Abstract
The emergence of the SARS‐CoV‐2 strain of the human coronavirus has thrown the world into the midst of a new pandemic. In the human body, the virus causes COVID‐19, a disease characterized by shortness of breath, fever, and pneumonia, which can be fatal in vulnerable individuals. SARS‐CoV‐2 has characteristics of past human coronaviruses, with close genomic similarities to SARS‐CoV, the virus that causes the disease SARS. Like these related coronaviruses, SARS‐CoV‐2 is transmitted through the inhalation of droplets and interaction with contaminated surfaces. Across the world, laboratories are developing candidate vaccines for the virus – with vaccine trials underway in the United States and the United Kingdom – and considering various drugs for possible treatments and prophylaxis. Here, we provide an overview of SARS‐CoV‐2 by analyzing its virology, epidemiology, and modes of transmission while examining the current progress of testing procedures and possible treatments through drugs and vaccines.
Keywords: ACE2, coronavirus, COVID‐19, CRISPR, MERS, MERS‐CoV, SARS, SARS‐CoV, SARS‐CoV‐2, vaccine
This review was written for the purpose of educating the public about the scientific aspects of SARS‐CoV‐2 that may be difficult to gather in the media. This review contains background information of SARS‐CoV‐2 as well as other coronaviruses. It also gives an overview of more technical topics, such as the structure of the virus and how scientists are working to create methods for testing, treatment, and further prevention of outbreaks.

Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ATP
adenosine triphosphate
- CAS‐12
CRISPR‐associated protein 12
- CAS‐13
CRISPR‐associated protein 13
- CDC
Centers for Disease Control and Prevention
- cDNA
complementary DNA
- COVID‐19
coronavirus disease 2019
- CRISPR
clustered regularly interspaced short palindromic repeats
- crRNA
CRISPR RNA
- CT
computed tomography
- DETECTR
DNA endonuclease‐targeted CRISPR trans reporter
- DNA
deoxyribonucleic acid
- DNase
deoxyribonuclease
- ELISA
enzyme‐linked immunosorbent assays
- EUA
emergency use approval
- FDA
Food and Drug Administration
- HIV
human immunodeficiency virus infection
- HR1
Heptad repeat 1
- HR2
Heptad repeat 2
- MERS
Middle East respiratory syndrome
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- mRNA
messenger ribonucleic acid
- NIAID
National Institute of Allergy and Infectious Diseases
- PACMAN
prophylactic antiviral CRISPR in huMAN cells
- PCR
polymerase chain reaction
- R 0
basic reproduction number
- RBD
receptor‐binding domain
- RdRp
RNA‐dependent RNA polymerase
- RNA
ribonucleic acid
- RNase
ribonuclease
- RT‐qPCR
quantitative reverse transcription‐PCR
- S protein
homotrimeric spike glycoprotein
- SARS
severe acute respiratory syndrome
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SHERLOCK
specific high sensitivity enzymatic reporter UnLOCKing
- VRI
viral respiratory tract infections
- WHO
World Health Organization
Introduction
Near the end of 2019, cases of an unknown upper respiratory tract infection began appearing in Wuhan, Hubei Province, China [1]. The illness spread rapidly throughout the city and eventually to the entire country, with scientists and doctors having no answers or solutions for its transmission or pathology. By early January 2020, it was determined that these infections were caused by a novel coronavirus SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2), with the disease being named COVID‐19 (coronavirus disease 2019) [2, 3].
Global efforts to contain the virus were mixed, with some countries and/or communities enacting stricter actions than others [4, 5]. As a result, the virus soon found its way around the world, and by the beginning of March 2020, the World Health Organization (WHO) officially labeled the disease as a pandemic [6]. Unprecedented measures were taken to slow the spread – major cities and even entire nations implemented lockdowns, restrictions on travel and gatherings, and closures of businesses and schools [7]. All these measures have stifled financial and economic confidence, sparking fears of a global recession.
As the numbers of infections and deaths from COVID‐19 exponentially rise each day, it is imperative to understand the workings of SARS‐CoV‐2 and the possible treatment routes to combat this latest pandemic. Here, we compare SARS‐CoV‐2 to previous coronaviruses in terms of epidemiology and molecular biology, discuss current and prospective testing mechanisms, evaluate the currently available and novel treatments for COVID‐19, and provide details on the types of potential SARS‐CoV‐2 vaccines under development. Additionally, while not a topic of this review, the immunopathogenesis of COVID‐19 has been studied extensively [8].
History of coronaviruses
Unlike viruses such as influenza, smallpox, and polio, coronaviruses have only recently been discovered to infect the human population. When they were first discovered in the 1960s, there was almost no epidemiological, genomic, or pathogenic information about these viruses – only that they contained RNA surrounded by a membrane composed of ‘spike’‐shaped proteins [9]. The crown‐like appearance of these surface ‘spike’ proteins gave the virus family the name – ‘corona’ being Latin for crown [10]. Viruses with that specific shape and structure belong to the family of Coronaviridae, which are grouped into four genera using their phylogeny: alpha‐CoV, beta‐CoV, gamma‐CoV, and delta‐CoV [11, 12]. As of 2020, the US‐based Centers for Disease Control and Prevention (CDC) recognizes seven coronavirus strains that can infect humans [13]. In general, they are classified as single‐stranded, positive‐sense RNA genome‐bearing viruses [12]. Their genome is estimated to be around 26–32 kilobases (for comparison, the human genome is 3 000 000 kilobases) [14].
The first identified coronaviruses in the human population were human CoV‐229E (HCoV‐229E) and HCoV‐OC43 [15]. These viruses were found to cause common upper respiratory tract diseases, such as the common cold, and infections caused by the viruses have low levels of severity. After the emergence of the first two coronavirus strains, two other strains were identified: HCoV‐HKU1 and HCoV‐NL63 [16]. Three other coronavirus strains that have been identified in the human population since are SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 [13]. All three of these coronavirus strains vary from the four common strains as they can cause severe illnesses that may result in death.
One characteristic that distinguishes HCoV‐229E, HCoV‐C43, HCoV‐HKU1, and HCoV‐NL63 from the severe strains of coronavirus is their extremely low basic reproduction numbers (R 0) [17]. The basic reproduction number is used to describe how transmissible or contagious a pathogen is [18]. The value is not fixed and can be affected by intervention methods such as social distancing and vaccination. The R 0 value is used to signify the potential amount of people a single infected person can infect [19]. The higher the R 0 number is, the higher the chance that infected individuals will spread the pathogen to others. Thus, pathogens that are deemed extremely infectious have R 0 values greater than 1, while some pathogens that have low R 0 values can be contained without any need of isolation of known cases and potentially infected individuals [20].
While coronaviruses have only been known in the human population for six decades, they have come to the forefront of research and news due to the outbreak of SARS‐CoV [12], which demonstrated in the early 2000s that this virus family has the potential to cause a pandemic [21]. The four common, nonsevere human coronaviruses are distributed globally, with a low density in any given local population [12]. Regarding the three severe coronavirus strains, infections from the SARS‐CoV strain were localized in China, with small outbreaks in other countries. MERS‐CoV infections, which have been ongoing since 2012, are localized in the Middle East. SARS‐CoV‐2, which causes COVID‐19, is a global pathogen of pandemic proportions. When first discovered, human coronaviruses were found only to cause mild illnesses; however, research and new strains have proven otherwise [22].
Rise of pathogenic coronaviruses
Between SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2, the former two have been extensively studied and do not pose a significant global threat at the moment; however, there is still much to learn about SARS‐CoV‐2.
SARS‐CoV
The SARS‐CoV strain emerged in November of 2002 in Guangdong Province, China. The strain causes the disease severe acute respiratory syndrome (SARS), with common symptoms of infection being fever, cough, chills, and fatigue [23]. Many of the cases also exhibited shortness of breath and the development of pneumonia [23]. Fatal cases ended in respiratory distress and lung failure [24]. Patient outcomes were affected by host factors such as pre‐existing conditions and age. The SARS‐CoV strain was found to have an incubation period of 2–10 days [25]. It specifically targets the epithelial cells in the respiratory tracts causing damage throughout the organ, especially in the alveoli [26].
Before its containment, SARS cases were reported in 26 countries, with a majority of the cases concentrated in China [27]. At the height of the epidemic, there were approximately 8000 cases with 774 deaths reported [27]. Social distancing and traveler screening were used to mitigate the outbreak [28]. The virus is thought to have originated from palm civet cats, which suggests that there was a zoonotic shift to the human population [29]. SARS cases have been dormant since 2004 when the last naturally occurring case was reported. Quarantine measures and isolation led to the control of the SARS pandemic [30].
MERS‐CoV
In 2012, a betacoronavirus emerged as the second pathogenic coronavirus in the human population and originated in Saudi Arabia in September 2012 [31, 32]. This strain of betacoronavirus is currently the most lethal strain, with a mortality rate of ~ 32–33% [12]. Twenty‐seven countries have reported cases of Middle East respiratory syndrome (MERS) caused by this virus, MERS‐CoV, with 80% of these cases occurring in Saudi Arabia. As of January 2020, there have been 2519 cases of MERS with 866 associated deaths [33. There is evidence that MERS originated from camels and spread to humans via a zoonotic shift [34. The incubation period of MERS is approximately 5–6 days; however, symptoms can persist from anywhere between 2 and 14 days [35. Symptoms include fever, cough, and shortness of breath. More severe cases of the viral infection developed pneumonia and kidney failure.
Similar to SARS, patient outcomes were affected by host factors such as medical history and age. There were higher levels of mortality associated with patients that had a pre‐existing medical condition which weakened their immune system [33. Quick quarantine and isolation led to the control of the MERS pandemic [36].
SARS‐CoV‐2
On December 31, 2019, the first case of COVID‐19 in Wuhan, China, was reported to the WHO. On March 11, 2020, the virus was declared a global pandemic. As of April 2020, coronavirus has affected 214 countries and territories, spreading extremely quickly (Fig. 1) [37. There are over four million cases of COVID‐19 worldwide and over 270 000 deaths [38]. Similar to SARS and MERS, patient outcomes are affected by factors such as pre‐existing conditions and age. China's authorities reported that the highest mortality rate was for those above 80 years of age [39]. Although the origin of this pandemic is uncertain, it is widely believed that the disease spread from bats, which act as intermediate hosts between the virus and humans; this idea is being tested in ongoing research [40]. Additionally, there is a possibility that pangolins were the intermediate between bats and humans for SARS‐CoV‐2 transmission [41]. There is no evidence that this virus was made in a laboratory, and the overwhelming evidence suggests a zoonotic shift from animals to humans [42]. The incubation period of COVID‐19 can be up to 2 weeks with a median of 5 days; during this period, the virus can be transmitted to others [39].
Fig. 1.
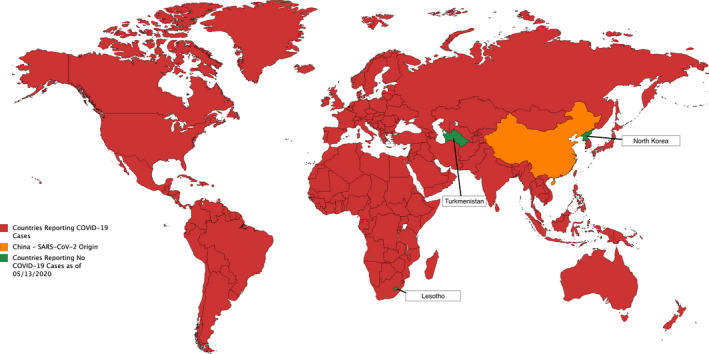
Reported cases of COVID‐19 by country adapted from CDC. Red represents China, the SARS‐CoV‐2 origin. Orange represents countries reporting COVID‐19 cases. Green represents major countries reporting no cases of COVID‐19 and includes Lesotho, North Korea, and Turkmenistan. Figure reproduced from [155].
Symptoms of the virus are similar to MERS and SARS and include fever, cough, and shortness of breath [39]. COVID‐19 is significantly more infectious than SARS and MERS in terms of human‐to‐human transmission, causing the number of cases to skyrocket and outweigh both MERS and SARS [43]. The R 0 for SARS is about 3 and for MERS 0.45, and early estimates placed this value for COVID‐19 at 2.2 to 3.11 [32, 44, 45]. While both SARS and COVID‐19 appear to have similar R 0, COVID‐19 has a higher viral load in the nose and throat of patients before symptoms develop, whereas SARS has a presence more directly tied to symptoms [46]. This suggests that COVID‐19 can be transmitted before the development of symptoms, making it harder to isolate and control [46].
Epidemiology of SARS‐CoV‐2
In over 80% of cases of COVID‐19, symptoms present as a mild fever, dry cough, and shortness of breath. Severe cases displayed symptoms such as dyspnea (shortness of breath) in 44% of patients, hypoxia (oxygen depletion in body tissues) in about 50% of patients, and a high fever in around 14% of total patients [47, 48, 49, 50]. Depending on the age, hospitalization rates in the United States range from 0.1% for ages 5–17 to 17.2% for ages 85 or above with 5% of total cases experiencing critical conditions such as shock and multi‐organ failure [47, 51]. There are two main clinical symptoms that appear for critically ill patients with COVID‐19: low levels of oxygen due to poor breathing (acute respiratory distress syndrome) and fevers [52, 53]. This lack of oxygen is usually addressed through mechanical ventilation in non‐COVID‐19 patients [54]. Additionally, it is thought that later phases of COVID‐19 bring a lack of oxygen due to low long compliance and sedation has been suggested in support of mechanical ventilation due to the large demand of drugs such as oxycodone and hydromorphone [54, 55, 56, 57].
However, in some cases, those confirmed with COVID‐19 are asymptomatic yet can still spread the disease [58]. For this reason and the long incubation period of up to 2 weeks, COVID‐19 has demanded extreme safety precautions to be implemented to minimize transmission and morbidity. This ‘flattens the curve’ of the projected number of infections and aims to stop healthcare services to be overwhelmed with so many new cases at once. Such precautions include social distancing (upholding 2 m distance from others), which would reduce the median estimated number of infections by 78% by some models [59, 60]. Despite the implementation of these measures, many countries are still experiencing infections at an exponential rate.
Notably, infection and death rates are not the same between countries, age‐groups, or even races [61]. Despite the worldwide mortality rate of 6.9%, death rates range from ~ 0.1% in Chile and Israel to 14% in Italy (note these are estimates based on numbers reported by the WHO as of May 8, 2020, and do not take account for asymptomatic or those infected who have not been tested) [44]. Countries are in various stages of their outbreaks, but there are still many factors that lead to the infection and death rate disparity such as population density, healthcare system, testing policy, and age structure of the country. Testing alone has proved to be a major determinant of the success of a country's response to this outbreak. The decision on whether to engage in mass testing has contributed to the quick control of the outbreak in countries such as Germany and South Korea, whereas the reluctance to provide mass testing of those exhibiting symptoms of infection done in the UK and United States has, arguably, prolonged the outbreak [62, 63]. A nation's testing system is especially pertinent given the possibility of disease transmission through asymptomatic carriers [64].
Moreover, the severity and onset of COVID‐19 vary dramatically for different ages with worsening symptoms with age [65]. The lowest risk is observed in those under 19 years of age with a mortality rate ranging from 0% to 0.1%, whereas those between 75 and 84 years of age have mortality rates ranging from 4.3% to 10.5%. The highest risk is seen in those aged 85 or above, with a mortality rate of 10.4–27.3% [65]. Underlying health conditions including diabetes, cardiovascular disease, or a suppressed immune system also increase the fatality rate [66].
Considering its high mortality rate in certain groups and high transmission rate, without proper treatment, the thousands of reported deaths from this virus might turn into millions without a coordinated and strategy‐based, worldwide response.
Structure of SARS‐CoV‐2
Like other coronaviruses, SARS‐CoV‐2 is a single‐stranded, positive‐sense RNA virus that uses spike proteins to bind to human lung epithelial cells (Fig. 2) [67]. The structure of the receptor‐binding region that the virus binds to, the angiotensin‐converting enzyme 2 (ACE2) receptor (Fig. 3), is the same target of the SARS‐CoV outbreak of 2003 [68]. It is these receptors that act as docking sites for the spike proteins of SARS‐CoV‐2 to bind to, allowing the viral and cellular membranes to fuse (Fig. 3). From there, the virus ‘hijacks’ the cell; it integrates its RNA into the cell's own replication machinery, facilitating propagation of the virus. The virus is then able to proliferate throughout the body, creating immune responses, and causing the person to become infected [69, 70].
Fig. 2.
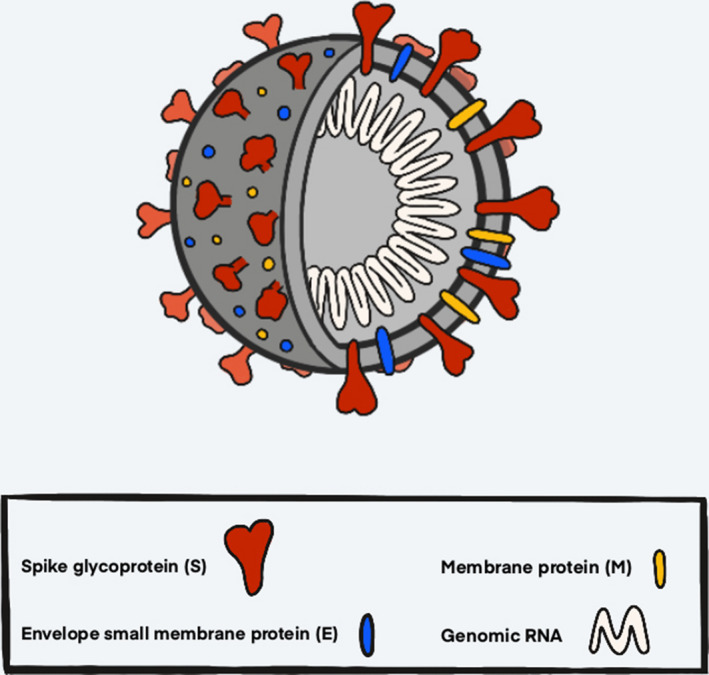
Diagram of SARS‐CoV‐2 virus structure. The spike glycoprotein (red) is the protein that binds to the ACE2 receptor of host cells and mediates viral entry. Additionally, this protein is what gives the virus its crown‐like (Latin ‘corona’) appearance. The membrane proteins (yellow) and the envelope small membrane proteins (blue) are important structurally as well as mechanistically. The genomic RNA (white) comprises the genetic material that the virus uses to propagate itself once inside its host.
Fig. 3.
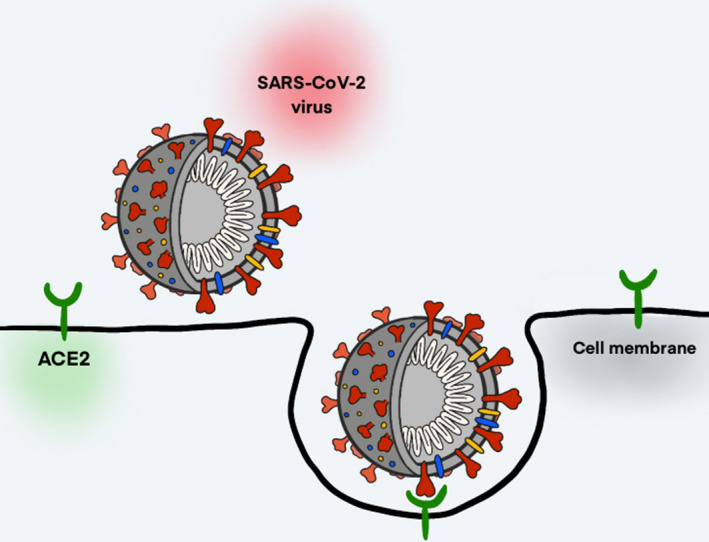
Diagram of SARS‐CoV‐2 entry into host cell. The spike glycoprotein (red), which consists of two subunits, binds to the ACE2 receptor (green) of host cells to merge the viral and cellular membranes and insert the viral genomic RNA (white) into the host cell. This induces endocytosis and the merging of the viral and cellular membranes, causing the viral genomic RNA to be inserted into the host cell and thus allowing the virus to replicate. The binding of SARS‐CoV‐2 to the ACE2 receptor causes a downregulation of this receptor, disrupting its normal function in maintaining immune homeostasis and leading to pro‐inflammatory effects that can cause lung injury.
A commonality among coronaviruses is the use of their envelope‐anchored homotrimeric spike glycoprotein (S protein) to mediate binding with host cell receptors (Fig. 3) [71]. The S protein is cleaved into two subunits during cell infection – the S1 subunit, which contains two receptor‐binding domains (RBDs) that allow the virus to bind to its host cell, and the S2 subunit, which is critical for membrane fusion [72].
The host cell receptor for the S1 subunit of the S protein, ACE2, is a transmembrane protein located on the cells of epithelial tissue in the lungs, heart, kidneys, and intestine [73]. Its primary physiological function is to regulate the maturation of the peptide hormone angiotensin, which helps regulate vasoconstriction and blood pressure [74]. Thus, the expression of ACE2 has anti‐inflammatory effects that can protect against lung injury, while its downregulation by the binding of SARS‐CoV or SARS‐CoV‐2 has pro‐inflammatory effects, which promote the severe acute lung injury symptomatic of infection by these viruses [75, 76]. Beyond its potential role in the pathology of COVID‐19, this receptor is directly linked to the infectivity and transmissibility of the SARS‐CoV‐2 virus. The fact that infectivity and transmissibility of SARS‐CoV‐2 are higher than those of SARS‐CoV is a result of the 10‐ to 20‐fold higher binding affinity of the SARS‐CoV‐2 RBD to the ACE2 receptor due to differences in the amino acid sequence of the SARS‐CoV‐2 RBD region which allow more interactions between the S protein and the host cell receptor [77, 78]. The binding affinity of the virus to host cell ACE2 receptors also dictates the pathway of intermediate host organisms that the virus could infect before being transmitted to humans and, therefore, which organisms could be used to study the virus [79, 80].
After the S1 subunit binds to the ACE2 receptor of the target cell, the heptad repeat 1 (HR1) and 2 (HR2) domains of the S2 subunit combine to create a six‐helix bundle core that brings the viral and host cell membranes within close proximity of each other for fusion to take place [80, 81]. Upon membrane fusion, the RNA of the coronavirus genome is released into the host cell cytoplasm via an early endosome – unlike SARS‐CoV, which employs a late endosome and therefore must cross higher barriers of antiviral host immunity – where it is translated into a replication–translation complex that in turn translates subgenomic RNA into accessory and structural proteins (Fig. 3) [82, 83, 84. These proteins form viral particle buds that are exocytosed to spread the virus to surrounding cells, thus spreading the infection throughout the host organism.
Mechanism of infection
Between infected hosts, COVID‐19 is primarily transmitted through contact with droplets that contain viral particles [85]. Droplets are any medium in which a human can release the virus, such as coughs, sneezes, and mucous. They generally cannot travel more than 2 m from their origin, though simulations investigating the effects of aerodynamics on the spread of these droplets suggest that fast physical activity, such as running or cycling, increases the distance they can travel [86, 87]. While it is generally thought that droplets do not linger in the air, in one study, SARS‐CoV‐2 was found to last in the air for 3 h in experimental conditions [88]. This disease can also spread by a person touching contaminated surfaces then subsequently touching their facial area (fomite‐mediated transmission). Depending on the material, the surfaces were shown to be infectious from several hours on cardboard to 3 days on plastics or stainless steel [88]. Despite these similarities to previous outbreaks and viruses, the ability of SARS‐CoV‐2 to stay on surfaces and affected individuals to not immediately display symptoms has made it difficult to contain and trace the virus, leading to the current global situation.
An understanding of the molecular mechanisms of this virus not only explains its increased transmissibility and the subsequent symptoms of infections, but also opens the door for a wide range of testing and treatment mechanisms that target the entry mechanism or epitopes of the virus.
Testing for the virus
RT‐qPCR‐based tests
The most prevalent form of testing right now utilizes quantitative reverse transcription–polymerase chain reaction (RT‐qPCR) [89]. This form of testing is widely used to combat the outbreak in places such as the United States, Hong Kong, Germany, Italy, and South Korea [90, 91, 92]. The test requires a nasopharyngeal swab to gather genetic material that will reveal whether or not the patient has the virus. The testing mechanism first requires the isolation of RNA and then the production of a single‐stranded complementary DNA (cDNA) copy of the RNA [93]. Finally, PCR is performed to amplify the cDNA for analysis, which, in all, takes hours to complete [94].
An important consideration for this testing method is navigating potential false‐negative and false‐positive results, which generally result from sample contamination [95]. In some instances, cases that have been suspected to be positive for COVID‐19 via computed tomography (CT) images were not diagnosed as positive by RT‐qPCR [95]. China has employed a high‐resolution CT to supplement their testing to ward out false negatives [96].
The challenge with carrying out this procedure on a global scale is that the current supply for swabs is limited and hard to distribute. Swab production facility locations can lead to delays and shipment issues, inhibiting the volume of testing [6]. Some countries, including the United States and Iceland, are struggling to find suppliers that will provide authorized nasal swabs for mass testing [97].
Recently, molecular testing has been made more accessible to organizations that are outside of the traditional hospital setting through the US‐based biotechnology company Abbott and the German technology company Bosch Healthcare Solutions. The Abbott RealTime SARS‐CoV‐2 Assay, which is their new automation systematic machine, reduces processing time from a few days to 15 min, because it reduces hands‐on time and increases patient analysis flexibility. Each test conducted for this machine is one cartridge and each cartridge elicits one test, which means that there will be a low throughput [98, 99]. The Vivalytic VRI (viral respiratory tract infections) COVID‐19 Test System pioneered by Bosch and Randox Laboratories is similar to the Abbott RealTime SARS‐CoV‐2 Assay in that it reduces hands‐on time and can confirm a positive test within 2.5 h with a reported 95% accuracy [100].
Serological tests
One of the most common and fastest forms of testing is the lateral flow immunoassay, which takes a genetic sample from the patient and measures whether or not they have antibodies against a virus [101]. This test, however, is not for determining whether the patient has the disease currently, but instead, it measures whether an individual has antibodies for a disease, which would indicate that they have mounted an immune response against the pathogen in the past. Companies like Abbott also have antibody test, and recently, England has approved a serological test pioneered by the Swiss biotechnology company Roche Diagnostics [102, 103]. Serological tests have a much higher success rate with these companies both boasting successes above 99% accuracy [104].
Another viable option is the enzyme‐linked immunosorbent assay (ELISA)‐based testing, because it is sensitive and is amenable to high‐throughput assays [105]. However, some of these serological assays introduced early in the pandemic lacked specificity, which may result in the generation of false positives, leading to overestimates of rates of infection [106]. More specific assays have now emerged that are proving very useful in providing a fuller picture of the rates of asymptomatic or mild SARS‐CoV‐2 infection, through detection of antiviral antibodies that persist for months and even years after the virus has been cleared [107].
Altogether, serological tests can serve as an indicator for how widespread the virus is, while RT‐qPCR tests can show who currently has the disease.
CRISPR‐based testing
Clustered regularly interspaced short palindromic repeats (CRISPR) technology has promise as a tool for disease detection [108]. Two enzymes that work in conjunction with CRISPR, Cas13 and Cas12, can be activated when specific sequences of RNA are detected. Once the enzymes are activated, they exhibit local RNase or DNase behavior, respectively, breaking down strands of RNA or single‐stranded DNA nearby; in a test scenario, this is usually measured with a fluorescent signal or activity on a lateral flow strip, which acts similarly to a pregnancy test. If the CRISPR‐Cas complex is combined with a reporter molecule, the enzyme breaks down the nearby reporter molecule, triggering a response.
When Cas13 is used, a process called SHERLOCK can detect the presence of an RNA virus such as SARS‐CoV‐2 [109]. Cas13 is activated when it is guided to the specified sequence and starts to break down nearby RNA strands. When a RNA sequence reporter molecule is combined with the CRISPR‐Cas13 sequence, Cas13 will break it down, signaling the presence of a specific RNA strand, such as a virus, through fluorescence or a lateral flow strip. Cas12 can also be used in a process very similar to SHERLOCK, called DETECTR, that shows the presence of a virus by activating Cas12 to break down a DNA‐based reporter molecule [110]. DETECTR technology is faster than SHERLOCK, but both are being researched and tried as future testing methods. Recently, the company Sherlock Biosciences has received FDA emergency use approval (EUA) for its SHERLOCK‐based COVID‐19 test, which the first EUA with CRISPR technology [111].
Drug development for the treatment of COVID‐19
Currently, there is no available cure or vaccine for COVID‐19 and the fastest timescale for development of a vaccine is estimated to be between 12 and 18 months [112]. In the absence of these long‐term and sustainable treatments, doctors and healthcare professionals are working on managing critical respiratory symptoms (dyspnea and hypoxia) from COVID‐19 through the use of mechanical ventilation [113]. In a case study on 2634 hospitalized patients in New York, about 12% of the patients required ventilation and the mortality among those receiving ventilation was 88% [113]. The repurposing of drugs has also shown promising results for a possible treatment of COVID‐19 symptoms: The WHO has outlined four drugs as potential therapeutic candidates for COVID‐19 in their Solidarity Project: remdesivir, lopinavir/ritonavir, interferon beta‐1a, and hydroxychloroquine/chloroquine [114]. Of these, remdesivir currently shows the most promise [115]. However, further studies have shown the promise of other drugs – EIDD‐2801 and favipiravir [116, 117]. Analyzing the pathways of these drugs not only provides a greater depth of understanding of the virus, but also reveals possible targets for the creation of an antiviral drug.
Remdesivir
A drug originally created to be used against the Ebola virus, remdesivir has recently been discovered to be effective in preventing the proliferation of SARS‐CoV and MERS‐CoV [115]. The structure of remdesivir prevents the propagation of viruses by blocking a crucial piece of RNA duplication machinery – the RNA‐dependent RNA polymerase (RdRp) [118].
In a study of remdesivir, the drug was administered to mouse cell lines infected with SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 and was able to inhibit the spread of the SARS‐CoV‐2 virus [115]. For this reason, there is excitement about the potential of this drug. Currently, several phase II/III trials are underway with one highly powered (large sample size) trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) in the United States, reporting patients had a 31% faster time to recover when taken compared to a placebo [119, 120]. This has been one of the few trials highly powered with a use of a placebo to generate positive results leading to its emergency authorization use by the FDA [121]. As a result, remdesivir has become one of the most promising drugs to fight COVID‐19, being tested around the world by countries such as China, Germany, Italy, and South Korea, among others [122]. Further studies and results will be needed to determine its efficacy.
Favipiravir
Favipiravir (also known as Avigan) similarly selectively inhibits RdRp through preventing it from binding to its energy source (ATP), thus making it nonfunctional [118]. Its efficacy is promising, with China's Science and Technology Ministry calling it an ‘effective treatment’ with ‘a high degree of safety’, as many symptoms such as fever and cough subsided quicker when taking the drug [122]. Even given these positive results, Japan is one of few countries heavily evaluating the drug with Fujita Health University performing phase II trials on the drug [122].
Hydroxychloroquine/chloroquine
Hydroxychloroquine and chloroquine are two safe, inexpensive drugs known mainly for their FDA‐approved treatment against malaria and autoimmune diseases, but also have been found to have antiviral activity [123, 124]. Viruses usually enter the body and infect cells through endocytosis, which packages the virus and brings it into the cell via an endosome. However, hydroxychloroquine/chloroquine increases the endosome's pH, making it too basic for other viruses to survive and replicate [115]. Given its hydrophobic structure, the drug can circulate throughout the blood and the effects can spread throughout the body including the lungs, making it a strong candidate for treatment against COVID‐19 [115]. Tests of hydroxychloroquine and chloroquine against COVID‐19 in China and France have proven promising but only studied small sample sizes [125].
Nevertheless, due to the minimal known side effects of the drug, the FDA has authorized for emergency use by healthcare professionals [126]. There are future hopes that this is a drug that everyone could take to prevent COVID‐19 (prophylaxis approach) and federal regulators in the United States have removed many regulations that have allowed for testing to hit ‘warp speed’ [127]. However, there are other studies suggesting no/limited effect of chloroquine, or even severe side effects [128, 129, 130, 131]. Some hospitals have also stopped using this drug for these reasons.
Lopinavir/ritonavir
Lopinavir and ritonavir, referred to as Kaletra when combined, are mainly used to combat human immunodeficiency virus (HIV) infection by acting as a protease inhibitor that would prevent the cutting of certain proteins needed for HIV replication [132]. After initial reports of success from China and South Korea, scientists were excited by the potential of lopinavir/ritonavir as a treatment against COVID‐19 [133]. Yet, when tested on hospitalized infected adults in isolation from other drugs, lopinavir/ritonavir showed no significant benefit/improvement, indicating that the protease inhibition was not the same for SARS‐CoV‐2 as for HIV, ceasing future testing of the drug [132, 133].
Interferon beta‐1a
Interferons are a natural component of the immune system that activate antiviral machinery in order to inhibit replication and increase immune response [134]. This process can be sped up via interferon injection [134]. Given its ability to stimulate active immunity, previous studies have tested alpha‐ and beta‐types of interferons against MERS and SARS [135]. It was identified that the type 1 interferon of a beta‐subtype was most effective against coronaviruses as it upregulates anti‐inflammatory cells in the lungs [135]. Early testing of interferons in human epithelial cell lines showed a reduction in viral concentration by injection and a reduction in infection rates by administration of interferons by spray, in support of interferon‐based prophylaxis [135, 136]. Currently, interferon beta‐1a is a specific type of interferon available in the market to help mitigate the symptoms of multiple sclerosis, but further research is needed to see its effects in humans [135].
EIDD‐2801
EIDD‐2801 can both serve as a preventable and treatable medicine through oral intake. In contrast to remdesivir, which may treat COVID‐19 by inhibiting RdRp [102], EIDD‐2801 induces lethal mutagenesis by accumulating deleterious transition mutations in the viral RNA while having many off‐target effects [116]. The results of EIDD‐2801 have shown the greatest promise out of all these drugs, having been shown to reduce the concentration of SARS‐CoV‐2 as well as other coronaviruses (MERS and SARS) when taken prophylactically [116]. While these results have only been observed in mice, its ability to be easily taken orally and as a potential preventative treatment makes it a strong candidate for continued research. As of April 7, 2020, the FDA has given the company responsible for EIDD‐2801 approval to perform human trials [137].
Overall, the antiviral activities of these drugs, with the exception of lopinavir/ritonavir, have shown some level of efficacy against COVID‐19 using different pathways. EIDD‐2801, remdesivir, and favipiravir all mitigate SARS‐CoV‐2 by stopping the RNA‐dependent RNA polymerase from replicating the virus further. By contrast, hydroxychloroquine/chloroquine targets endosomes, while interferon beta‐1a takes advantage of the body's innate immunity increasing inflammatory responses. Trying to evaluate the level of success of these drugs against COVID‐19 is difficult due to their different points of research; however, remdesivir is the one of the few drugs to have a high‐powered experiment that favors positive results, which has led it to become one of the more promising and frequently tested drugs around the world [122]. While there is no definite answer yet, testing of these drugs has garnered greater understanding of the viral mechanism that could further aid other drug repurposing or synthesis for a possible cure.
Other possible treatments for COVID‐19
CRISPR
Along with possible drugs, new technologies have been utilized preemptively to counter the symptoms of SARS‐CoV‐2. CRISPR‐Cas13 has been utilized to target essential parts of the SARS‐CoV‐2 virus, through an approach called PACMAN (Prophylactic Antiviral CRISPR in huMAN cells) [138]. Similar to SHERLOCK‐based virus detection, PACMAN utilizes Cas13, which has RNase activity that can be used for both the detection (see above) and the destruction of SARS‐CoV‐2 (Fig. 4) [139]. This aspect of Cas13 can be utilized to destroy the virus with pinpoint accuracy, while also knocking out the RdRps. The main roadblock to using this technology is delivery. Some of the problems associated with the delivery of CRISPR‐Cas13 have been researched, and possible liposomal delivery systems have been established [138]. While these treatments still require further testing, there is promising research in the use of CRISPR as a means of targeting the virus and stopping symptoms, as well as utilizing PACMAN to prevent future pandemics.
Fig. 4.
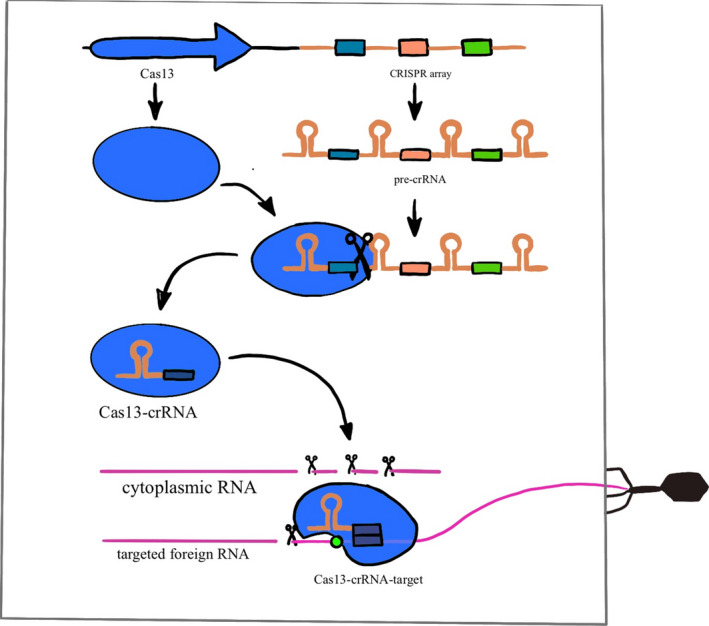
The top of the drawing represents the DNA encoding for the Cas 13 protein and the CRISPR array, which contains the targets for Cas13 cleavage and subsequent degradation. This array is transcribed into pre‐crRNA. The Cas13 protein turns this transcript into mature crRNAs, forming a crRNA‐Cas13 complex that in turns searches along existing RNA transcripts for matching sequences known as protospacers. Once this complementary protospacer is found, Cas13 undergoes a conformational change to enhance binding and activates the RNA cleavage activity of Cas13, which can then be used to degrade foreign viral RNA entering the cell as shown on the right side of the image. Adapted from [156].
Antibodies
While antibodies indicate that a person has developed an immune response to the virus, there is no evidence that shows how long the person will be protected, if they are protected at all, from reinfection of COVID‐19. However, in a study of 23 patients that recovered from COVID‐19, it was found using ELISA that antibody concentration was associated with neutralizing activity [140]. This has led healthcare professionals to consider blood transfusion of convalescent plasma as a possible treatment. In a case study of five critically ill patients treated with plasma, four out of the five patients exhibited normalized body temperatures and decreased viral load, suggesting that the antibodies have antiviral activity [141].
Using ELISA, scientists from Utrecht University in the Netherlands were able to identify a reactive human monoclonal antibody (47D11) that blocks SARS‐CoV‐2 infection [141]. Similar to the antibodies found in the blood plasma transfusions, 47D11 antibody is able to reduce viral activity, while also having the capability to be synthesized in a laboratory. This leads to significant advantages compared to plasma as nothing has to be stored and there is not a limit to availability. With the recent evidence of 47D11's efficacy in cells, pharmaceutical companies are in a rush to produce a monoclonal antibody treatment as the world waits for an answer.
Vaccines against SARS‐CoV‐2
The vaccine development process is known to take years for full approval for mass production. However, many leaders in this area such as Anthony Fauci, the director of the US National Institute of Allergy and Infectious Diseases, have recently suggested that this process could take less than a year given the current need to combat COVID‐19. Meanwhile, researchers at Oxford University's Edward Jenner Institute for Vaccine Research have started testing a vaccine in humans and could have results of its efficacy by June [142, 143]. For most vaccines, this rapid progression would be seen as unrealistic, but the pressure needed to put this pandemic to halt has led to many regulatory agencies to speed up their approval process.
Typically, vaccine trials are three‐phase processes that are run over the course of several years. The first phase is meant to assess the safety of a vaccine, and so, it is injected into a small group of healthy volunteers. Next, in the second phase, the efficacy of the vaccine is tested in several hundred volunteers, including at‐risk groups. Finally, the third phase moves to testing in thousands of patients if the results are promising, and only then can a vaccine be mass‐produced. However, governments worldwide have encouraged companies to launch production of vaccine candidates despite the fact that none of them have completed the clinical trial process [144]. There are several different types of vaccines that scientists are researching to combat COVID‐19.
Live vaccines
This type of vaccine aims to provoke an immune response in vaccinated people by introducing a weakened version of the virus into their bodies. These live, attenuated vaccines are the closest thing to a natural infection, and so, they are very effective at creating an immune response [145]. The vaccines for measles, mumps, and chickenpox use this approach. However, children and immunocompromised individuals can have difficulties handling the live attenuated vaccines and evoking an immune response [146].
Inactivated vaccines
Another method of vaccination is to use a dead or inactivated virus, like in the polio vaccine. One upside to these vaccines is that there is no concern about the virus regaining pathogenicity and causing disease. However, booster shots are needed for continued protection because these vaccines trigger smaller immune responses [147].
Subunit vaccines
Rather than including the entire virus, subunit vaccines work by including only parts of the virus' structure or subunits [148]. For example, a subunit vaccine for a coronavirus strain may only include the S protein or parts of the S protein (Fig. 2). Similar to the inactivated vaccine, side effects are less common but booster shots are needed. Additionally, subunit vaccines may not be the most cost‐effective method of acquiring immunity against the coronavirus since their production is very costly.
Nucleic acid vaccines
The previously mentioned approaches to vaccine development are labor‐intensive and require scientists to work long hours cultivating and modifying the specific virus in the laboratory. Due to the urgency for a SARS‐CoV‐2 vaccine, researchers are trying novel approaches to vaccine development, with one such being a nucleic acid vaccine.
One of the first COVID‐19 vaccines to be approved for clinical trials was a RNA vaccine first tested in Seattle [149]. Rather than injecting a weakened or other altered forms of the virus into patients, this vaccine works by injecting mRNA directly into the patient. The mRNA then codes for viral proteins that would lead to an immune response from the body and thus giving individual protection from the disease should they come into contact with the virus again. Other types of nucleic acid‐based vaccines code for material that interferes with protein translation that is essential to the virus' propagation [150]. However, despite the fact that this technology has been around for about 30 years, nucleic acid vaccines have not yet earned approval since an effective delivery system is needed in order for these vaccines to match the efficacy of a live vaccine [151].
Viral vector vaccines
In clinical trials, research is also being conducted for viral vector vaccines for COVID‐19 [152]. In this particular vaccine, scientists engineer nonpathogenic viruses – or a viral vector – with the particular sequence that codes for the virus' antigen. This approach is being used by researchers at Oxford University, who engineered a nonreplicating chimpanzee adenovirus with the spike sequence of the SARS‐CoV‐2 spike protein [153]. This vaccine is particularly promising since it can elicit a strong immune response [154]. However, patients may have pre‐existing immunity to the vector, rendering the vaccine useless.
Even with all of these options available, developing a vaccine that can be distributed in mass to combat COVID‐19 is not without its challenges. Vaccines are developed from scratch for each target, and vaccines against similar coronaviruses cannot be simply repurposed for new uses.
It is important to note that while vaccines were developed for both the SARS‐CoV and MERS‐CoV outbreaks, they were not utilized in the public sphere because they did not complete clinical trials. Both of the SARS‐CoV and MERS‐CoV outbreaks were largely controlled due to containment, patient isolation, and social distancing. Nonetheless, as the SARS‐CoV‐2 continues to spread farther than its predecessors, the need for a vaccine only becomes more urgent.
Conclusions and perspectives
The 2020 SARS‐CoV‐2 pandemic has sparked advancement in coronavirus research. Data from previous coronavirus outbreaks have been used to enhance the study of treatment and prevention measures for COVID‐19. Studying the structure of the virus has allowed scientists to design testing processes rapidly, and new systems of testing are currently being designed to increase the speed and specificity of the process. The testing process is rapidly improving, yielding greater knowledge of who is or has been infected. These data could greatly advance regional decision‐making concerning when to lift restrictions and deciding when individuals can resume their usual day‐to‐day activities.
Treatments for COVID‐19 are also being rapidly developed to treat the symptoms of the disease, since no vaccine has currently been approved for mass distribution. Out of all the drugs tested, remdesivir has shown the most promising results. This drug has shown potential in low‐powered studies, but hopefully, further research will shine light on its efficacy in the short term. Vaccine production is underway, and many types of vaccines are currently being researched and tested so that a vaccine can be available as soon as possible. There are many options for possible treatments of COVID‐19, and, ultimately, it boils down to what is most effective, efficient, and available in the near future.
The combination of increased testing and efficient symptom treatment is an encouraging response to the pandemic. Currently, the spread of this virus shows promising decline, but these data will become more accurate as more people are tested and more time has passed. It is essential for governments to consider these factors before initiating the return to normal life.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
CLA, IM, RDPM, TMO, JP, KTLT, and HES reviewed the literature, wrote the manuscript, and revised the manuscript. CLA, IM, RDPM, TMO, JP, KTLT, and HES contributed equally to this manuscript. TPC carefully revised and cowrote the manuscript.
Acknowledgements
Before this pandemic occurred, all of these authors were undergraduate students in a discovery‐based research laboratory focused on projects centered on performing novel CRISPR‐Cas9 genetic knockouts in zebrafish under the discretion of Senior Lecturer Dr Thomas Clements at Vanderbilt University. When the Spring 2020 semester went online, the class decided to focus their efforts on learning everything they could about COVID‐19. We would like to thank Dr James Pask, the Introductory Biology Lab Coordinator, for creating and fostering an environment that encourages us to explore these endeavors. Additionally, we are grateful for the Biological Sciences Department at Vanderbilt University for funding our experiments. Lastly, we would like to thank the Ta Family for helping revise the citations.
References
- 1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY et al. (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med 382, 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol 5, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang X‐L, Wang X‐G, Hu B, Zhang L, Zhang W, Si H‐R, Zhu Y, Li B, Huang C‐L et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong JEL, Leo YS & Tan CC (2020) COVID‐19 in Singapore—current experience: critical global issues that require attention and action. JAMA 323, 1243–1244. [DOI] [PubMed] [Google Scholar]
- 5. Remuzzi A & Remuzzi G (2020) COVID‐19 and Italy: what next? Lancet 395, 1225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization Coronavirus. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019
- 7. Kelso JK, Milne GJ & Kelly H (2009) Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 9, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tay MZ, Poh CM, Rénia L, MacAry PA & Ng LFP (2020) The trinity of COVID‐ 19: immunity, inflammation and intervention. Nat Rev Immunol 20, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vabret A, Mourez T, Dina J, van der Hoek L, Gouarin S, Petitjean J, Brouard J & Freymuth F (2005) Human coronavirus NL63, France. Emerg Infect Dis 11, 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahn JS & McIntosh K (2005) History and recent advances in coronavirus discovery. Pediatr Infect Dis J 24, S223. [DOI] [PubMed] [Google Scholar]
- 11. Siddell SG, Anderson R, Cavanagh D, Fujiwara K, Klenk HD, Macnaughton MR, Pensaert M, Stohlman SA, Sturman L & van der Zeijst BAM (1983) Coronaviridae. Intervirology 20, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y & Gao GF (2016) Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC (2020) Coronavirus|Human coronavirus types. CDC, Atlanta, GA. [Google Scholar]
- 14. Masters PS (2006) The molecular biology of coronaviruses. In Advances in Virus Research (Kielian M, Mettenleiter T & Roossinck M, eds), pp. 193–292. Academic Press, Cambridge, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wevers BA & van der Hoek L (2009) Recently discovered human coronaviruses. Clin Lab Med 29, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaunt ER, Hardie A, Claas ECJ, Simmonds P & Templeton KE (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real‐time PCR method. J Clin Microbiol 48, 2940–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delamater PL, Street EJ, Leslie TF, Yang YT & Jacobsen KH (2019) Complexity of the basic reproduction number (R0). Emerg Infect Dis 25, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Driessche P & Watmough J (2008) Further notes on the basic reproduction number. In Mathematical Epidemiology (Brauer F, van den Driessche P & Wu J, eds), pp. 159–178. Springer, Berlin, Heidelberg. [Google Scholar]
- 19. Andreasen V (2011) The final size of an epidemic and its relation to the basic reproduction number. Bull Math Biol 73, 2305–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dietz K (1993) The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res 2, 23–41. [DOI] [PubMed] [Google Scholar]
- 21. Xu R‐H, He J‐F, Evans MR, Peng G‐W, Field HE, Yu D‐W, Lee C‐K, Luo H‐M, Lin W‐S, Lin P et al. (2004) Epidemiologic clues to SARS origin in China. Emerg Infect Dis 10, 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singhal T (2020) A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr 87, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CDC (2019) SARS|Basics factsheet. CDC, Atlanta, GA. [Google Scholar]
- 24. Breugelmans JG, Zucs P, Porten K, Broll S, Niedrig M, Ammon A & Krause G (2004) SARS transmission and commercial aircraft. Emerg Infect Dis 10, 1502–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau ALD, Chi I, Cummins RA, Lee TMC, Chou K‐L & Chung LWM (2008) The SARS (severe acute respiratory syndrome) pandemic in Hong Kong: effects on the subjective wellbeing of elderly and younger people. Aging Ment Health 12, 746–760. [DOI] [PubMed] [Google Scholar]
- 26. Peiris J, Lai S, Poon L, Guan Y, Yam L, Lim W, Nicholls J, Yee W, Yan W, Cheung M et al. (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361, 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan‐Yeung M & Xu R‐H (2003) SARS: epidemiology. Respirology 8, S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell DM (2004) Public health interventions and SARS spread, 2003. Emerg Infect Dis 10, 1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bolles M, Donaldson E & Baric R (2011) SARS‐CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr Opin Virol 1, 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S & Styra R (2004) SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis 10, 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du L, Yang Y, Zhou Y, Lu L, Li F & Jiang S (2017) MERS‐CoV spike protein: a key target for antivirals. Expert Opin Ther Targets 21, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Killerby ME, Biggs HM, Midgley CM, Gerber SI & Watson JT (2020) Middle east respiratory syndrome coronavirus transmission. Emerg Infect Dis 26, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization Regional Office for the Eastern Mediterranean WHO EMRO | MERS outbreaks | MERS‐CoV | Health topics. http://www.emro.who.int/health‐topics/mers‐cov/mers‐outbreaks.html
- 34. Nowotny N & Kolodziejek J (2014) Middle East respiratory syndrome coronavirus (MERS‐CoV) in dromedary camels, Oman, 2013. Eurosurveillance 19, 20781. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed AE (2018) Estimating survival rates in MERS‐CoV patients 14 and 45 days after experiencing symptoms and determining the differences in survival rates by demographic data, disease characteristics and regions: a worldwide study. Epidemiol Infect 146, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zumla A, Hui DS & Perlman S (2015) Middle East respiratory syndrome. Lancet 386, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CDC (2020) International locations with confirmed COVID‐19 cases. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 38. Coronavirus update (Live): 3,916,338 cases and 270,711 deaths from COVID‐19 virus pandemic – Worldometer. https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1?%22%20%5Cl%20%22countries%3Ca%20href=
- 39. Rothan HA & Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun 109, 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020) The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID‐19) — China. China CDC Weekly 2, 113–122. [PMC free article] [PubMed] [Google Scholar]
- 41. Wang C, Liu Z, Chen Z, Huang X, Xu M, He T & Zhang Z (2020) The establishment of reference sequence for SARS‐CoV‐2 and variation analysis. J Med Virol 92, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andersen KG, Rambaut A, Lipkin WI, Holmes EC & Garry RF (2020) The proximal origin of SARS‐CoV‐2. Nat Med 26, 450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Y, Gayle AA, Wilder‐Smith A & Rocklöv J (2020) The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med 27, taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization COVID‐19 situation reports. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports
- 45. Riou J & Althaus CL (2020) Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐nCoV), December 2019 to January 2020. Eurosurveillance, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J et al. (2020) SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382, 1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garg S (2020) Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 – COVID‐NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 69, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michelen M, Jones N & Stavropoulou C. In patients of COVID‐19, what are the symptoms and clinical features of mild and moderate cases? CEBM. https://www.cebm.net/covid‐19/in‐patients‐of‐covid‐19‐what‐are‐the‐symptoms‐and‐clinical‐features‐of‐mild‐and‐moderate‐case/
- 49. Auwaerter PG. Coronavirus COVID‐19 (SARS‐CoV‐2) | Johns Hopkins ABX guide. https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540747/all/Coronavirus_COVID_19__SARS_CoV_2
- 50. Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo‐Dannenburg G, Thompson H, Walker PGT, Fu H et al. (2020) Estimates of the severity of coronavirus disease 2019: a model‐based analysis. The Lancet Infectious Diseases 20, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M et al. (2020) Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 8, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L et al. (2020) Covid‐19 in critically Ill patients in the Seattle region – case series. N Engl J Med 382, 2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L & Camporota L (2020) COVID‐19 pneumonia: different respiratory treatments for different phenotypes?. Intensive Care Medicine, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gattinoni L, Quintel M & Marini JJ (2020) “Less is more” in mechanical ventilation. Intensive Care Med 46, 780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young PJ, Bailey MJ, Bass F, Beasley RW, Freebairn RC, Hammond NE, van Haren FMP, Harward ML, Henderson SJ, Mackle DM et al. (2019) Randomised evaluation of active control of temperature versus ordinary temperature management (REACTOR) trial. Intensive Care Med 45, 1382–1391. [DOI] [PubMed] [Google Scholar]
- 57. Ryan M & Levy MM (2003) Clinical review: fever in intensive care unit patients. Crit Care 7, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y et al. (2020) Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci 63, 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lewnard JA & Lo NC (2020) Scientific and ethical basis for social‐distancing interventions against COVID‐19. Lancet Infect Dis 20, 631–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anderson RM, Heesterbeek H, Klinkenberg D & Hollingsworth TD (2020) How will country‐based mitigation measures influence the course of the COVID‐19 epidemic? Lancet 395, 931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Dorn A, Cooney RE & Sabin ML (2020) COVID‐19 exacerbating inequalities in the US. Lancet 395, 1243–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Black JRM, Bailey C, Przewrocka J, Dijkstra KK & Swanton C (2020) COVID‐19: the case for health‐care worker screening to prevent hospital transmission. Lancet 395, 1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Al‐Muharraqi MA (2020) Testing recommendation for COVID‐19 (SARS‐CoV‐2) in patients planned for surgery – continuing the service and ‘suppressing’ the pandemic. Br J Oral Maxillofac Surg 20, 30164–30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gandhi M, Yokoe DS & Havlir DV (2020) Asymptomatic transmission, the Achilles' Heel of current strategies to control covid‐19. N Engl J Med 382, 2158–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Onder G, Rezza G & Brusaferro S (2020) Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA 323, 1775–1776. [DOI] [PubMed] [Google Scholar]
- 66. CDC People who are at higher risk for severe illness. CDC, Atlanta, GA. [Google Scholar]
- 67. Poltronieri P, Sun B & Mallardo M (2015) RNA viruses: RNA roles in pathogenesis, coreplication and viral load. Curr Genomics 16, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N‐H, Nitsche A et al. (2020) SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fehr AR & Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frieman M & Baric R (2008) Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation. Microbiol Mol Biol Rev 72, 672–685, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Astuti I & Ysrafil (2020) Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): an overview of viral structure and host response. Diabetes Metab Syndr Clin Res Rev 14, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walls AC, Park Y‐J, Tortorici MA, Wall A, McGuire AT & Veesler D (2020) Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell 181, 281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yan R, Zhang Y, Li Y, Xia L, Guo Y & Zhou Q (2020) Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science 367, 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kuba K, Imai Y & Penninger JM (2006) Angiotensin‐converting enzyme 2 in lung diseases. Curr Opin Pharmacol 6, 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li F (2015) Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol 89, 1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Verdecchia P, Cavallini C, Spanevello A & Angeli F (2020) The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. European Journal of Internal Medicine 20, 30151–30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C‐L, Abiona O, Graham BS & McLellan JS (2020) Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science 367, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. He J, Tao H, Yan Y, Huang S‐Y & Xiao Y (2020) Molecular mechanism of evolution and human infection with SARS‐CoV‐2. Viruses 12, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Qiu Y, Zhao Y‐B, Wang Q, Li J‐Y, Zhou Z‐J, Liao C‐H & Ge X‐Y (2020) Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS‐CoV‐2. Microbes Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S et al. (2020) Inhibition of SARS‐CoV‐2 (previously 2019‐nCoV) infection by a highly potent pan‐coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gao J, Lu G, Qi J, Li Y, Wu Y, Deng Y, Geng H, Li H, Wang Q, Xiao H et al. (2013) Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J Virol 87, 13134–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Burkard C, Verheije MH, Wicht O, van Kasteren SI, van Kuppeveld FJ, Haagmans BL, Pelkmans L & Rottier PJM (2014) Coronavirus cell entry occurs through the endo‐/lysosomal pathway in a proteolysis‐dependent manner. PLoS Pathog 10, e1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shirato K, Kawase M & Matsuyama S (2018) Wild‐type human coronaviruses prefer cell‐surface TMPRSS2 to endosomal cathepsins for cell entry. Virology 517, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo Y‐R, Cao Q‐D, Hong Z‐S, Tan Y‐Y, Chen S‐D, Jin H‐J, Tan K‐S, Wang D‐Y & Yan Y (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak – an update on the status. Mil Med Res 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dietz L, Horve PF, Coil DA, Fretz M, Eisen JA & Wymelenberg KVD(2020) 2019 Novel coronavirus (COVID‐19) pandemic: built environment considerations to reduce transmission. mSystems 5. https://msystems.asm.org/content/5/2/e00245‐20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. CDC (2020) Coronavirus disease 2019 (COVID‐19) – transmission. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 87. Blocken B, Malizia F, van Druenen T & Marchal T (2020). Towards aerodynamically equivalent COVID19 1.5 m social distancing for walking and running. Eindhoven, the Netherlands: Eindhoven University of Technology. Retrieved from http://www.urbanphysics.net/COVID19.html (version 21 April 2020). [Google Scholar]
- 88. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI et al. (2020) Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med 382, 1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bridge JA (2017) Reverse transcription‐polymerase chain reaction molecular testing of cytology specimens: pre‐analytic and analytic factors. Cancer Cytopathol 125, 11–19. [DOI] [PubMed] [Google Scholar]
- 90. Wong SC‐Y, Kwong RT‐S, Wu TC, Chan JWM, Chu MY, Lee SY, Wong HY & Lung DC (2020) Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 20, 30174–30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Konrad R, Eberle U, Dangel A, Treis B, Berger A, Bengs K, Fingerle V, Liebl B, Ackermann N & Sing A (2020) Rapid establishment of laboratory diagnostics for the novel coronavirus SARS‐CoV‐2 in Bavaria, Germany, February 2020. Eurosurveillance 25, 2000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lorusso A, Calistri P, Mercante MT, Monaco F, Portanti O, Marcacci M, Cammà C, Rinaldi A, Mangone I, Di Pasquale A et al. (2020) A “One‐Health” approach for diagnosis and molecular characterization of SARS‐CoV‐2 in Italy. One Health 10, 100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Freeman WM, Walker SJ & Vrana KE (1999) Quantitative RT‐PCR: pitfalls and potential. Biotechniques 26, 112–125. [DOI] [PubMed] [Google Scholar]
- 94. Romsos EL & Vallone PM (2015) Rapid PCR of STR markers: applications to human identification. Forensic Sci Int Genet 18, 90–99. [DOI] [PubMed] [Google Scholar]
- 95. Tahamtan A & Ardebili A (2020) Real‐time RT‐PCR in COVID‐19 detection: issues affecting the results. Expert Rev Mol Diagn 20, 453–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xia T, Li J, Gao J & Xu X (2020) Small solitary ground‐glass nodule on CT as an initial manifestation of coronavirus disease 2019 (COVID‐19) pneumonia. Korean J Radiol 21, 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Askham P (2020) From Iceland – COVID‐19 screening swab shortage continues. Reyk Grapevine. https://grapevine.is/news/2020/03/25/covid‐19‐screening‐swab‐shortage‐continues/
- 98. Comparison of two commercial molecular tests and a laboratory‐developed modification of the CDC 2019‐nCOV RT‐PCR assay for the qualitative detection of SARS‐CoV‐2 from upper respiratory tract specimens. medRxiv. https://www.medrxiv.org/content/10.1101/2020.05.02.20088740v1 [DOI] [PMC free article] [PubMed]
- 99. Abbott RealTime SARS‐CoV‐2 assay (EUA). Abbott Molecular. https://www.molecular.abbott/us/en/products/infectious‐disease/RealTime‐SARS‐CoV‐2‐Assay
- 100. VRI multiplex test. Vivalytic. https://www.bosch‐vivalytic.com/en/product/vivalytic‐tests/vri‐multiplex‐test/
- 101. Raeisossadati MJ, Danesh NM, Borna F, Gholamzad M, Ramezani M, Abnous K & Taghdisi SM (2016) Lateral flow based immunobiosensors for detection of food contaminants. Biosens Bioelectron 86, 235–246. [DOI] [PubMed] [Google Scholar]
- 102. Abbott launches COVID‐19 antibody test. https://www.abbott.com/corpnewsroom/product‐and‐innovation/abbott‐launches‐covid‐19‐antibody‐test.html
- 103. Roche's COVID‐19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark. Diagnostics. https://www.roche.com/media/releases/med‐cor‐2020‐05‐03.htm
- 104. Roche takes on Abbott in Covid‐19 antibody testing (2020). Evaluate.com. https://www.evaluate.com/vantage/articles/news/policy‐and‐regulation/roche‐takes‐abbott‐covid‐19‐antibody‐testing
- 105. Perera RA, Mok CK, Tsang OT, Lv H, Ko RL, Wu NC, Yuan M, Leung WS, Chan JM, Chik TS et al. (2020) Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), March 2020. Eurosurveillance 25, 2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Infantino M, Damiani A, Gobbi FL, Grossi V, Lari B, Macchia D, Casprini P, Veneziani F, Villalta D, Bizzaro N et al. (2020) Serological assays for SARS‐CoV‐2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J 22, 203–210. [PubMed] [Google Scholar]
- 107. Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J et al. (2020) A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med 1–4. 10.1038/s41591-020-0913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vidyasagar A (2018) What is CRISPR? livescience.com. https://www.livescience.com/58790‐crispr‐explained.html
- 109. Zhang F, Abudayyeh OO & Gootenberg JS. A protocol for detection of COVID‐19 using CRISPR diagnostics. 8. https://www.broadinstitute.org/files/publications/special/COVID‐19%20detection%20(updated).pdf
- 110. Chen JS, Ma E, Harrington LB, Costa MD, Tian X, Palefsky JM & Doudna JA (2018) CRISPR‐Cas12a target binding unleashes indiscriminate single‐stranded DNase activity. Science 360, 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sherlock Biosciences receives FDA emergency use authorization for CRISPR SARS‐CoV‐2 rapid diagnostic. Sherlock Biosciences. https://sherlock.bio/sherlock‐biosciences‐receives‐fda‐emergency‐use‐authorization‐for‐crispr‐sars‐cov‐2‐rapid‐diagnostic/
- 112. CDC (2020) Coronavirus disease 2019 (COVID‐19) situation summary. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 113. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell COVID‐19 Research Consortium , Barnaby DP, Becker LB, Chelico JD et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 323, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kupferschmidt K & Cohen J (2020) Race to find COVID‐19 treatments accelerates. Science 367, 1412–1413. [DOI] [PubMed] [Google Scholar]
- 115. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W & Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 30, 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, Leist SR, Schäfer A, Dinnon KH, Stevens LJ et al. (2020) An orally bioavailable broad‐spectrum antiviral inhibits SARS‐CoV‐2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 12. https://stm.sciencemag.org/content/12/541/eabb5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Furuta Y, Komeno T & Nakamura T (2017) Favipiravir (T‐705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 93, 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tchesnokov EP, Feng JY, Porter DP & Götte M (2019) Mechanism of inhibition of ebola virus RNA‐dependent RNA polymerase by remdesivir Viruses 11, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gilead Sciences Remdesivir Clinical Trials. https://www.gilead.com/purpose/advancing‐global‐health/covid‐19/remdesivir‐clinical‐trials
- 120. Gilead Sciences . Gilead announces results from phase 3 trial of investigational antiviral remdesivir in patients with severe COVID‐19. https://www.gilead.com/news‐and‐press/press‐room/press‐releases/2020/4/gilead‐announces‐results‐from‐phase‐3‐trial‐of‐investigational‐antiviral‐remdesivir‐in‐patients‐with‐severe‐covid‐19
- 121. Subramanian S (2020) Some FDA approved drugs exhibit binding affinity as high as ‐16.0 kcal/mol against COVID‐19 Main Protease (Mpro): a molecular docking study IndiaRxiv. https://indiarxiv.org/t7jsd/
- 122. Ito K, Ohmagari N, Mikami A & Sugiura W (2020) Major ongoing clinical trials for COVID‐19 treatment and studies currently being conducted or scheduled in Japan. Global Health & Medicine 2, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. CDC (2020) Coronavirus disease 2019 (COVID‐19). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 124. Savarino A, Di Trani L, Donatelli I, Cauda R & Cassone A (2006) New insights into the antiviral effects of chloroquine. Lancet Infect Dis 6, 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gao J, Tian Z & Yang X (2020) Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends 14, 72–73. [DOI] [PubMed] [Google Scholar]
- 126. FDA emergency use authorization. FDA. https://www.fda.gov/emergency‐preparedness‐and‐response/mcm‐legal‐regulatory‐and‐policy‐framework/emergency‐use‐authorization
- 127. Stricker RB & Fesler M (2020) Pre‐exposure prophylaxis with hydroxychloroquine for COVID‐19 disease. Open Science Framework. https://www.trialsitenews.com/pre‐exposure‐prophylaxis‐for‐covid‐19‐lessons‐from‐the‐past‐and‐the‐search‐for‐new‐options/
- 128. Suranagi UD, Rehan HS & Goyal N (2020) Review of current evidence of hydroxychloroquine in pharmacotherapy of COVID‐19. medRxiv. 10.1101/2020.04.16.20068205 [DOI]
- 129. Shamshirian A, Hessami A, Heydari K, Alizadeh‐Navaei R, Ebrahimzadeh MA, Yip GW, Ghasemian R, Behnamfar M, Baradaran H, Aboufazeli E et al. (2020) Hydroxychloroquine versus COVID‐19: a rapid systematic review and meta‐analysis. medRxiv 10.1101/2020.04.14.20065276 [DOI]
- 130. Mahevas M, Tran V‐T, Roumier M, Chabrol A, Paule R, Guillaud C, Gallien S, Lepeule R, Szwebel T‐A, Lescure X et al. (2020) No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID‐19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv 10.1101/2020.04.10.20060699 [DOI]
- 131. Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS & Ambati J (2020) Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid‐19. medRxiv, 10.1101/2020.04.16.20065920 [DOI] [PMC free article] [PubMed]
- 132. Cvetkovic RS & Goa KL (2003) Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs 63, 769–802. [DOI] [PubMed] [Google Scholar]
- 133. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M et al. (2020) A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med 382, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rönnblom L & Leonard D (2019) Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med 6, e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sallard E, Lescure F‐X, Yazdanpanah Y, Mentre F & Peiffer‐Smadja N (2020) Type 1 interferons as a potential treatment against COVID‐19. Antiviral Res 178, 104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R & Menachery VD (2020) SARS‐CoV‐2 is sensitive to type I interferon pretreatment. bioRxiv 10.1101/2020.03.07.982264. [DOI] [PMC free article] [PubMed]
- 137. Halford B. An emerging antiviral takes aim at COVID‐19. Chem Eng News. https://cen.acs.org/pharmaceuticals/drug‐development/emerging‐antiviral‐takes‐aim‐COVID‐19/98/web/2020/05
- 138. Abbott TR, Dhamdhere G, Liu Y, Lin X, Goudy L, Zeng L, Chemparathy A, Chmura S, Heaton NS, Debs R et al. (2020) Development of CRISPR as a prophylactic strategy to combat novel coronavirus and influenza. bioRxiv 10.1101/2020.03.13.991307 [DOI] [PMC free article] [PubMed]
- 139. O'Connell MR (2019) Molecular mechanisms of RNA targeting by Cas13‐containing type VI CRISPR‐Cas systems. J Mol Biol 431, 66–87. [DOI] [PubMed] [Google Scholar]
- 140. To KK‐W, Tsang OT‐Y, Leung W‐S, Tam AR, Wu T‐C, Lung DC, Yip CC‐Y, Cai J‐P, Chan JM‐C, Chik TS‐H et al. (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis 20, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, van Kuppeveld FJM, Haagmans BL, Grosveld F & Bosch B‐J (2020) A human monoclonal antibody blocking SARS‐CoV‐2 infection. Nat Commun 11, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kuznia R.The timetable for a coronavirus vaccine is 18 months. Experts say that's risky. CNN. https://www.cnn.com/2020/03/31/us/coronavirus‐vaccine‐timetable‐concerns‐experts‐invs/index.html
- 143. Kamisar B. Oxford scientist says its vaccine is making headway, could show efficacy by June. NBC News. https://www.nbcnews.com/politics/meet‐the‐press/oxford‐scientist‐says‐its‐vaccine‐making‐headway‐could‐show‐efficacy‐n1198946
- 144. Loftus P (2020) WSJ news exclusive | Drugmaker moderna delivers first experimental coronavirus vaccine for human testing. Wall Str J. https://www.wsj.com/articles/drugmaker‐moderna‐delivers‐first‐coronavirus‐vaccine‐for‐human‐testing‐11582579099
- 145. Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K & Roberts A (2005) Long‐term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV‐based vaccine. Virology 340, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Detmer A & Glenting J (2006) Live bacterial vaccines – a review and identification of potential hazards. Microb Cell Fact 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Prompetchara E, Ketloy C & Palaga T (2020) Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38, 1–9. [DOI] [PubMed] [Google Scholar]
- 148. He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M & Jiang S (2004) Receptor‐binding domain of SARS‐CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 324, 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lanese N (2020) First coronavirus vaccine trial in the US is recruiting volunteers. livescience.com. https://www.livescience.com/us‐coronavirus‐vaccine‐trial‐recruiting.html
- 150. Jin H, Xiao C, Chen Z, Kang Y, Ma Y, Zhu K, Xie Q, Tu Y, Yu Y & Wang B (2005) Induction of Th1 type response by DNA vaccinations with N, M, and E genes against SARS‐CoV in mice. Biochem Biophys Res Commun 328, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tan L & Sun X (2018) Recent advances in mRNA vaccine delivery. Nano Res 11, 5338–5354. [Google Scholar]
- 152. Amanat F & Krammer F (2020) SARS‐CoV‐2 vaccines: status report. Immunity 52, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. About the COVID‐19 vaccine trials. Univ Oxf COVID‐19 Oxf Vaccine Tral. http://www.ox.ac.uk/news/2020‐05‐22‐oxford‐covid‐19‐vaccine‐begin‐phase‐iiiii‐human‐trials
- 154. Bråve A, Ljungberg K, Wahren B & Liu MA (2007) Vaccine delivery methods using viral vectors. Mol Pharm 4, 18–32. [DOI] [PubMed] [Google Scholar]
- 155. CDC (2020) International locations with confirmed COVID‐19 cases. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 156. Burmistrz M, Krakowski K & Krawczyk‐Balska A (2020) RNA‐targeting CRISPR‐Cas systems and their applications. Int J Mol Sci 21, 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]


