INTRODUCTION
COVID‐19, the disease caused by the novel coronavirus (SARS‐CoV2), has been declared a global pandemic by the World Health Organization. Severe acute respiratory distress syndrome may be a consequence of COVID‐19, and veno‐venous (V‐V) extracorporeal membrane oxygenation (ECMO) may be offered to patients. This strategy is being used at specialized centers for the treatment of severe cases unable to be supported by traditional mechanical ventilation. 1 Typically, the patient's venous system is drained through a single femoral vein, and an internal jugular vein is then utilized to return oxygenated blood to the system, bypassing the injured lungs. This process requires chronic anticoagulation to avoid thrombosis and circuit failure. There are limited reports of epistaxis and/or upper airway hemorrhage management in ECMO patients in the literature, and none detailing protocols for actively infected patients in the midst of a global pandemic. 2
CASE SERIES
Among five COVID‐19 patients at our institution being supported by V‐V ECMO at the time of this writing, three required consultation by the otolaryngology service for management of epistaxis and/or oropharyngeal bleeding and are reported here. All were anticoagulated with bivalirudin, which was briefly held during the course of bleeding in all cases. In all cases, bleeding was successfully controlled after measures were taken as detailed. Table I details a summary of the cases including relevant laboratory values on the day of consultation.
TABLE I.
Summary of COVID‐19 Patients on Extracorporeal Membrane Oxygenation Requiring Otolaryngology Intervention for Airway Bleeding.
| Case | Age | Sex | Hgb (g/dL) | Platelets (K/μL) | INR/PTT (sec) | Anticoagulant | Method of Hemostasis | Length of Time Anticoagulation Held (d) |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | 11.8 | 146 | 1.85/71.4 | Bivalirudin | Oropharyngeal gauze, absorbable nasal (gelatin/thrombin matrix, gelatin sponge) | 5 |
| 2 | 46 | M | 13.2 | 103 | 1.38/61.8 | Bivalirudin | Absorbable nasal (cellulose hemostat, gelatin sponge) | 1 |
| 3 | 34 | M | 12.4 | 144 | 1.66/65.8 | Bivalirudin | Absorbable nasal (gelatin sponge, gelatin/thrombin matrix) | 3 |
Hgb = hemoglobin; INR = international normalized ratio; PTT = partial thromboplastin time.
Case 1
A 53‐year‐old intubated male had spontaneous bleeding from the nasal and oral mucosa 7 days after V‐V ECMO initiation. An absorbable gelatin sponge (Surgifoam; Ethicon, Somerville, NJ) and gelatin/thrombin matrix (Surgiflo; Ethicon) were packed in the bilateral nasal cavities. Gauze oropharyngeal packing was placed and exchanged every 2 days until removal on the 6th day with resolution of bleeding.
Case 2
A 46‐year‐old male had epistaxis 14 days after initiation of V‐V ECMO. He was extubated and hypertensive (189/105 mm Hg), with a nasogastric tube in the left nasal cavity at the time of evaluation. Bilateral nasal cavities were bleeding and were packed with an oxidized cellulose absorbable hemostat (Surgicel Original, Ethicon) and absorbable gelatin sponge. Bleeding resolved, and anticoagulation was resumed the day after packing.
Case 3
A 34‐year‐old male had bilateral epistaxis 8 days after initiation of V‐V ECMO. He had been extubated prior to evaluation. A nasogastric tube was in the left nasal cavity. A gelatin/thrombin matrix was placed in the bilateral nasal cavities. There was also mild bleeding from the lips and oral cavity mucosa that did not require intervention and had resolved by the following day. The bedside nursing staff was instructed to apply petroleum jelly to the lips twice daily to prevent dryness. All bleeding was controlled, and anticoagulation was resumed after being held for 3 days.
DISCUSSION
The etiology of upper aerodigestive hemorrhage in patients treated with ECMO is multifactorial, and the incidence of hemorrhage has been estimated at between 5% and 30% in this population. 2 , 3 Patients who are placed on V‐V ECMO may be at high risk for hemorrhage secondary to anticoagulation, known underlying hematologic abnormalities, and/or coagulopathy associated with critical illness. Early reports suggest that patients with severe COVID‐19 have distinct hematologic findings consistent with alterations to the coagulation cascade, though these are not yet well understood. 4 Instrumentation of the upper airway is commonly required in critically ill patients and often causes mucosal trauma that triggers bleeding. Additionally, mechanical obstruction of the internal jugular vein or superior vena cava by large ECMO cannulas may cause venous congestion, increasing the propensity for persistent hemorrhage.
Although unfractionated heparin (UFH) is commonly utilized for ECMO anticoagulation, early experiences with ECMO in patients with COVID‐19 at our institution showed maintaining therapeutic anticoagulation at typical doses was challenging. Recent literature suggests that bivalirudin better maintains ECMO anticoagulation within a therapeutic range than UFH, without increasing incidence of bleeding events. 5 Bivalirudin is a direct thrombin inhibitor, as opposed to heparin, which indirectly inhibits thrombin by activating antithrombin. As we highlight in this series, there is still a risk of bleeding events requiring intervention with bivalirudin use.
Extra precautions are required in the treatment of COVID‐19 patients with upper airway bleeding to prevent viral transmission by aerosolization of secretions. Only a single provider was in the patient room during the procedure. The personal protective equipment (PPE) used by the physician included disposable surgical cap, gown, gloves, and powered air‐purifying respirator with face shield or N95 mask with eye/face protection (Fig. 1). Providers were trained in proper donning and doffing protocols for PPE. A handheld light source and overhead lighting were used in all cases. A headlight was not used because a model compatible with the PPE was not immediately available. A headlight may also be less advantageous because adjustments during the procedure increase the risk of contamination. All possible supplies necessary were carried into the room to minimize need for exit and reentry. Because of the diffuse nature of the bleeding encountered, endoscopy was of little utility and was avoided, as it could exacerbate bleeding and cause further aerosolization of secretions. Suctioning was also avoided for the same reasons. Placement of absorbable packing was preferred to limit the number of procedures required to temporize the nasal bleeding. This minimizes the need to remove permanent packing, such as petrolatum gauze or polyvinyl alcohol sponge (Merocel), which could risk rebleeding and requires further instrumentation. Nasal sprays, such as oxymetazoline, were not recommended in these cases to minimize aerosolization. If needed, it should be applied using soaked pledgets rather than spraying.
Fig. 1.
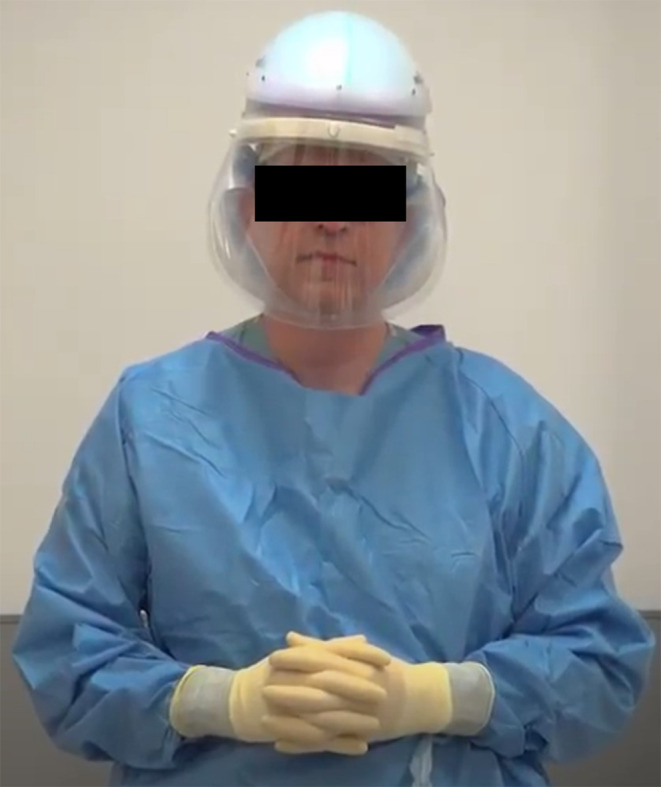
Photograph of provider having donned recommended personal protective equipment. An N95 mask with eye and face protection may be substituted for the powered air‐purifying respirator shown in this photograph.
CONCLUSION
As the number of severe COVID‐19 cases continue to increase, more patients will likely require ECMO. Otolaryngologists may be required to intervene on these patients who are at risk for bleeding in the upper aerodigestive tract. Bleeding was noted in a high percentage of ECMO cases at our institution. Although use of anticoagulation is a major factor, it must be restarted soon after hemorrhage control to avoid thrombus formation near the ECMO cannula sites. Further refinements to our strategy are expected as we continue to encounter additional ECMO cases.
Supporting information
Supporting Data
Editor's Note: This Manuscript was accepted for publication on May 26, 2020.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID‐19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med 2020;8:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazzeffi M, Kiefer J, Kon Z, Wolf J. Severe epistaxis during adult extracorporeal membrane oxygenation: not your average nosebleed. J Thorac Dis 2015;7:E564–E565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Makdisi G, Wang IW. Severe epistaxis in patients on extracorporeal membranous oxygenator support occurrence and management. J Thorac Dis 2015;7:E566–E567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2 [published online April 3, 2020]. J Thromb Thrombolysis 2020. 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaseer H, Soto‐Arenall M, Sanghavi D, et al. Heparin vs bivalirudin anticoagulation for extracorporeal membrane oxygenation. J Card Surg 2020;35:779–786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Data


