To the Editor,
The most common presenting symptoms of COVID‐19 include fever and cough, with neurologic complications becoming more widely recognized as presenting symptoms. 1 We report the case of a patient who presented with suspected drug overdose and was diagnosed with COVID‐19 induced encephalopathy manifesting as choreiform movements. Choreiform movements appear to be a rare manifestation of Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and may be due to direct viral central nervous system (CNS) invasion or, more likely, autoimmune‐mediated toxicity precipitated by the initial viral infection.
A 36 years old otherwise healthy homeless male with known polysubstance abuse presented on 3/24/2020 for suspected drug overdose. On presentation, his vitals were normal except oxygen saturation was 99% on two liters per minute of oxygen, which he was weaned off of. Physical exam was limited because he received intramuscular midazolam before arrival. On examination, the patient was obtunded, with slurred speech and pinpoint pupils. Urine drug screen was positive for cocaine, opiates, and benzodiazepines, while blood alcohol level was undetectable. Laboratory studies showed a normal white blood cell count, decreased lymphocytes, mildly increased C‐reactive protein, and erythrocyte sedimentation rate. A noncontrast computed tomography scan of the head was done which showed no acute infarct or intracranial hemorrhage. He began to regain consciousness in the emergency room but due to agitation was given further sedatives. He continued to have intermittent episodes of agitation for which he was given IV diazepam for presumed withdrawal symptoms. He was noted to have intermittent rapid, irregular, and nonpurposeful movements of the bilateral upper extremities for which a cause was not immediately identified. On day two he became febrile to 39.4°C, raising concern for an underlying infectious process. Chest X‐ray (CXR) did not show any active disease. Over concern for bacterial meningitis, he was then started on vancomycin and ceftriaxone. He continued to spike fevers and remained encephalopathic with choreiform movements, becoming increasingly hypoxic. Repeat CXR on day 4 showed a hazy opacity in the left lung base. Blood cultures remained negative. His oxygen requirement increased further, prompting suspicion for COVID‐19 and subsequent reverse transcriptase‐polymerase chain reaction (RT‐PCR) assay for SARS‐CoV‐2 was sent and was positive. Vancomycin and ceftriaxone were discontinued as it was felt he did not have bacterial meningitis. Workup including human immunodeficiency virus, syphilis, malaria, vitamin B12, lupus anticoagulant, anticardiolipin, antinuclear antibodies, heavy metals, Mayo clinic encephalitis, and paraneoplastic panel were unremarkable.
On day 8 his mentation slowly improved, however, his choreiform movements worsened and subsequently involved all extremities. A brain magnetic resonance imaging scan (MRI) was obtained which demonstrated multiple focal enhancing lesions primarily affecting the bilateral medial putamen and left cerebellum. There were also several cortical and subcortical lesions including the hippocampus, primarily on the left side, along with punctate restricted diffusion in the right basal ganglia. The MRI did not show any necrosis or hemorrhage (Figure 1). Lumbar puncture (LP) done on the same day was negative for any bacterial or viral organisms, showing mildly elevated white blood cells, lymphocyte predominance, and increased myelin basic protein. On day 13 he was given one dose of 500 mg IV Solu‐Medrol followed by intravenous immunoglobulin (IVIG) 2 g/kg on day 14, and then continued on Solu‐Medrol 500 mg IV for four additional days (days 15‐18). Improvement in the patient's choreiform movements was first noted on day 15 and they gradually improved daily, with near resolution on day 22. He was subsequently started on a prednisone taper with adjustment based on response. He is still undergoing therapy.
Figure 1.
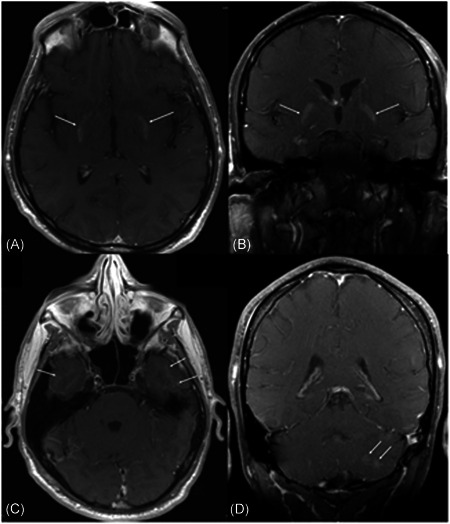
T1‐weighted postcontrast MR on the brain in axial (A) and coronal (B) views. There is an enhancement of bilateral medial putamen (arrows). C, Axial T1‐weighted postcontrast MR of the brain. There is a subtle faint enhancement of bilateral inferior temporal cortical and subcortical regions with involvement of the hippocampi, left greater than right. D, T1‐ weighted coronal postcontrast MR of the brain showing two enhancing lesions within the left cerebellum. MR, magnetic resonance
Specimens sent for COVID‐19 were nasopharyngeal and used RT‐PCR assay. Swabs obtained on days 4, 14, and 21 were positive. Swab obtained on day 25 was negative, however, repeat swab the following day was positive. False negatives have been reported, attributed to operator error, differential viral shedding, or the sensitivity and specificity of the test itself. 2 , 3 Due to this, the negative test on day 25 is believed to be a false negative.
Numerous diseases can cause damage to the function and structure of the CNS. Examples include encephalitis from viral infections, encephalopathy from severe systemic infections, or postinfectious acute demyelinating processes. Neurologic complications such as encephalopathy have been seen in SARS‐CoV‐1, MERS‐CoV, and now COVID‐19. 4 The first case of COVID‐19 encephalitis in the United States was in March 2020. 5 A female airline worker with a 3‐day history of fever, cough, and altered mental status was found to be COVID‐19 positive. MRI of the brain revealed hemorrhagic lesions within the bilateral thalami and temporal lobes and she was diagnosed with COVID‐19 associated acute necrotizing encephalitis. Since then, CNS symptoms have been an increasing complication noticed in patients with COVID‐19. 6 , 7 Our patient did not have hemorrhagic lesions and to our knowledge, this is the first report of a patient with COVID‐19 presenting with choreiform movements. His choreiform movements, MRI findings, and rapid improvement with high dose glucocorticoids are suggestive of an autoimmune‐mediated process such as acute disseminated encephalomyelitis (ADEM). ADEM is an autoimmune condition which commonly occurs after viral or bacterial infections including, but not limited to, coronavirus OC43, herpes simplex, or legionella. 8 Diagnosis is based on characteristic MRI findings and symptomatology as no definite test exists. MRI findings are variable but include multiple lesions commonly in the cortical and subcortical white matter and periventricular white matter of the cortex and thalami as were seen in our patient. Other causes for the MRI findings include other viral infection, toxic leukoencephalopathy, metabolic disorders, and trauma. 9 Evaluating his entire clinical picture, all of the previous differentials had either been ruled out, or were considered to be much less likely. Myelin basic protein, a sign of active myelin breakdown in the CSF, is commonly elevated in ADEM, as it is in our patient. Treatment is not well defined, with some patients responding to IVIG or glucocorticoids, as did our patient. 10
Coronaviruses like SARS‐CoV‐1 and MERS‐CoV have been detected in the CSF of patients, suggesting direct neuroinvasive potential. 11 The pathologic mechanism of CNS invasion of COVID‐19 may be similar to SARS‐CoV‐1, MERS‐CoV, and various other respiratory viruses, but still requires further investigation. 12 Angiotensin‐converting enzyme 2 (ACE2) has been recognized as a functional receptor for COVID‐19, and ACE2 receptors have been found in various human organs including the nervous system, which may provide a direct route for viral entry after destruction of the blood‐brain barrier. 13 , 14 It remains unclear if pre‐existing neurological conditions can predispose patients to CNS manifestations as demonstrated in a case where SARs‐CoV‐2 was isolated from brain tissue post‐mortem in a patient with Parkinson's disease. 15 For the patient with COVID‐19 and neurologic symptoms, brain imaging and LP are helpful in the diagnosis. Glucocorticoids should be considered on a case by case basis in patients with COVID‐19 as they have been associated with worse clinical outcomes in certain patient populations. The long term effect of this complication remains unknown.
Neurologic complications in COVID‐19 may be due to direct viral invasion or autoimmune‐mediated toxicity precipitated by the initial viral infection. In patients with CNS involvement secondary to COVID‐19, the prognosis remains unknown at this time but is believed to be poor, resulting in rapid clinical deterioration. COVID‐19 can present with a wide spectrum of symptoms, and while encephalopathy is becoming an increasingly important presenting symptom, to our knowledge this is the first case of COVID‐19 presenting as choreiform movements with encephalopathy. A patient who is positive for COVID‐19 with persistent neurological symptoms should prompt specialist investigation as well as brain imaging and LP.
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: (Zhou et al. 2020) a retrospective cohort study. The Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prinzi A. False Negatives and Reinfections: the Challenges of SARS‐CoV‐2 RT‐PCR Testing [Internet]. 2020; Available from: https://asm.org/Articles/2020/April/False-Negatives-and-Reinfections-the-Challenges-of
- 3. Cao S, Wu A, Li J, Li Y, Xia M, Wu J. Recurrent recurrence of positive SARS‐CoV‐2 RNA in a COVID‐19 patient. Int J Infect Dis. 2020;93:927‐299. 10.1016/j.ijid.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pleasure S, Green A, Josephson S. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines [published online ahead of print April 10, 2020]. JAMA Neurology. 10.1001/jamaneurol.2020.1065 [DOI] [PubMed] [Google Scholar]
- 5. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online ahead of print March 31, 2020]. Radiology. 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin R, Feng W, Wang T, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019 [published online ahead of print April 15, 2020]. J Med Virol. 10.1002/jmv.25888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finsterer J, Stollberger C. Causes of hypogeusia/hyposmia in SARS‐CoV2 infected patients [published online ahead of print April 20, 2020]. J Med Virol. 10.1002/jmv.25903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menge T, Hemmer B, Nessler S, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62(11):1673‐1680. 10.1001/archneur.62.11.1673 [DOI] [PubMed] [Google Scholar]
- 9. Schmahmann J, Smith E, Eichler F, Filley C. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann NY Acad Sci. 2008;1142:266‐309. 10.1196/annals.1444.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marchioni E, Tavazzi E, Minoli L, et al. Acute disseminated encephalomyelitis. Neurol Sci. 2008;29:286‐288. [DOI] [PubMed] [Google Scholar]
- 11. Arabi YM, Balkhy HH, Hayden FG, et al. Middle east respiratory syndrome. N Engl J Med. 2017;376(6):584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The emergence of a novel coronavirus (SARS‐CoV‐2) . disease and their neuroinvasive propensity may affect in COVID‐19 patients. Yashavantha Rao HC, Jayabaskaran C [published online ahead of print April 22, 2020]. J Med Virol. 10.1002/jmv.25918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Bai W, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552‐555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus ‐2 (SARS‐CoV‐2) [published online ahead of print May 8, 2020]. J Med Virol. 10.1002/jmv.25991 [DOI] [PMC free article] [PubMed] [Google Scholar]


