Abstract
During the COVID‐19 pandemic, incidence rates for dental diseases will continue unabated. However, the intent to prevent the spread of this lethal respiratory disease will likely lead to reduced treatment access due to restrictions on population movements. These changes have the potential to increase dental‐related emergency department visits and subsequently contribute to greater viral transmission. Moreover, dentists experience unique challenges with preventing transmission due to frequent aerosol‐producing procedures. This paper presents reviews and protocols implemented by directors and residents at the Dental College of Georgia to manage a dental emergency clinic during the COVID‐19 pandemic. The methods presented include committee‐based prioritization of dental patients, a multilayered screening process, team rotations with social and temporal spacing, and modified treatment room protocols. These efforts aid in the reduction of viral transmission, conservation of personal protective equipment, and expand provider availability. These protocols transcend a university and hospital‐based models and are applicable to private and corporate models.
Keywords: COVID‐19, dentistry, emergency
1. INTRODUCTION
Evidence suggests that the dental profession poses a greater risk for transmitting illnesses due to aerosol‐producing procedures. 1 , 2 , 3 As such, dental professionals pose unique epidemiological risks during a pandemic such as coronavirus disease 2019 (COVID‐19); the disease is caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 4 At the same time, dental providers take on moral and ethical responsibilities that require balancing individual patient needs with these epidemiological considerations. In the case of the COVID‐19 outbreak, social distancing (maybe more aptly described as “spatial” or “physical” distancing) has been recommended by the Centers for Disease Control (CDC) to “flatten” the epidemiological curve.
Social distancing requires individuals to restrict group sizes to 10 or less and maintain a physical distance of 6 feet or greater. This widely adopted protocol was designed to achieve a manageable ratio of COVID‐19 cases to available healthcare resources (i.e., “flatten the curve”). 5 , 6 , 7 Several countries that have implemented social distancing demonstrate its potential for success. Evidence from Singapore, China, and South Korea suggests decreased rates of transmission with the implementation of social distancing. 8 , 9 , 10 , 11 Social distancing is particularly important for COVID‐19. Inoculated individuals are reported to have up to 14 days of asymptomatic viral shedding resulting in rapid viral spread. 12 , 13 , 14 , 15 As a result, some health experts recommend self‐quarantining for 2 weeks after possible virus exposure. 16 Observing these protocols in the healthcare field are nearly impossible challenges, especially for healthcare providers that likely create vectors from biological aerosols during routine procedures. 17 , 18 , 19
In dental clinics, high‐speed handpieces and ultrasonic scalers are the primary aerosol producers; however, other less invasive dental treatments such as oral exams and intraoral radiographs may produce infectious droplets through gagging and coughing. 20 , 21 , 22 Literature shows that up to 97% of these aerosols may be removed from the field with use of high volume evacuation (HVE) suction; however, HVE is not always routinely used during these procedures. 23 , 24 During a pandemic with a contagion such as SARS‐CoV‐2, dental providers must implement greater precautions to mitigate the risk of viral spread via biological aerosols.
Unfortunately, social distancing has no effect on reducing rates of dental disease in the population. Therefore, it is reasonable to expect the incidence of dental emergencies to persist at known or greater levels throughout the pandemic period. 25 However, reduced access to dental care suggests an expected increase in dental‐related hospital emergency department visits, meaning otherwise healthy dental patients may be subjected to greater risk of COVID‐19. 26 Specific methods and protocols unique to dental providers are needed in order to provide necessary dental treatment to patients, observe recommended guidelines, reduce hospital emergency visits, and protect dentists and staff.
This paper presents literature reviews, methods, and protocols implemented by the Dental College of Georgia at Augusta University (DCG) in order to provide emergency dental care. Our protocols are designed to conserve personal protective equipment (PPE) and minimize provider exposure. This includes our novel temporal spacing protocol along with other protocols frequently used in hospitals. Moreover, these protocols can be adapted to private and corporate models.
2. REPORT
2.1. Defining dental emergencies
Defining emergencies was essential to limiting exposure and prioritizing patient care during the COVID‐19 pandemic. The American Dental Association (ADA) issued guidelines for emergency dental procedures. 27 These guidelines were developed in response to several states that mandated dental providers to perform emergency treatments only. 28 These ADA guidelines categorize dental procedures as either emergent, urgent, or nonemergent. According to the ADA, dental emergencies include but are not limited to pain from pulpitis, pericoronitis, alveolar osteitis, tooth fractures, abscesses/local swellings, dental trauma, or loss of temporary restorations. 27 In contrast, general healthcare emergencies are considered life‐threatening conditions. Urgent conditions are not life threatening; however, failure to address urgent conditions in a timely manner may lead to an emergency. 29 , 30 Therefore, the DCG chose to define emergencies as potential life‐threatening conditions, and limited procedures to infections with spatial swelling, trismus, and/or trauma (Figure 1). These differentiations were necessary due to the large patient base that included cases from students, faculty, and seven specialty programs (approximately 13 000 active patients) as well as emergency referrals of nonrecord patients. The goal for this protocol was to immediately address all patients with true dental emergencies. Once achieved, urgent cases were scheduled based on the evaluation of the Emergency Clinic Triage Committee (ECTC) (Figure 1). This committee prioritized urgent cases with the greatest likelihood of becoming an emergency. Once prioritized, these cases were placed on either the treatment schedule, screening schedule, or a nonemergent waitlist. DCG practitioners acted as teledentistry representatives. This allowed greater communication with patients and allowed for adequate screening prior to the patient arriving at the dental office. In many cases, this process alleviated the need for in‐office visits by recommending over‐the‐counter pain management regiments and in select cases antibiotic prescriptions. Antibiotics were prescribed based on the antibiotic stewardship guidelines from the ADA. 31 Urgent but not emergent patients would only be scheduled on days that emergency patients as defined above were scheduled. The above protocols were performed in order to preserve N95 masks and maintain a sustainable emergency clinic workflow.
FIGURE 1.
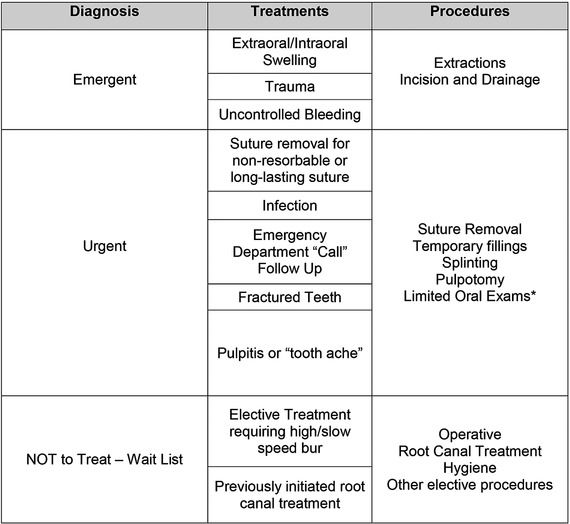
Emergency Clinic Triage Committee (ECTC) triage table. This multidisciplinary committee included all departments and specialties. Collectively, departmental representatives developed these necessary standards to ensure that all respective patients received appropriate care and limited provider exposure. All procedures were evaluated by the committee to determine severity and priority, with a list of examples procedures appropriate for each diagnosis. High‐speed handpieces were used at provider discretion. However, this was not a determining factor in determining patient priority. Patients were treated based on disease severity, and appropriate treatment modalities were used as needed
2.2. Pandemic‐related emergency clinic workflow
The workflow of the DCG emergency clinic during the COVID‐19 pandemic was important to conserve limited N95 masks, treat as many emergencies as possible, and limit staff and provider exposure. Therefore, all clinics at DCG were redirected to the General Practice Residency (GPR) clinic for emergencies. All patients that received treatment were based on a multilayered triage process (see below). Two to four dental practitioners were scheduled daily to provide treatment for all emergencies during their scheduled week, and each practitioner received a single N95 mask. The scheduled practitioners were selected from rotating teams (described below). Our emergency clinic triage process involved three levels (Figure 2).
FIGURE 2.
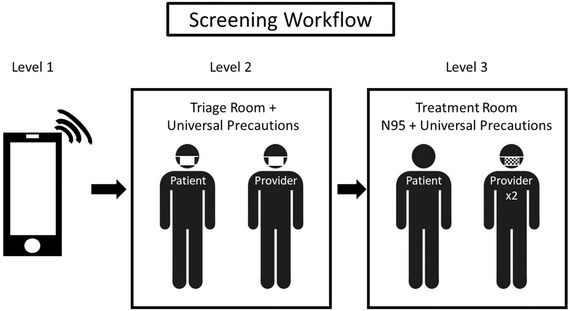
Screening workflow. Level 1 screenings are phone interviews. Level 2 are pretreatment physical follow‐ups in a triage room. Level 3 requires a predetermined treatment room with designated dailyproviders wearing N95 masks and using modified treatment and PPE protocols
Level 1 consisted of a phone interview. Open‐ended questions were scripted by the ECTC committee to assist with the phone interviews. This calibrated practitioners and ensured that patients met established criteria for emergent or urgent treatment. In addition, phone interviews helped to determine a patient's possible exposure to COVID‐19, determine if there were any active or positive COVID‐19 tests, and/or if the patient exhibited COVID‐19 symptoms. COVID‐19 symptoms include fever, dry cough, and shortness of breath. 32 COVID‐19 positive or symptomatic patients were referred to Augusta University Hospital for nondental related care prior to any dental treatment. All dental emergencies that were asymptomatic to COVID‐19 proceeded to Level 2 (Figure 2).
Level 2 screenings included physical assessments in a designated room separate from treatment rooms. This designated room was similar to a triage room used in hospitals. Level 2 screenings required patients to wear level 1 masks (i.e., surgical masks), have vitals recorded, and patients were cursorily evaluated by a dental provider (Figure 2). This provider wore universal precautions with a level 1 mask. Evidence supports prevention of viral transmission from contagious individuals that wear level 1 masks conserving N95 masks. 33
Following physical evaluation, patients were then brought to a separate designated treatment area (Level 3). The treatment providers (usually one oral surgery resident and one general practice resident) at Level 3 wore disposable gown, surgical cap, shoe coverings, and N95 masks covered with a secondary mask with either an attached face shield or visor supported face shield to further evaluate and provide treatment (Figure 2). These providers treated all scheduled patients, and alternated as operators and assistants based on clinical expertise. For example, oral surgery residents were the primary providers for facial fractures, infections that jeopardized patient airway, and oral surgery specific postoperative follow‐ups. In contrast, general practice residents served as primary for implant and periodontal postoperative surgery, endodontic related issues, pediatric patients older than 14 years, infections that did not threaten airway, and other odontogenic related issues. Either resident on the operator team could extract teeth. The operator team treated all scheduled patients during their scheduled week. This method does not reduce provider exposure. Instead, it limits the number of exposed providers. All PPE was doffed (see protocol below) in the patient room except for the N95 mask. Extended use guidelines were applied to the N95 masks, and these masks were sterilized by ultraviolet germicidal irradiation (UGI) at the end of each day. 34 Due to limited supply of N95 masks, the ideal single use was not permissible. New PPE would be donned before entering a new patient room. In addition to these screening modifications, procedure modifications reduced biological aerosols.
2.3. Modifications for emergency and urgency treatment
This section includes general recommendations to reduce aerosols for all pandemic‐related emergent and urgent dental procedures. Due to logistics of implementing all recommendations, not all were used at the DCG. In order to control potential clinic exposure and preserve PPE, the ECTC developed a list of urgent patients. On days with emergency patients, these urgent patients were appointed concomitantly. Triage rooms, described above, were used for all patients. All patients receiving treatment rinsed for 30 seconds with 0.12% chlorhexidine (CHX) and 30 seconds with 1.5% hydrogen peroxide solution. Preoperative rinses with CHX demonstrate a significant reduction in colony forming units and postoperative infections. 35 , 36 , 37 Other literature indicates that SARS‐CoV‐2 may be more vulnerable to oxidative destruction. 22 , 38 The use of CHX mouth rinse is primarily intended to reduce the bacterial load in the mouth and resulting aerosols. It is commonly utilized at the DCG prior to any surgical procedure. As such, CHX was not abandoned in favor of a hydrogen peroxide rinse; rather, the hydrogen peroxide was added to the preoperative regiment due to its potential effectiveness against SARS‐CoV‐2. Intraoral radiographs may cause gagging and were replaced, when possible, with extraoral imaging modalities such as cone beam computed tomography and/or a panoramic radiograph. 26 HVE suction and rubber dams have been shown to reduce biological aerosols during dental procedures by up to 99%, and they were implemented whenever possible. 3 , 23 , 39 , 40 All elective treatment was deferred, especially prophylactic treatments that use ultrasonic scalers. Ultrasonic instruments and handpieces create the greatest amount of biological aerosols. 3 Handpieces should be used only when absolutely necessary. Examples include, tooth sectioning, bone troughing, and pulpotomies. Resorbable sutures are recommended for all surgical procedures in order to reduce postoperative follow‐up appointments. At the DCG, suture removal cases were managed with phone interviews unless a case specific issue necessitated an urgency appointment. For example, nonresorbable sutures (e.g., nylon) placed prior to COVID‐19 restrictions were treated as urgent cases. All patients at DCG were considered as potential asymptomatic carriers, therefore, other general recommendations include flushing water lines and modifying room ventilation when possible. Although it is not recommended to use the air‐water combination due to aerosolization, waterlines and suctions, as recommended by the CDC, should be discharged for 30 seconds after each patient. 41 Negative pressure rooms are frequently used in hospitals in order to reduce aerosol transmissible infections. 42 The authors recognize that negative pressure rooms are neither widely available nor practical in many dental operatories, but they are the standard of care for airborne precautions according to the CDC's Environmental Infection Control Guidelines. 43 A more pragmatic method, especially due to the currently understood transmission of the SARS‐CoV‐2 virus via droplet transmission, includes the use of air filtration systems that utilize high‐efficiency particle air (HEPA) filtration with or without the addition of UGI. Properly set up, these units can remove up to 99% of airborne pathogens in as little as 23 minutes leading to improved air quality for providers and decreased time required for safe room turnover. 44 Note that the DCG was unable to implement negative pressure rooms or air filtration devices at the time of submission for this article, instead we relied on time between room uses to reduce concentration of aerosol infection particles.
2.4. Team rotations and temporal spacing
The CDC recommends social distancing and self‐quarantining of potentially exposed persons. 5 All patients were assumed asymptomatic carriers, and the greatest risk of exposure was assumed with high speed handpiece use. Therefore, the directors and residents at DCG restructured provider care in order to comply with the CDC recommendations. In addition, we took advantage of our large group of providers by developing teams and temporal spacing (Figure 3). This novel technique is designed based on the incubation period of the COVID‐19 and the above recommendations.
FIGURE 3.
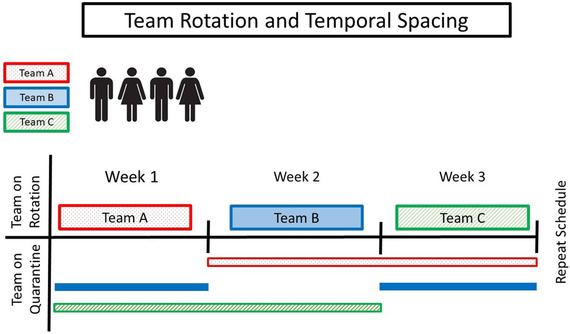
Temporal spacing through team rotations. Three selected teams placed on a 3‐week cycle. Each team provides dental care for 1 week and is then placed in self‐quarantine for 2 weeks
Due to the COVID‐19 incubation period of 1‐14 days, three on‐service teams were created. At the DCG, these teams consisted of two oral surgery residents and four general practice residents per week; however, efforts were made to restrict the number of providers for direct patient care. Our teams and their corresponding sizes were based on equal division of available residents. The authors acknowledge that this will vary among institutions. Additional residents in the on‐service team and the remaining residency programs served as support (i.e., calling, screening, and scheduling patients). The DCG is an educational setting, and clinical faculty were available for consultation, but they did not interact with patients directly reducing their potential exposure. Each team served for 1 week, followed by 2 weeks of self‐isolation (Figure 3). As discussed, COVID‐19 has been shown to transmit asymptomatically. 12 , 13 , 14 , 15 Therefore, spatial and temporal separation of these teams reduced the probability of exposing an entire department from a single infected team member and cause complete prevention of patient care. In the case of provider infection, the entire on‐service team was directed to self‐isolate until testing can be completed or >14 days elapses with no symptom development. If any team member exhibited symptoms consistent with COVID‐19, the remaining unexposed teams have the capacity to split into smaller teams (minimum of two) in order to provide patient care during the rotation schedule.
2.5. Treatment room disinfection
SARS‐CoV‐2 has been demonstrated to be viable, aerosolized, and infectious for at least 3 hours. 13 Moreover, contaminated clinical surfaces may serve as fomites for several days. 45 Careful disinfection of a treatment room is important to prevent cross‐contamination among staff. The literature supports a surface disinfection protocol using 0.1% sodium hypochlorite solution or 62‐71% ethanol for smaller surfaces. 38 The authors recommend postoperative room disinfection to be completed by the operators to conserve PPE and to limit the use of any single unventilated room to once every 24 hours. During the COVID‐19‐modified clinic workflow, the DCG treated 4‐7 true emergencies daily. The size of our institution permits a single room to be used once every 24 hours. This time period allows for droplets or aerosols to settle onto surfaces with the intent to again disinfect prior to using those surfaces. 46 Other practical methods for clinics with a limited number of operatories include utilizing air filtration units as described above.
2.6. Modified PPE use
During COVID‐19, all patients should be considered asymptomatic carriers. This is due to reports demonstrating viral transmission from asymptomatic carriers during the 1‐14 day incubation period. 15 In order to provide safe treatment, appropriate PPE is required for both patients and providers. Clinically acceptable PPE includes disposable booties, full length gown, head cover, gloves that overlap the sleeves of the gown, N95 respirator, surgical mask, and face shield. Goggles are not currently required as the conjunctiva is not a preferred gateway for SARS‐CoV‐2 to infect the respiratory system. 47 Providers are required to wear primary masks rated N95 or greater as conventionally used Level 1 surgical masks do not provide adequate aerosol particle protection. More specifically, N95 masks demonstrate an approximate 95% filtration of airborne particles of 0.3 μm in size or greater and form an air‐tight peripheral seal. 48 Occupational Safety and Health Administration (OSHA) (29 CFR 1910.134) requires annual fit tests to confirm the peripheral seal on the wearer's face before use in the workplace. Due to the frequently reported COVID‐19‐related shortages of PPE, conservation of N95 masks is paramount. Although the ideal scenario would be to have single use N95 masks, a more pragmatic approach is required in times of extreme scarcity. The CDC provided guidelines for extended use of N95 masks by either treating multiple patients without removing the mask or by reusing the mask once doffed and donned again prior to treating the next patient. 49 Extended use is preferred whenever possible, due to reduced risk of cross‐contamination. Note that a secondary mask with attached face shield or a secondary mask with a visor supported face shield is recommended to be placed over the N95 mask to reduce contamination. The authors recommend exercising caution with the secondary mask due to potential violation of the N95 mask peripheral seal.
The PPE protocol at the Dental College of Georgia emergency clinic was modified for two providers per day with a single N95 mask per provider. These providers followed the CDC extended use guidelines during the daily workflow of treating multiple patients per day. Assigned providers doffed and discarded PPE except the N95 mask following each patient. N95 masks were sterilized at the end of the day with UGI. This prolongs the use of a single mask; however, the maximum number of sterilization cycles is dependent on mask model and UGI dose. 50 Moreover, the providers modified the recommended CDC PPE donning and doffing procedure in order to prevent indirect contamination of the N95 mask or their hands. 51 The modified protocol sequence is as follows:
Remove soiled gloves.
Wash hands.
Don clean gloves.
Remove overlying surgical mask with face shield (minimize contact with N95 mask).
Remove gown, booties, and surgical cap.
Remove gloves.
Exit treatment room.
Wash hands.
Remove N95 mask and place in plastic bag marked with provider's name for later use (unless catastrophically damaged/contaminated during treatment).
Wash hands.
3. DISCUSSION
3.1. Recommendations for community dentists
The above protocols are modifiable for private and corporate models. Community dentists can create interoffice teams in order to cover each other's emergencies and enter into self‐isolation should the need arises. This is similar to current practices of community dentists covering clinics for extended periods of absence (i.e., vacation, illness, etc.). Therefore, the protocols described above are viable strategies during a pandemic like COVID‐19. In the experience of the DCG residents and directors, the emergency patient volume never exceeded designated provider capacity with the above protocols.
3.2. A look to the immediate future
At the time of this article, the course of the pandemic will continue to evolve. For our State of Georgia, cases of COVID‐19 are expected to peak by the approximate date of April 23, 2020. 52 Projections suggest that the COVID‐19 case load will potentially exceed the existing capacity of Georgia's healthcare system in terms of ICU and total hospital bed capacity. It is possible that the Dental College of Georgia's facilities could be repurposed for overflow treatment of nonemergent COVID‐19 symptoms (i.e., shortness of breath, dehydration). This is possible due to plumbed oxygen and monitors typically used during IV sedation in the GPR operatories. Hopefully, this will not be required, and the Dental College can continue to function as the provider of emergency dental services throughout the outbreak. Epidemiologic projections will guide the DCG in triaging and treating dental emergencies. However, there has been greater emphasis on teledentistry and expanding care to patients of non‐record. Therefore, maintaining the protocols, mission, and focus of treating potentially life‐threatening emergencies is of greatest importance until Georgia is beyond its COVID‐19 peak. Rapid COVID‐19 detection test kits would provide a significant benefit during patient screenings at large dental providers that will likely encounter a relatively high volume of emergency cases. Such a test system was recently approved by the FDA, and it can deliver results in less than 15 minutes. 53 Rapid testing would allow confirmation of the health of both the provider and the patient prior to treatment allowing providers to treat with confidence as well as allow dentists to meaningfully participate in tracking this public health crisis. However, the authors acknowledge that current tests demonstrate a false negative rate of approximately 15% and insist that this is an important area for improvement. 54 Although current rapid testing demonstrates a high false negative rate, it is expected to decrease over time as new testing methods become available.
3.3. Conclusions
The COVID‐19 pandemic will continue to stress healthcare systems all over the world for the foreseeable future until a vaccine or herd immunity develops. Dental providers are challenged to provide care while reducing their vector potential due to frequent aerosolizing procedures. These issues necessitate protocols that reduce disease transmission, but also be readily adaptable as more information comes available regarding COVID‐19. The protocols implemented by the DCG were immediate responses to calls for action due to the growing threats associated with the virus and the dental field. The strategic implementation of modified patient screenings, prioritizing cases, team rotations with temporal spacing, and modified PPE protocols are effective measures in the event of a pandemic. These modifications can help expand access to emergency care, reduce patient exposure, and manage provider and supporting staff exposure.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGMENTS
We thank our program directors, administrators, and residents for their commitment to serve patients.
ETHICAL STATEMENT
This report is consistent with the Declaration of Helsinki.
Long RH, Ward TD, Pruett ME, Coleman JF, Plaisance MC. Modifications of emergency dental clinic protocols to combat COVID‐19 transmission. Spec Care Dentist. 2020;40:219–226. 10.1111/scd.12472
REFERENCES
- 1. Heir J, Ziccardi VB. Transmission of infectious disease in the dental setting. Mt Sinai J Med. 1998;65(5‐6):378‐382. [PubMed] [Google Scholar]
- 2. Araujo MW, Andreana S. Risk and prevention of transmission of infectious diseases in dentistry. Quintessence Int. 2002;33(5):376‐382. [PubMed] [Google Scholar]
- 3. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence [published online ahead of print February 25, 2020]. J Med Virol. 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The CDC Coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/index.html. Accessed April 6, 2020.
- 6. Fisher D, Heymann D. Q&A: the novel coronavirus outbreak causing COVID‐19. BMC Med. 2020;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamvar Z, Cai J, Pulliam J, Schumacher J, Jombart T. Epidemic curves made easy using the R package incidence. F1000Res. 2019;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID‐19 in South Korea. Int J Infect Dis. 2020;93:339‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewnard J, Lo NC. Scientific and ethical basis for social‐distancing interventions against COVID‐19. Lancet Infect Dis. 2020;S1473‐3099(20):30190‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johns Hopkins Coronavirus Resource Center . https://coronavirus.jhu.edu/data/new-cases. Accessed April 5, 2020.
- 11. Lai C, Shih T, Ko W, Tang H, Hsueh P. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu S, Lin J, Zhang Z, et al. Alert for non‐respiratory symptoms of coronavirus disease 2019 (COVID‐19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID‐19 patients. J Med Virol. 2020. 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- 15. Si‐Hui Luo, Wei Liu, Zhen‐Jun Liu, et al. A confirmed asymptomatic carrier of 2019 novel coronavirus (SARS‐CoV‐2). Chin Med J (Engl). 2020;133:1123‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coronavirus, Social and Physical Distancing and Self‐Quarantine . https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-social-distancing-and-self-quarantine. Accessed April 7, 2020.
- 17. Zemouri C, de Soet H, Crielaard W, Laheij A. A scoping review on bio‐aerosols in healthcare and the dental environment. PLoS One. 2017;12(5):e0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tellier R, Li Y, Cowling B, Tang J. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones R, Brosseau L. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57(5):501‐508. [DOI] [PubMed] [Google Scholar]
- 20. Szymańska J. Dental bioaerosol as an occupational hazard in a dentist's workplace. Ann Agric Environ Med. 2007;14(2):203‐207. [PubMed] [Google Scholar]
- 21. Al‐Sehaibany FS. Middle East respiratory syndrome in children. Dental considerations. Saudi Med J. 2017;38(4):339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019‐nCoV and controls in dental practice. Int J Oral Sci. 2020;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrel SK, Barnes JB, Rivera‐Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J Periodontol. 1996;67(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 24. Harrel S, Barnes J, Rivera‐Hidalgo F. Aerosol reduction during air polishing. Quintessence Int. 1999;30(9):623‐628. [PubMed] [Google Scholar]
- 25. Rozier R, White B, Slade G. Trends in oral diseases in the U.S. population. J Dent Educ. 2017;81(8):eS97‐eS109. [DOI] [PubMed] [Google Scholar]
- 26. Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID‐19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99:481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ADA Website Guidance on Emergency vs. Non Emergency . https://www.ada.org/en/publications/ada-news/2020-archive/march/ada-develops-guidance-on-dental-emergency-nonemergency-care. Accessed April 7, 2020.
- 28. ADA Website on COVID‐19 State Mandates and Recommendations . https://success.ada.org/en/practice-management/patients/covid-19-state-mandates-and-recommendations?utm_source=adaorg&utm_medium=covid-resources-lp&utm_content=stateaction&utm_campaign=covid-19. Accessed April 7, 2020.
- 29. Fong R, Glen W, Mohamed Jamil A, Tam WWS, Kowitlawakul Y. Comparison of the emergency severity index versus the patient acuity category scale in an emergency setting. Int Emerg Nurs. 2008;41:13‐18. [DOI] [PubMed] [Google Scholar]
- 30. Mirhaghi A, Kooshiar H, Esmaeili H, Ebrahimi M. Outcomes for emergency severity index triage implementation in the emergency department. J Clin Diagn Res. 2015;9(4):OC04‐OC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lockhart PB, Tampi MP, Abt E, et al. Evidence‐based clinical practice guideline on antibiotic use for the urgent management of pulpal‐ and periapical‐related dental pain and intraoral swelling: a report from the American Dental Association. J Am Dent Assoc. 2019;150(11):906‐921.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lake M. What we know so far: COVID‐19 current clinical knowledge and research. Clinical Medicine (Lond). 2020;20(2):124‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nogee D, Tomassoni A. Concise communication: covid‐19 and the N95 respirator shortage: closing the gap. Infect Control Hosp Epidemiol. 2020:1 10.1017/ice.2020.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sawhney A, Venugopal S, Babu G, et al. Aerosols how dangerous they are in clinical practice. J Clin Diagn Res. 2015;9(4):52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reddy S, Prasad M, Kaul S, Satish K, Kakarala S, Bhowmik N, et al. Efficacy of 0.2% tempered chlorhexidine as a pre‐procedural mouth rinse: a clinical study. J Indian Soc Periodontol. 2012;16(2):213‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halabi D, Escobar J, Alvarado C, Martinez N, Muñoz C. Chlorhexidine for prevention of alveolar osteitis: a randomised clinical trial. J Appl Oral Sci. 2018;26:e20170245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cochran MA, Miller CH, Sheldrake MA. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. J Am Dent Assoc. 1989;119:141‐144. [DOI] [PubMed] [Google Scholar]
- 40. ADA Council on Scientific Affairs and ADA Council on Dental Practice . Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1996;127(5):672‐680. [DOI] [PubMed] [Google Scholar]
- 41. Sehulster L, Chinn RYW. Guidelines for environmental infection control in health‐care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR‐10):1‐48. [PubMed] [Google Scholar]
- 42. Baek JH, Seo YB, Choi WS, et al. Guideline on the prevention and control of seasonal influenza in healthcare setting. Korean J Intern Med. 2014;29(2):265‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. CDC Environmental Infection Control Guidelines . https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html. Accessed April 29, 2020.
- 44. Medical Advisory Secretariat . Air cleaning technologies: an evidence‐based analysis. Ont Health Technol Assess Ser. 2005;5(17):1‐52. [PMC free article] [PubMed] [Google Scholar]
- 45. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of HCoV‐19 (SARS‐CoV‐2) compared to SARS‐CoV‐1. N Engl J Med. 2020;382:1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Utrup L, Frey A. Fate of bioterrorism‐relevant viruses and bacteria, including spores, aerosolized into an indoor air environment. Exp Biol Med. 2004;229(4):345‐350. [DOI] [PubMed] [Google Scholar]
- 47. Liu Z, Sun CB. Conjunctiva is not a preferred gateway of entry for SARS‐CoV‐2 to infect respiratory tract [published online ahead of print April 10, 2020]. J Med Virol. 2020. 10.1002/jmv.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. CDC Website Frequently Asked Questions about Personal Protective Equipment . https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirator-use-faq.html. Accessed March 31, 2020.
- 49. CDC Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings . https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. Accessed March 31, 2020.
- 50. Lindsley WG, Martin SB, Thewlis RE, et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12(8):509‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. CDC PPE Sequence Poster . https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf. Accessed April 7, 2020.
- 52. Institute for Health Metrics and Evaluation ‐ COVID‐19 Projections . https://covid19.healthdata.org/projections. Accessed April 1, 2020.
- 53. Detect COVID‐19 in as Little as 5 Minutes . https://www.abbott.com/corpnewsroom/product-and-innovation/detect-covid-19-in-as-little-as-5-minutes.html. Accessed April 1, 2020.
- 54. Study Raises Questions About False Negatives From Quick COVID‐19 Test . https://www.npr.org/sections/health-shots/2020/04/21/838794281/study-raises-questions-about-false-negatives-from-quick-covid-19-test. Accessed April 29, 2020.


