority, with 15 in the 'red' risk category, 15 in the 'amber' category, 10 in the 'green' category and nine on active surveillance (Table 2). Following careful risk assessment and case stratification, we decided to re‐start out prostate biopsy programme, but needed to carefully consider the logistics and how to proceed safely for both our patients and staff.
Table 2.
Risk stratification for biopsies following PSA and MRI during the COVID‐19 pandemic.
| Risk category | PSA density (ng/mL/cc) | MRI LIKERT score | Clinical stage | Management |
|---|---|---|---|---|
| Green (low risk) | <0.15 |
2–3 No lesion |
T1c to T2a |
Delay biopsy, repeat PSA within the next 4‐‐6 months Low priority |
| Amber (intermediate risk) | <0.2 |
3–4–5 Lesion |
>T2b N0 M0 |
Early biopsy, within 3 months and within 1 month Intermediate priority |
| Red (high risk) | >0.2 |
3–4–5 Lesion |
≥T2c N1 M0 T3a/b N0 M0 |
Urgent biopsy, within 1/12 High priority |
General Anaesthesia Transperineal Biopsy
Aerosol‐generating procedures such as general anaesthesia (GA) are a significant risk for COVID‐19 transmission to healthcare professionals [2]. COVID 19 RT‐PCR has a sensitivity of only 83%, making a negative test unreliable, therefore, the safest approach is to assume all patients are positive, and full personal protective equipment (PPE) should be worn for intubation. As GA TP biopsy lists were cancelled, and anaesthetists were redeployed to support intensive care, it was unlikely that GA TP biopsy lists would restart soon.
Local Anaesthesia Transrectal Biopsies
With limited access to GA, local anaesthesia (LA) TRUS biopsy is an alternative, but carries a high risk of post‐biopsy‐related sepsis. At a time when intensive care beds are scarce, a 1.3% or greater risk of severe sepsis, as reported by the National Prostate Cancer Audit, is unjustifiable [3, 4]. There are further concerns that COVID‐19 can be identified in faeces, but this risk is yet to be fully quantified.
COVID‐19 appears to exploit angiotensin‐converting‐enzyme 2 (ACE2) for entry into target cells. ACE2 can be found in extra‐pulmonary sites, such as the gastrointestinal tract and kidney. Viral RNA may persist within faeces after symptom resolution [5]. In a study of 74 patients, Wu et al. [5] collected oropharyngeal and faecal samples for RT‐PCR after two sequential positive oropharyngeal samples. Although 33 (54%) faecal samples were negative, 41 (55%) remained positive for 27.9 (sd 10.7) days after symptom onset compared to 16.7 (sd 6.7) days for oropharyngeal samples. Presence of detectable viral RNA within faeces [6] does not imply infectivity, as swallowed viral RNA in respiratory secretions might pass through the gastrointestinal tract, but might not host active viral replication; however, Wang et al. [6] detected live virus in faecal samples using electron microscopy in those without gastrointestinal symptoms. Viral replication within the gastrointestinal tract could have consequences for transmission routes and urological practice, particularly TRUS biopsy.
Local Anaesthesia Transperineal Biopsies
Because of the safety concerns discussed above, a feasible solution is LA TP biopsy in the outpatient setting. A total of 70% of TP prostate biopsies in our hospital are performed under LA, with only 20% requiring i.v. sedation and 10% requiring GA. Approximately half are delivered in the outpatients department, the operator wearing simple gloves and an apron for what is considered a clean rather than a sterile procedure. We were aware that other urology centres across the UK were continuing to offer LA TP biopsies much as before, but, in our centre in London, in the midst of the COVID‐19 pandemic, it seemed to us that simple gloves and an apron might not be sufficient protection for either staff or patients. We had to assume that all patients might be COVID‐19‐positive or recovering, with potentially live virus in their faeces. For a LA TP biopsy, a TRUS probe is required to visualize the prostate and the rectum is filled with ultrasound gel, so the risk of faecal spillage should be considered. The operator’s head and shoulders would be less than 1 m from the patients’ mouth and nose, and the assistant would be within 2 m. Staff and patient safety are paramount and, for this reason, it was decided that initially the procedure should be performed with full PPE precautions, until timely robust testing becomes widely available.
The theatre matron, outpatient sister, disinfection and infection control teams were consulted for advice. Within 24 h a 'secure area' was established in the outpatients department, with four rooms designated: a donning room; a cold room for trolley preparation; a hot procedure room for the biopsy; and a doffing room (Fig. 1). With more outpatient clinics transitioning to remote telephone clinics, there was capacity for this layout. More recently, a separate doffing room may be not be required, as doffing can safely be done in the hot procedure room. This is an evolving model, in line with Public Health England and Trust guidelines. Initially, the operator and assistant used full PPE for each case, but in the interests of conserving PPE, this has been modified. The operator wears the same FFP3 mask and visor for the entire 4‐h session, along with a waterproof plastic apron over theatre scrubs, with plastic shoes and double gloves, which are changed after each case. The assistant wears gloves, a plastic apron, surgical face mask and visor.
Fig. 1.
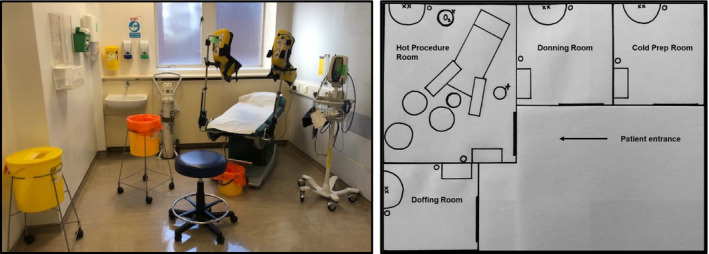
Line diagrams of our four‐room layout. The hot procedure room should contain only the essential equipment to carry out the biopsy procedure and dispose of used disposables safely.
The first list was commenced on 1 April; five patients, stratified as in the red category based on their PSA density and MRI, with no evidence of metastatic disease, were scheduled at hourly intervals. Patients were initially screened by telephone, which included asking if they were isolating for any reason. Seven of 49 patients were being shielded because of comorbidities, therefore, are being delayed. Patients were advised to drive or travel by taxi if possible. On arrival, they were screened with temperature, routine observations and a COVID‐19 health questionnaire. Men were considered low risk for COVID‐19 infection if they were apyrexial, and had no symptoms or any history of household/occupational exposure. All patients wore a surgical mask. To facilitate flow and training, two tandem teams comprising Surgeon, Clinical Nurse Specialist and Healthcare Assistant alternated. Equipment and surfaces in the hot room were cleaned between cases, according to infection control guidance, and ‘dirty’ disposables bagged securely in this room. All biopsies were completed under LA with no immediate complications. All patients received telephone follow‐up within 1 week of the procedure. One patient attended the emergency department with a fever and cough 2 days post‐biopsy, had a normal chest X‐ray and was COVID‐19 test‐negative. After these first patients, we successfully carried out 16 biopsies over the following 4 weeks (Table 3). Of these 16 biopsies, 14 (88%) were 2‐week‐wait referrals, with one patient on active surveillance and one with post‐brachytherapy PSA increase. Both had lesions on MRI. Five of the 16 patients were black. Most men underwent a target + systematic biopsy, while 3/16 underwent a target biopsy only. The cancer detection rate was 11/16, and 10/16 patients had International Society of Urological Pathology grade ≥2 disease. The median (range) maximum cancer core length was 10 (2–17) mm (Table 3).
Table 3.
Patient demographics, biopsy results and management plans for patients managed with local anaesthetic transperineal biopsy during the COVID‐19 pandemic.
| Patient demographics | |
| Total number of patients | 16 |
| Median (range) age, years | 60 (46–68) |
| Median PSA, ng/mL | 10.3 |
| Median PSA density, ng/mL/cc | 0.26 |
| Median Likert score | 4 |
| Results | |
| Median number of cores taken | 16 |
| Benign, n (%) | 5 (38) |
| ISUP 1, n (%) | 1 (8) |
| ISUP ≥2, n (%) | 10 (63) |
| ISUP ≥3, n (%) | 4 (25) |
| Management plans, n (%) | |
| MDT decision pending | 3 (19) |
| Discharged | 5 (31) |
| Active surveillance | 1 (6) |
| Not for hormones, active treatment after COVID‐19 | 4 (25) |
| Bicalutamide initially, consider active treatment after COVID‐19 | 3 (19) |
ISUP, International Society of Urological Pathology; MDT, multidisciplinary team.
Whilst the net number of patients needing biopsies at our hospital over the next 8–12 weeks remains relatively small, scaled up across the whole cancer network, this may represent a significant number. The South East London Cancer Alliance stopped all TRUS biopsies in March 2019 and all five centres offer LA TP biopsies. In the COVID‐19 era, it seems illogical for all centres to deliver a few TP biopsies in a potentially unsafe environment, without access to a three‐ or four‐room outpatient set‐up. We have identified two sites within our Cancer Alliance that can carry out LA TP biopsies in a COVID‐secure environment supported by a urology network nurse specialist and consultant to continue delivering a limited, but carefully triaged, closely audited and safe prostate diagnostic capability for the entire cancer network.
Delivering a National Cancer Network Response to Prostate Cancer Diagnostics
As urologists, we need to plan how to deliver timely and safe prostate biopsies in the COVID‐19 era, but with little indication of the length of time that routine diagnostic services will be suspended. Across the UK there are more than 27 centres that already have extensive experience (>100 procedures) in delivering outpatient LA TP biopsies, amounting to >7500 LA TP biopsies. The 18 Cancer Alliances have an opportunity to harness this experience and should be encouraged to support high‐quality prostate diagnostics under LA in outpatient settings, despite the challenges imposed by the pandemic. There are opportunities to establish longitudinal studies in these patients, of whom up to half, by definition, would either be asymptomatic or have recovered from COVID‐19. We could nationally determine whether faecal transmission is a true or hypothetical risk, but until we are certain, we strongly advocate that all TP biopsies should be offered under LA with appropriate PPE, to minimize the risk of COVID‐19 transmission.
Conflicts of Interest
Rick Popert is a Fellow for the NHS Innovation Accelerator programme and has received honoraria from BXT‐Accelyon. There are no conflicts of interest for any of the other authors.
Abbreviations
- ACE2
angiotensin‐converting‐enzyme 2
- GA
general anaesthesia
- LA
local anaesthesia
- PPE
personal protective equipment
- TP
transperineal
Acknowledgements
With thanks to Thomasia Azavedo (Urology Centre Sister), John Francis (Disinfection Officer), Sonia Barber (Healthcare Assistant), Minahil Farrukh (Patient Pathway Co‐ordinator).
References
- 1. British Association of Urological Surgeons, Section of Oncology . COVID‐19 Strategy for the Interim Management of Prostate Cancer. BAUS Section of Oncology; 2020. (1). [Google Scholar]
- 2. Association of Anaesthetists . COVID‐19 Guidance. 2020; Available at: https://anaesthetists.org/Home/Resources‐publications/COVID‐19‐guidance. Accessed March, 2020.
- 3. Olvera‐Posada D, Welk B, McClure JA, Winick‐Ng J, Izawa JI, Pautler SE. A population‐based cohort study of the impact of infectious complications requiring hospitalization after prostate biopsy on radical prostatectomy surgical outcomes. Urology 2018; 121: 139–46 [DOI] [PubMed] [Google Scholar]
- 4. The Royal College of Surgeons . National Prostate Cancer Audit. 2017. Accessed April, 2020.
- 5. Wu Y, Guo C, Tang L et al. Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5: 434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang W, Xu Y, Gao R et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020; 323: 1843–4. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]


