1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) pandemic has challenged healthcare systems around the world. Unfortunately, failure has become evident with the collapse of high‐income countries where excellent‐quality and efficient systems—with enough resources and advanced scientific technology to establish strong public health strategies based on mathematical models adjusted to serve COVID‐19 1 —have undergone significant strain.
Currently, there are no effective pharmacological interventions with sufficient scientific evidence to treat COVID‐19. Fueled by the epidemiological and clinical impact of its rapid spread, and the alarming fatality rates of over 10% in some countries, the need for in‐hospital management—especially in intensive care units (ICU)—geared public health attention towards previous pharmacological experiences evaluated in other coronavirus strains. 2 , 3 , 4
2. CHLOROQUINE AND COVID‐19
Chloroquine phosphate (CQ) stood out among potential personalized pharmacological therapies for COVID‐19 due to the antiviral effect demonstrated in preclinical studies and its high specificity towards the SARS‐CoV‐2 receptor: angiotensin‐converting enzyme 2 (ACE‐2). High distribution within lung tissue (the main organ affected in COVID‐19), 5 and the results described in case series of critically ill COVID‐19 patients with favorable clinical outcomes after being treated with CQ have suggested its possible use as a suitable pharmacological option. 6 , 7 Based on published evidence, Colombia, China, India, United States, and other countries have included CQ within the management guidelines for COVID‐19 8 or as an extreme measure in patients with a high risk of death. However, its effectiveness against COVID‐19 has recently been questioned, 9 and studies suggest that CQ should be used with caution in the treatment of severe presentations of disease based on its potential cardiotoxic side effects and increased lethality. 10
In response to this initiative, the World Health Organization issued a warning on how the use of unproven drugs could generate shortages to treat other diseases for which efficacy has already been proven, referring to chloroquine, commonly used to treat malaria and autoimmune disorders. 11
3. CHLOROQUINE REPURPOSING AND RHEUMATIC AUTOIMMUNE DISEASE IN COLOMBIA
Recently, the Colombian government established that "after finding scientific evidence, the use of chloroquine and hydroxychloroquine is considered given the possibility that medical personnel may consider its use for the treatment of COVID‐19". 12 However, this would be an off‐label indication given that the effectiveness of chloroquine in treating patients with COVID‐19 is supported mainly by in‐vitro studies, animal models, anecdotic evidence and by ongoing clinical trials without definitive results. 6 Another problem is the limited sample size of many of these trials, and the possible selection bias towards critically ill patients with pneumonia. 6 , 7 , 13 Chloroquine and hydroxychloroquine are first‐line medications to treat systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren syndrome (SS). They are also indicated in primary antiphospholipid syndrome, erythema nodosum associated with autoimmunity, septal panniculitis of autoimmune etiology, sarcoidosis, among other rheumatic autoimmune diseases (RADs). 14 According to data from the Comprehensive Social Protection Information System, the number of patients diagnosed in Colombia with SLE, RA, or SS increased by 78.8% between 2015 and 2019 (Figure 1A,B).
Figure 1.
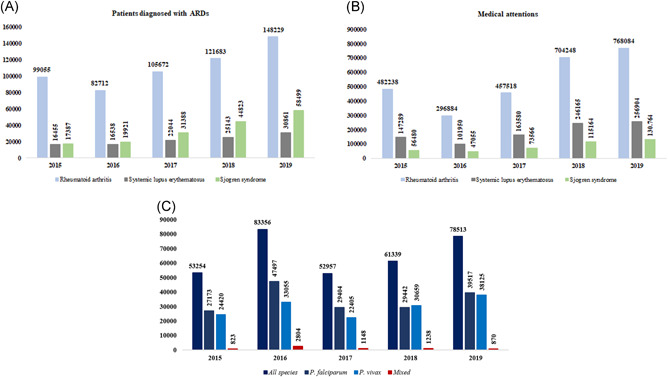
Frequency of patients with neglected autoimmune and infectious diseases susceptible to Chloroquine shortages in Colombia. A, Annual frequency of patients diagnosed with autoimmune rheumatic diseases (ARDs). B, Annual medical visits to patients with Rheumatoid arthritis, Systemic lupus erythematosus or Sjogren's syndrome. C, Annual frequency of diagnosed cases of malaria
4. MALARIA IN COLOMBIA AND THE REGION
Data from the Colombian Public Health Surveillance System showed a 47.4% increase in confirmed malaria cases between 2015 and 2019; the increase in P. vivax and P. falciparum malaria cases was 56.1% and 45.4%, respectively 15 (Figure 1C) with an annual parasite index of 4.98 cases for every 1000 inhabitants in 2019. 16 Based on annual projections, and anticipating the wide use of CQ during the mitigation phase of COVID‐19, the Ministry of Health and Social Protection established that necessary provisions of this drug should be reserved so as not to deprive susceptible population from malaria treatment. 12 Colombia is one of the countries with the highest reports of malaria in the region (117 650) 15 , 17 and indeed requires a large stash of this drug. Including CQ as a drug‐of‐choice for COVID‐19 could result in a drastic reduction in malaria treatment availability in the country. Taking this into account, recommending an off‐label medication to healthcare personnel that lacks approval, regulation, and evidence of efficacy against COVID‐19 pneumonia could generate misinformation among the general population, promoting massive panic‐buying behaviors and creating an over‐cost and shortage problem that may lead to a serious public health issue.
On the other hand, a "kill two birds with one stone" approach would be favorable in particular epidemiological settings such as COVID‐19 and malaria overlapping areas. This is the case of the Colombian Amazon department—the 7th most affected by COVID‐19 and one of the five Colombian departments without ICU services 18 —where long‐time prevalent malaria 15 and the abrupt increase in COVID‐19 cases 19 have started to overlap. Capitalizing on the use of CQ to treat both conditions could result in advantageous and is definitely a topic that deserves further investigation.
5. CURRENT STATUS OF CHLOROQUINE AND FINAL CONSIDERATIONS
As of 25th April 2020, there are 23 clinical trials registered for the management of COVID‐19 using chloroquine in "recruiting" (n: 11), "not yet recruiting" (n: 10) and "enrolling by invitation" (n: 2) phases, and to date, the two most rigorous clinical trials published with partial or complete results have not shown favorability, with one of them having to be suspended 6 days after beginning due to elevated rates of adverse cardiac effects. 13 , 20 In addition to the nonsupporting evidence on the use of chloroquine to manage COVID‐19, the impact of medicine shortages in patients with RADs could translate into an increased risk of relapses and exacerbations; and in the case of SLE, patients could be at risk for intensive care requirement or even death. The same stands for malaria, where Latin‐American countries such as Colombia, Venezuela, and Brazil report the highest prevalence rates of Plasmodium infection and where prompt treatment must be put in place to reduce disease burden. 15 , 16 Reconsideration from the Colombian national government on the final decision of choosing chloroquine as a first‐line treatment for the management of COVID‐19 should be encouraged.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Teherán AA, Camero G, Hernández C, Perez‐Garcia L, Gúzman R, Paniz‐Mondolfi A, Ramírez JD. Potential negative effects of the free use of chloroquine to manage COVID‐19 in Colombia. J Med Virol. 2020;92:2254–2256. 10.1002/jmv.26059
REFERENCES
- 1. Bloomers L, Van Leener M, Van Der Kaay HJ. Impact of non‐pharmaceutical interventions (NPIs) to reduce COVID‐19 mortality and healthcare demand. ImperialAcUk. 2020:3‐20. 10.25561/77482 [DOI] [Google Scholar]
- 2. Mizumoto K, Chowell G. Estimating risk for death from 2019 novel coronavirus disease, China, January‐February 2020. Emerg Infect Dis. 2020;26(6):1251‐1256. 10.3201/eid2606.200233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuan J, Li M, Lv G, Lu ZK. Monitoring transmissibility and mortality of COVID‐19 in Europe. Int J Infect Dis. 2020;95:311‐315. 10.1016/j.ijid.2020.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kapoor KM, Kapoor A. Role of chloroquine and hydroxychloroquine in the treatment of COVID‐19 infection‐ a systematic literature review [published online ahead of print March 30, 2020]. medRxiv. 10.1101/2020.03.24.20042366 [DOI] [Google Scholar]
- 5. Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet. 1996;31(4):257‐274. 10.2165/00003088-199631040-00003 [DOI] [PubMed] [Google Scholar]
- 6. Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID‐19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr Clin Res Rev. 2020;14(3):241‐246. 10.1016/j.dsx.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L‐Y, Cui J‐J, OuYang Q‐Y, et al. Genetic profiles in pharmacogenes indicate personalized drug therapy for COVID‐19 [published online ahead of print March 30, 2020]. medRxiv. 10.1101/2020.03.23.20041350 [DOI] [Google Scholar]
- 8. Saavedra Trujillo CH. Resumen: Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS‐COV‐2/COVID‐19 en establecimientos de atención de la salud ‐ Recomendaciones basadas en consenso de expertos e informadas en la evidencia. Infectio. 2020;24(3). 10.22354/in.v24i3.852 [DOI] [Google Scholar]
- 9. Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid‐19. BMJ. 2020;369(April):9‐10. 10.1136/bmj.m1432 [DOI] [PubMed] [Google Scholar]
- 10. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. JAMA Netw Open. 2020;3(4.23):e208857. 10.1001/jamanetworkopen.2020.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO Director‐General's opening remarks at the media briefing on COVID‐19 ‐ 27 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---27-march-2020. Accessed on May 10, 2020.
- 12. Hidroxicloroquina y cloroquina se podrán usar para tratamiento de covid – 19. https://www.minsalud.gov.co/Paginas/Hidroxicloroquina-y-cloroquina-se-podran-usar-para-tratamiento-de-covid---19.aspx. Accessed on May 10, 2020.
- 13. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial [published online ahead of print April 10, 2020]. medRxiv. 2020;7. 10.1101/2020.03.22.20040758 [DOI] [Google Scholar]
- 14. Rainsford KD, Parke AL, Clifford‐Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231‐269. 10.1007/s10787-015-0239-y [DOI] [PubMed] [Google Scholar]
- 15. Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S, Lacerda MVG. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J. 2017;16(1):1‐18. 10.1186/s12936-017-1925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salas Botero D. Informe de evento Malaria Colombia, primer semestre 2019. 2019:1‐14.
- 17. Feged‐Rivadeneira A, Ángel A, González‐Casabianca F, Rivera C. Malaria intensity in Colombia by regions and populations [published online ahead of print September 12, 2018]. PLoS One. 2018;13(9):e0203673. 10.1371/journal.pone.0203673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Díaz‐guio DA, Villamil‐gómez WE, Dajud L, et al. Will Colombian intensive care units collapse due to the COVID‐19 pandemic? [published online ahead of print May 16, 2020]. Travel Med Infect Dis. 2020:101746. 10.1016/j.tmaid.2020.101746 [DOI] [Google Scholar]
- 19. Coronavirus en Colombia . https://www.ins.gov.co/Noticias/Paginas/Coronavirus.aspx. Accessed on May 10, 2020.
- 20. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid‐19 [published online ahead of print April 23, 2020]. medRxiv. 10.1101/2020.04.16.20065920 [DOI] [PMC free article] [PubMed] [Google Scholar]


