Abstract
Objective
The clinical manifestations of coronavirus disease (COVID‐19) run from asymptomatic disease to severe acute respiratory syndrome. Older age and comorbidities are associated to more severe disease. A role of obesity is suspected.
Methods
Patients hospitalized in the medical COVID‐19 ward with severe acute respiratory syndrome coronavirus 2–related pneumonia were enrolled. The primary outcome of the study was to assess the relationship between the severity of COVID‐19 and obesity classes according to BMI.
Results
A total of 92 patients (61.9% males; age 70.5 [13.3] years) were enrolled. Patients with overweight and obesity were younger than patients with normal weight (68.0 [12.6] and 67.0 [12.6] years vs. 76.1 [13.0] years, P < 0.01). A higher need for assisted ventilation beyond pure oxygen support (invasive mechanical ventilation or noninvasive ventilation) and a higher admission to intensive or semi‐intensive care units were observed in patients with overweight and obesity (P < 0.01 and P < 0.05, respectively) even after adjusting for sex, age, and comorbidities (P < 0.05 and P < 0.001, respectively) or when patients with dementia or advanced cancer were removed from the analysis (P < 0.05).
Conclusions
Patients with overweight and obesity admitted in a medical ward for severe acute respiratory syndrome coronavirus 2–related pneumonia, despite their younger age, required more frequently assisted ventilation and access to intensive or semi‐intensive care units than normal weight patients.
Study Importance.
What is already known?
-
►
Clinical manifestations of COVID‐19 run from asymptomatic disease to severe acute respiratory syndrome, with older age and the presence of comorbidities reported as risk factors for more severe disease.
-
►
A role of obesity in determining the severity of COVID‐19 manifestations has been suspected, but more data are still needed.
What does this study add?
-
►
Overweight and obesity were frequent in a cohort of patients admitted in an internal medicine ward dedicated to the care of COVID‐19.
-
►
Patients with overweight and obesity and SARS‐CoV‐2 pneumonia were 10 years younger than patients with normal weight.
-
►
Patients with overweight and obesity, despite their younger age, required more frequently assisted ventilation, and they were more prone to be admitted to intensive or semi‐intensive care units.
-
►
Patients with overweight and obesity had a higher need for noninvasive ventilation during SARS‐CoV‐2 pneumonia than normal weight patients without dementia or cancer.
How might these results change the focus of clinical practice?
-
►
Patients with overweight and obesity are at higher risk for more severe COVID‐19, even after adjustment for age, sex, and comorbidities.
-
►
Patients with overweight and obesity should very strictly follow prevention measures during the current pandemic disease, and they need to be monitored more closely in case of infection.
Introduction
In December 2019, a new coronavirus causing a severe acute respiratory syndrome (SARS‐CoV‐2) emerged in Wuhan, China (1). The virus spread rapidly worldwide affecting many other countries during the first months of year 2020. In Italy, by February 15, 2020, only three cases of coronavirus disease 2019 (COVID‐19) were identified in people who recently traveled to China. However, on February 20, 2020, a severe case of pneumonia caused by SARS‐CoV‐2 was diagnosed in a young man living in northern Italy’s Lombardy region, without any possibility of exposure abroad. Since then, the epidemic spread very rapidly in our country, with a total of 156,363 cases and 19,901 deaths registered up to April 13, 2020 (2).
The clinical manifestations of COVID‐19 run from asymptomatic disease to severe acute respiratory infection requiring hospitalization and oxygen support and to admission to an intensive care unit for assisted ventilation (3). Older age and the presence of comorbidities have been reported as risk factors for more severe disease and death both in China (3, 4) and in Italy (5). In particular, in Chinese patients with COVID‐19, the prevalence of diabetes and hypertension was 20% and 30%, respectively (4, 6). According to a recent meta‐analysis, diabetes may not increase the risk of SARS‐CoV‐2 infection but can worsen its outcome (7). Diabetes and hypertension are typical complications of overweight and obesity. In addition, obesity was recognized as an independent risk factor for systemic complications from influenza during the 2009 H1N1 influenza pandemic (8). Although obesity was not listed among the comorbidities associated to SARS‐CoV‐2 infection, a possible role of obesity in determining the severity of COVID‐19 manifestations has been suspected (9). Very recently, a French study demonstrated a high frequency of obesity among patients admitted in intensive care unit for SARS‐CoV‐2–related pneumonia, with disease severity increasing with BMI (10). Nonetheless, more data on this possible association are still needed.
In this study, we report the prevalence of overweight and obesity in Italian patients hospitalized for SARS‐CoV‐2–related pneumonia in an internal medicine ward entirely dedicated to COVID‐19 patients during the Italian outbreak of the disease. The association between increased BMI and the severity of the disease was also evaluated.
Methods
Study setting
Veneto is an Italian region with about 5 million inhabitants located in the northeast of the country, very close to Lombardy. Initial cases of COVID‐19 in the Veneto region were detected shortly after the first Italian ones, and the outbreak spread rapidly, with 14,432 confirmed cases, 4,430 hospitalizations, and 800 deaths reported by April 14, 2020 (11). The burden for the Regional Health Care Service increased rapidly, reaching a peak of 2,068 patients hospitalized for COVID‐19 in April 1, 2020, 350 of whom were in intensive care units (ICU) (11). Initially, our hospital addressed the outbreak through the beds available in the ICU for patients requiring invasive mechanical ventilation (IMV), in a semi‐intensive respiratory unit for patients requiring noninvasive assisted ventilation (NIV), and in the Infectious Disease Unit for patients requiring only oxygen support. As the number of cases increased, the hospital reacted by increasing the number of beds in the ICU and in the semi‐intensive unit and by transforming some of the Internal Medicine Units to COVID‐19 wards. Our 50‐bed Internal Medicine Unit (Clinica Medica 3) was transformed to a COVID‐19 ward in March 14, 2020. Since then, our ward received patients with COVID‐19 requiring oxygen support from the Emergency Department and from the ICU and the semi‐intensive respiratory unit, in a continuous exchange of patients according to their modifying requirements for different levels of intensity of care. Discharge could occur through quarantine at home or in one of the community hospitals specifically identified by the region. Criteria for discharge, as agreed for all the COVID‐19 units of our hospital, were the following: oxygen saturation > 93% without oxygen support in the last 48 hours, no fever in the last 48 hours, respiratory rate < 22 acts/min, no worsening findings at chest x‐ray, duration of the disease > 7 days, and oxygen saturation > 92% during a 6‐minute walking test.
Study patients and data collection
For the present study, we analyzed the clinical data of all patients hospitalized in our COVID‐19 ward from March 23 (when we started to collect systematically anthropometric data) to April 11, 2020. All patients were positive to an oropharyngeal swab used for real‐time reverse‐transcriptase polymerase chain reaction assays specific for SARS‐CoV‐2, and they were diagnosed with COVID‐19 pneumonia. Data were extracted from the electronic clinical documentation, and patient confidentiality was protected by assigning an anonymous identification code. The following data were included in the database: sex, age, height, weight at hospital admission, BMI, days between the onset of symptoms and the hospital admission, previous comorbidities (cancer, dementia, type 2 diabetes, hypertension, cardiovascular diseases, chronic respiratory diseases, chronic kidney diseases, chronic liver diseases, chronic inflammatory diseases), most intensive form of ventilatory support required during the hospital stay (IMV, NIV, oxygen support), most intensive setting of care required during the hospital stay (ICU, semi‐intensive respiratory unit, medical ward), death, hospital discharge (at home or in a community hospital), and duration of hospital stay.
For a further assessment of the severity of COVID‐19, the following two additional separate indicators were considered: (1) need for IMV at any time during the hospital stay and (2) need for NIV at any time during the hospital stay. Patients were classified according to their BMI in three categories as normal weight (< 25 kg/m2), overweight (from 25 to < 30 kg/m2), and obesity (≥ 30 kg/m2).
The study was a retrospective cohort study not involving any active treatment or procedure outside the normal clinical care. The study complied with standard operating procedures in place, in accordance with the European Data Protection Directive (95/46/EC) and, upon its entry into force, Regulation (EU) 2016/679 (also referred to as the General Data Protection Regulation [GDPR]). The study has been notified to the local Institutional Review Board (Comitato Etico per la Sperimentazione Clinica della Provincia di Padova) (Prot. N° 0031090) in 20 May 2020.
Statistical analysis
All variables were tested by normality test (Shapiro‐Wilk test) and equal variance test (Brown‐Forsythe). One‐way ANOVA was used when normality test and equal variance test were passed (data are presented as mean value [SD]). χ2 test (or Fisher exact test) was carried out for categorical variables, and data are expressed as frequency (percentage). Different multiple logistic models were performed considering the following as dependent (dichotomous) variables: assisted ventilation (NIV + MV = 1) versus only oxygen support (= 0), considered as the most intensive ventilation; semi‐ICU (SEMI) plus ICU versus medical ward (SEMI + ICU = 1; medical ward = 0); and death (= 1) versus no death (= 0). We analyzed two models (A and B) for each dependent variable. In model A, we included as independent variables obesity categories (overweight and obesity, BMI ≥ 25, versus normal weight, BMI < 25), age (years), and sex (male = 1, female = 2); in model B, comorbidities were entered as independent variables beyond obesity categories, age, and sex. The small sample size included in this study precluded the analysis of the role of each baseline comorbidity as an independent predictor of outcomes in multiple logistic regression. Thus, we chose to analyze the presence of comorbidities that were statistically different among the three populations according to BMI (respiratory chronic diseases and dementia) and type 2 diabetes suggested to be a negative prognostic factor for SARS‐CoV‐2 infection outcome (7). P < 0.05 was considered significant. All statistical analyses were performed with the Systat Software SigmaPlot v.14.
Results
A total of 92 patients stayed at least 1 day in the COVID‐19 medical ward from March 23 to April 11, 2020. At the end of this study period, 50 patients (54.4%) had been discharged from the hospital (36 at home and 14 in a community hospital), 12 (13.0%) died, and 30 (32.6%) were still hospitalized in our ward or in other departments of the hospital (ICU, semi‐intensive respiratory unit, or Infectious Disease Unit). Mean total duration of the hospital stay was 14.6 (8.6) days (range, 3‐41 days) for patients discharged from the hospital and 10.8 (10.4) days (range, 1‐33 days) for the patients who died during hospitalization. The baseline characteristics, the clinical data during hospitalization, and the outcomes of the patients are reported in Table 1. The patients were predominantly males (61.9%), and their mean age was 70.5 (13.3) years (range, 40‐96 years). Baseline comorbidity burden was relevant. Assisted ventilation (NIV + IMV) beyond pure oxygen support was used in 34 patients (37.0%), with 9 patients requiring IMV and 25 patients requiring NIV. A total of 35 patients (38.0%) were treated during hospitalization in more intensive settings than the internal medicine COVID‐19 ward, with 16 patients requiring admission to an ICU and 19 patients requiring admission to the semi‐intensive respiratory unit.
TABLE 1.
Baseline characteristics, clinical data during hospitalization, and outcomes in 92 patients hospitalized for SARS‐CoV‐2–related pneumonia in COVID‐19 medical ward
| Demographics and anthropometry | ||
| Male sex | 57 (61.9%) | |
| Age, y | 70.5 ± 13.3 | Range: 40‐96 |
| BMI, kg/m2 | 27.2 ± 4.6 | Range: 17.7‐40.8 |
| Baseline comorbidities | ||
| Type 2 diabetes | 28 (30.4%) | |
| Hypertension | 59 (64.1%) | |
| Cardiovascular diseases | 29 (31.5%) | |
| Respiratory chronic diseases | 12 (13.0%) | |
| Renal chronic diseases | 5 (5.4%) | |
| Liver chronic diseases | 5 (5.4%) | |
| Inflammatory chronic diseases | 6 (6.7%) | |
| Cancer | 12 (13.0%) | |
| Dementia | 17 (18.5%) | |
| Most intensive ventilatory support | ||
| Invasive mechanical ventilation | 9 (9.8%) | |
| Noninvasive ventilation | 25 (27.7%) | |
| Oxygen support | 58 (63.0%) | |
| Most intensive setting of care | ||
| Intensive care units | 16 (17.4%) | |
| Semi‐intensive respiratory unit | 19 (20.6%) | |
| Medical ward | 57 (70.0%) | |
| Outcome | ||
| Death | 12 (13.0%) | |
| Hospital discharge | 50 (54.4%) |
Data presented as mean ± SD for continuous variables and as frequency (percentage) for categorical variables.
Mean BMI in our study population was 27.2 (4.6) (range, 17.7‐40.8). Distribution into the three BMI classes was as follows: 32 patients (34.8%) with normal weight, 31 (33.7%) with overweight, and 29 (31.5%) affected by obesity. Only one patient had severe obesity (BMI ≥ 40) and was included in the obesity class. The baseline characteristics, the clinical data during hospitalization, and the outcomes of the patients in the three BMI classes are compared in Table 2. Male gender was more prevalent in patients with overweight and obesity than in patients with normal weight (P < 0.05). Patients with normal weight were more than 10 years older than patients with higher BMI (P < 0.01), and 43.7% of them had dementia, with a statistically significant difference from the other two subgroups (P < 0.001). Furthermore, the presence of respiratory chronic diseases displayed a strong difference among the three classes (P < 0.01), with obesity category showing the most prevalence. Assisted ventilation (NIV + IMV) beyond pure oxygen support was used in 15.6% of patients with normal weight, in 54.8% of patients with overweight, and in 41.4% of patients with obesity (P < 0.01), with most of the difference being linked to an increased use of NIV (P < 0.05). More intensive settings (ICU or semi‐intensive respiratory unit) than the internal medicine COVID‐19 ward was requested by 18.7% of patients in the normal weight group, by 54.8% of patients in the overweight group, and by 41.3% of patients with obesity (P < 0.05). Death rate was significantly higher in the normal weight group (31.2%) than in patients with overweight (no deaths) or obesity (6.9%) (P < 0.001).
TABLE 2.
Baseline characteristics, clinical data during hospitalization, and outcomes in 92 patients hospitalized for SARS‐CoV‐2–related pneumonia in COVID‐19 medical ward according to BMI classes
| Normal (n = 32) | Overweight (n = 31) | Obesity (n = 29) | P value | |
|---|---|---|---|---|
| Demographics and anthropometry | ||||
| Male sex | 53.1% | 61.3% | 72.4% | < 0.05 |
| Age, y | 76.1 ± 13.0 | 68.0 ± 12.6 | 67.0 ± 12.6 | < 0.01 |
| BMI, kg/m2 | 22.3 ± 1.9 | 27.4 ± 1.5 | 32.4 ± 2.6 | < 0.001 |
| Baseline comorbidities | ||||
| Type 2 diabetes | 28.1% | 25.8% | 37.9% | 0.300 |
| Hypertension | 56.2% | 67.7% | 69.0% | 0.513 |
| Cardiovascular diseases | 31.2% | 29.0% | 34.5% | 0.901 |
| Respiratory chronic diseases | 6.2% | 3.2% | 31.0% | < 0.01 |
| Renal chronic diseases | 12.5% | 6.4% | 0% | 0.142 |
| Liver chronic diseases | 3.1% | 9.7% | 3.4% | 0.440 |
| Inflammatory chronic diseases | 9.4% | 9.7% | 0% | 0.228 |
| Cancer | 18.7% | 6.4% | 13.8% | 0.346 |
| Dementia | 43.7% | 0% | 10.3% | < 0.001 |
| Most intensive ventilatory support | ||||
| Invasive mechanical ventilation | 6.2% | 16.1% | 6.9% | 0.343 |
| Noninvasive ventilation | 10.3% | 38.7% | 34.5% | < 0.05 |
| Oxygen support | 84.5% | 45.2% | 58.6% | < 0.01 |
| Assisted ventilation (NIV + IMV) | 15.6% | 54.8% | 41.4% | < 0.01 |
| Most intensive setting of care | ||||
| Intensive care units | 6.2% | 22.6% | 24.1% | 0.119 |
| Semi‐intensive unit | 12.5% | 32.2% | 17.2% | 0.132 |
| Medical ward | 81.3% | 48.2% | 58.6% | < 0.05 |
| ICU + SEMI | 18.7% | 54.8% | 41.3% | < 0.05 |
| Outcome | ||||
| Death | 31.2% | 0% | 6.9% | < 0.001 |
| Hospital discharge | 43.7% | 64.5% | 55.2% | < 0.001 |
Data presented as mean ± SD for continuous variables and percentage for categorical variables. P < 0.05 considered significant.
Thus, with the aim to analyze the most intensive ventilatory support, the most intensive setting, and the mortality, adjusting the differences among the BMI categories, we developed multiple logistic regression models (Table 3). We analyzed two models (A and B) for each dependent dichotomous variable. In model A, we included as independent variables obesity categories (overweight and obesity versus normal weight), age, and sex; in model B, comorbidities were entered as independent variables beyond obesity categories, age, and sex. The association of the BMI categories with the need for assisted ventilation (NIV + IMV) as the most intensive ventilation versus only oxygen support remained statistically significant (P < 0.05; odds ratio [OR], 4.19; 95% confidence interval [CI]: 1.36‐12.89) in a multiple regression analysis in which age and sex were entered as independent variables (model A). Interestingly, when comorbidities were also included (model B), BMI categories continued to be significant (P < 0.05; OR, 3.62; 95% CI: 1.09‐11.97). The differences among the most intensive setting (SEMI + ICU vs. medical ward) were confirmed also in model A (P < 0.001; OR, 11.65; 95% CI: 3.88‐34.96) and model B (P < 0.001; OR, 12.46; 95% CI: 3.48‐44.54).
TABLE 3.
Different multiple logistic regression models considered as dependent variables
| Independent variables | Dependent (dichotomous) variables | ||||||
|---|---|---|---|---|---|---|---|
| NIV + IMV vs. only oxygen | SEMI + ICU vs. medical ward | Death vs. no death | |||||
| P | OR (95% CI) | P | OR (IC 95%) | P | OR (95% CI) | ||
| Model A | BMI ≥ 25 vs. < 25 | 0.012* | 4.19 (1.36‐12.89) | < 0.001* | 11.65 (3.88‐34.96) | 0.204 | 0.27 (0.03‐2.05) |
| Age (y) | 0.091 | 0.97 (0.93‐1) | 0.180 | 0.97 (0.93‐1.01) | 0.007* | 1.21 (1.05‐1.39) | |
| Sex (male = 1, female = 2) | 0.682 | 1.22 (0.47‐3.17) | 0.240 | 0.54 (0.19‐1.52) | 0.346 | 2.51 (0.37‐16.94) | |
| Model B | BMI ≥ 25 vs. < 25 | 0.035* | 3.62 (1.09‐11.97) | < 0.001* | 12.46 (3.48‐44.54) | 0.375 | 0.26 (0.01‐5.20) |
| Age (y) | 0.246 | 0.98 (0.94‐1.02) | 0.110 | 0.96 (0.92‐1.01) | 0.182 | 1.11 (0.95‐1.29) | |
| Sex (male = 1, female = 2) | 0.604 | 1.29 (0.49‐3.42) | 0.278 | 0.56 (0.19‐1.61) | 0.334 | 3.49 (0.28‐43.98) | |
| Respiratory chronic diseases vs. no | 0.580 | 1.48 (0.37‐5.88) | 0.111 | 4.86 (0.69‐33.96) | 0.157 | 9.8 (0.41‐230.38) | |
| Type 2 diabetes vs. no | 0.332 | 0.59 (0.2‐1.71) | 0.784 | 0.84 (0.25‐2.81) | 0.553 | 2.06 (0.19‐22.44) | |
| Dementia vs. no | 0.394 | 0.45 (0.07‐2.79) | 0.584 | 0.84 (0.25‐2.81) | 0.059 | 15.81 (0.9‐277.43) | |
Assisted ventilation (NIV + MV = 1) vs. only oxygen support (= 0), considered as the most intensive ventilation; semi‐ICU (SEMI) plus ICU vs. medical ward (SEMI + ICU = 1; medical ward = 0); and death (= 1) vs. no death (= 0). We analyzed two models (A and B) for each dependent variable.
The statistically significant differences of death rate among BMI classes disappeared in model A, in which the effects of sex and age were taken into account, with age being the only factor associated with death (P < 0.01; OR, 1.21; 95% CI: 1.05‐1.39). When we included comorbidities, age stopped being linked to mortality.
IMV was requested at any time during the hospital stay in 9 patients (9.8%) and NIV in 34 (37.0%) patients. The proportion of patients who required IMV or NIV at any time during the hospital stay according to BMI classes is reported in Figure 1A. No significant differences were observed in the proportion of patients requiring IMV (6.2% in patients with normal weight, 16.1% in patients with overweight, and 6.9% in patients with obesity). The proportion of patients requiring NIV at any time during the hospital stay was lower in patients with normal weight (15.6%) than in patients with overweight (54.8%) or obesity (41.4%) (P < 0.01). The proportion of patients receiving some form of assisted ventilation in the normal weight group could be lowered by the high prevalence in this group of frail elderly patients with dementia or advanced cancer, for whom intensive ventilatory support was deemed not appropriate and who died in the medical COVID‐19 ward. Therefore, we repeated this analysis removing 22 patients with dementia or advanced cancer (Figure 1B). No deaths were reported in this subgroup. In this subgroup, no differences were observed in the proportion of patients receiving IMV between the three BMI classes (14.3%, 17.2%, and 8.7%, respectively), whereas the prevalence of patients receiving NIV remained lower in patients with normal weight (28.6%) than in patients with overweight (55.2%) or obesity (43.5%) (P < 0.05).
Figure 1.
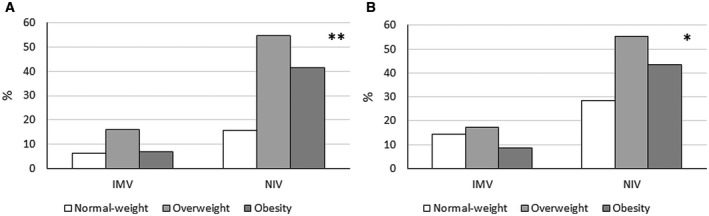
Proportion of patients who required invasive mechanical ventilation (IMV) or noninvasive assisted ventilation (NIV) at any time during the hospital stay according to BMI classes. Data were calculated in (A) all patients and in (B) patients without cancer and/or dementia only. Statistical analysis was performed with χ2 test (**P < 0.01, *P < 0.05).
Discussion
In this descriptive cross‐sectional study, we illustrated the baseline characteristics, the clinical data, and the outcomes of a cohort of patients hospitalized with a diagnosis of SARS‐CoV‐2–related pneumonia in a dedicated medical ward of a large university hospital located in the Veneto region of Italy. The cohort was an unselected group of patients representing the real‐life situation of patients hospitalized in our ward. Obesity and overweight were present in two‐thirds of patients.
Patients with overweight and obesity were younger than patients with normal weight, they more frequently required respiratory support beyond simple oxygen (IMV and NIV), and they were more frequently admitted to the ICU and semi‐intensive respiratory units. Older patients with normal weight were more frequently affected by dementia, and they had a higher mortality rate. The need for assisted ventilation and an increased admission to the ICU and SEMI remained more prevalent in patients with overweight and obesity than in patients with normal weight after adjustment for age, sex, and comorbidities. In particular, NIV use was demonstrated to be lower in patients with normal weight, even though patients with dementia or advanced cancer were removed from the analysis.
The prevalence of overweight and obesity in patients hospitalized for SARS‐CoV‐2–related pneumonia was 65.2% (33.7% for overweight and 31.5% for obesity, respectively). The last reported prevalence of overweight and obesity in adults 18 to 69 years old in the Veneto region was 40.0% (30.4% for overweight and 9.6% for obesity), with a progressive increase in prevalence when age increases (12). According to these epidemiologic data, age‐adjusted expected prevalence of overweight and obesity in our sample would be 39.6% and 15.3%, respectively (χ2 expected vs. observed prevalence = 6.8285; P < 0.05). Therefore, obesity in particular seems overrepresented in our COVID‐19 group of patients than in the general population of our region. However, the small sample included in our study limits the reliability of this observation. In a single French center, obesity was significantly more prevalent among 124 patients admitted in intensive care for SARS‐CoV‐2 than in historical controls admitted for no SARS‐CoV‐2–related respiratory distress (47.6% vs. 25.2%) (10).
The patients with overweight and obesity admitted in our COVID‐19 internal medicine ward for SARS‐CoV‐2–related pneumonia were 10 years younger than patients with normal weight admitted in the same ward with the same diagnosis and in the same period. In a recent prepublication report, Petrilli et al. (13) reported that age and obesity were among the most important risk markers for having symptoms severe enough to warrant hospitalization in 5,279 patients with COVID‐19 in New York City. In our study, patients with overweight and obesity, despite their younger age, required more frequently assisted respiratory support, and they were more prone to be admitted to intensive or semi‐intensive care unit. The association with an increased need of assisted ventilation and a greater admission in the ICU and SEMI remained significant even after adjustment for age, sex, and comorbidities. Moreover, patients with overweight and obesity required NIV more frequently even if compared with patients with normal weight without dementia or cancer. Our results are consistent with the findings of the French study, in which the proportion of patients who required IMV increased with BMI categories, and it was greatest in patients with BMI > 35 (10).
In our study, death rate was higher in patients with normal weight than in patients with overweight or obesity. This difference was totally explained by a difference in age, as confirmed by the regression model in which the effect of BMI categories was adjusted by age. Most of the deaths observed in this group occurred indeed in very old frail elderly patients with disability and multiple comorbidities, including dementia and cancer. When we included the presence of dementia in the regression model, we did not find a significant difference, even though the role of the age disappeared, probably because of the small size of our cohort. Frailty is a strong predictor of short‐term survival in older adults with or without comorbidities (14). We can suggest that the normal weight observed in this subgroup might be considered an indirect marker of sarcopenia and/or undernourishment in a setting of increased requirements linked to the presence of a severe acute inflammatory status. In Italy, more than 50% of the deaths related to the SARS‐CoV‐2 epidemic occurred in patients > 80 years old (15). Adequacy of nutritional support might be a crucial aspect in the subgroup of older patients with dementia or cancer hospitalized for COVID‐19 (16).
Patients with overweight and obesity enrolled in our study are mostly represented by middle‐aged men. The constraint of the clinical work in a confined ward precluded an assessment of fat distribution, but we can speculate that the majority of these patients were affected by visceral obesity. Currently, the mechanisms linking overweight and obesity to a more severe SARS‐CoV‐2–related pneumonia remain speculative. Obesity is associated with sleep apnea syndrome and with a restrictive ventilatory pattern, as well as to surfactant dysfunction, which might contribute to severe acute respiratory syndrome during COVID‐19 (16). Moreover, patients with central obesity and insulin resistance as well as patients with type 2 diabetes are frequently characterized by a chronic state of low‐grade inflammation, with increased levels of interleukin 6 and other proinflammatory cytokines. This chronic activation of the inflammatory pathways could induce an impaired immune response and an increased susceptibility to infections (17). Recent reports have suggested that the procoagulant pattern of patients with severe COVID‐19 may justify the high rate of thromboembolic complications and pulmonary embolism during the course of the disease (18). We cannot exclude that overweight and obesity could explain a more severe COVID‐19 considering that obesity per se is characterized by a disturbed hemostatic balance with increased coagulation and impaired fibrinolysis, which could trigger thrombosis (19). In our study, we did not observe a worsening of the severity of COVID‐19 with the increase in BMI class (obesity vs. overweight). This could be attributed to a protective effect of more severe obesity (“obesity paradox”) or to the fact that the association between overweight and obesity and severity of SARS‐CoV‐2–related pneumonia could be mediated by factors not related to BMI. Further studies may contribute to a better understanding of the mechanisms explaining the link between visceral obesity and COVID‐19 complications.
Our study presents several limitations. Our sample was relatively small and heterogeneous, limiting the power of the study. In particular, we were not able to analyze if each obesity‐related comorbidity influences the association between overweight and obesity and the severity of COVID‐19. This is clearly a very relevant question that would require a larger sample. On the contrary, we tried to test the presence of three important comorbidities in our population (respiratory chronic diseases, type 2 diabetes, and dementia). Moreover, the study was centered on patients admitted to a dedicated internal medicine ward, and this probably excluded some patients with the most severe manifestations of SARS‐CoV‐2 infection. However, our ward works in strict contact with the ICU and the semi‐intensive respiratory unit, and many of our cases were represented by patients originally admitted to more intensive clinical settings and then transferred to our ward after stabilization and partial recovery. Therefore, our sample covers the full spectrum of disease severity and provides some information about the characteristics of patients with less severe disease not included in previous studies limited to an intensive care setting (10).
In conclusion, our study and other available evidence suggest that patients with overweight and obesity have a higher risk of more severe clinical symptoms during SARS‐CoV‐2 infection. Patients with overweight and obesity require hospitalization more frequently (13), and we showed a greater admission in intensive and semi‐intensive care units, independent of age. Furthermore, patients with overweight and obesity present with a more frequent need of assisted ventilation during SARS‐CoV‐2–related pneumonia, as demonstrated in our study, and more frequently need IMV when in the ICU (10). Finally, we suggest that patients with overweight and obesity should be considered in a higher risk class and therefore be more protected from infection and monitored more closely in cases of SARS‐CoV‐2–related pneumonia.
Disclosure
The authors declared no conflict of interest.
Data sharing statement
The original data source used for this study is not publicly available, as they are part of the personal clinical documentation of the individual patients enrolled in the study, and they are protected by Italian privacy protection laws. An anonymized electronic database specific for this study is stored and locked in our center and available upon request.
References
- 1. Team NCPERE. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) – China. China CDC Wkly 2020;2:113‐222. [PMC free article] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . COVID‐19 situation update worldwide, as of 13 April 2020. https://www.ecdc.europa.eu/en/geographical‐distribution‐2019‐ncov‐cases. Accessed April 15, 2020.
- 3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy [published online March 23, 2020]. JAMA. doi:10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest 2020;43:867‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009;302:1896‐1902. [DOI] [PubMed] [Google Scholar]
- 9. Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the patient with obesity – The Editors speak out. Obesity (Silver Spring). 2020;28:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Regione del Veneto. Azienda Zero. Emergenza Coronavirus SARS‐CoV‐2 / COVID‐19. SARS‐CoV‐2 in Veneto. https://www.azero.veneto.it/‐/emergenza‐coronavirus. Accessed April 15, 2020.
- 12. Istituto Superiore di Sanità. Epicentro. La sorveglianzaPassi. I dati per l’Italia: Sovrappeso ed Obesità. https://www.epicentro.iss.it/passi/dati/sovrappeso#dati. Accessed April 15, 2020.
- 13. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. doi:10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zucchelli A, Vetrano DL, Marengoni A, et al. Frailty predicts short‐term survival even in older adults without multimorbidity. Eur J Intern Med 2018;56:53‐56. [DOI] [PubMed] [Google Scholar]
- 15. Istituto Superiore di Sanità. Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on July 22nd, 2020. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_22_july_2020.pdf
- 16. Puig‐Domingo M, Marazuela M, Giustina A. COVID‐19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine 2020;68:2‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37:333‐340. [DOI] [PubMed] [Google Scholar]
- 18. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost 2020;18:1747‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bladbjerg EM, Stolberg CR, Juhl CB. Effects of obesity surgery on blood coagulation and fibrinolysis: a literature review. Thromb Haemost 2020;120:579‐591. [DOI] [PubMed] [Google Scholar]


