Abstract
Coronavirus disease 2019 (COVID‐19) is a respiratory disorder caused by the highly contagious severe acute respiratory syndrome coronavirus 2. The immunopathological characteristics of patients with COVID‐19, either systemic or local, have not been thoroughly studied. In the present study, we analysed both the changes in the number of various immune cell types as well as cytokines important for immune reactions and inflammation. Our data indicate that patients with severe COVID‐19 exhibited an overall decline of lymphocytes including CD4+ and CD8+ T cells, B cells and natural killer cells. The number of immunosuppressive regulatory T cells was moderately increased in patients with mild COVID‐19. Interleukin‐6 (IL‐6), IL‐10 and C‐reactive protein were remarkably up‐regulated in patients with severe COVID‐19. In conclusion, our study shows that the comprehensive decrease of lymphocytes, and the elevation of IL‐6, IL‐10 and C‐reactive protein are reliable indicators of severe COVID‐19.
Keywords: coronavirus disease 2019, cytokines, immune cells, inflammation, severe acute respiratory syndrome coronavirus 2
COVID‐19 exhibited an overall decline of lymphocytes including CD4+ and CD8+ T cells, B cells and natural killer cells. Interleukin‐6 (IL‐6), IL‐10 and C‐reactive protein were remarkably up‐regulated in patients with severe COVID‐19. IL‐6, IL‐10 and C‐reactive protein are reliable indicators of severe COVID‐19.
Abbreviations
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CT
computed tomography
- IFN
interferon
- IL
interleukin
- IP‐10
interferon γ‐induced protein 10
- MCP‐1
monocyte chemoattractant protein‐1
- MIP‐1A
macrophage inflammatory protein 1α
- NK cells
natural killer cells
- NKT cells
natural killer T cells
- NLR
neutrophil‐to‐lymphocyte ratio
- PBS
phosphate‐buffered saline
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- Treg cells
regulatory T cells
- TNF
tumour necrosis factor
- WBC
white blood cells
Introduction
Coronavirus disease 2019 (COVID‐19) is a respiratory disorder caused by a new coronavirus termed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 This virus probably arose from an unidentified animal source and is subsequently transmitted from person to person. 2 Transmission of COVID‐19 is through the air by coughing and sneezing, close personal contact, or touching a virus‐contaminated object and then touching the mouth, nose, or possibly eyes. COVID‐19’s initial manifestations include cough, fever, and respiratory distress. The thorough clinical profile of COVID‐19 is not completely understood. Reported illnesses range from mild to severe and even death. The severity of COVID‐19 varies among individual patients during the same outbreak and patient groups during different outbreaks in different regions.
The immune response in COVID‐19, either systemic or local, has not been well studied even 1 month after the COVID‐19 outbreak in China. Although chest computed tomography (CT) scans have suggested progressive pneumonia in patients with COVID‐19, the inflammatory process and immune reaction have not been detailed. In one report, raised neutrophil number, serum interleukin‐6 (IL‐6) and C‐reactive protein (CRP) were found, together with lymphopenia, in patients with COVID‐19. 3 Other clinical studies indicate that patients with COVID‐19 develop lymphopenia and high levels of various cytokines such as granulocyte colony‐stimulating factor, interferon‐γ‐induced protein 10 (IP‐10), monocyte chemoattractant protein 1 (MCP‐1), macrophage inflammatory protein‐1α (MIP‐1A) and tumour necrosis factor‐α (TNF‐α). 4 , 5 The surge of pro‐inflammatory cytokines produced by the cytokine storm can induce viral sepsis and tissue/organ damage, and result in multiple organ failure. SARS‐CoV‐2‐specific antibodies seem to be produced in patients with COVID‐19, suggesting the mounting of humoral responses. 3 However, the comprehensive status of either innate immunity or adaptive immunity in individuals with COVID‐19 remains largely unknown.
In the current study, we analysed multiple cytokines and immune cell populations in the blood of Chinese patients with COVID‐19. Our study outlines the immunopathological profile in patients with COVID‐19 and may provide helpful information for developing future therapies against SARS‐CoV‐2 infection.
Materials and methods
Patients
The study was approved by the Ethics Committee of Guangzhou Eighth People’s Hospital. Thirty‐one individuals who were diagnosed with mild/moderate COVID‐19 symptoms (17 men, 14 women, average age 44·5 years), and 25 individuals who were diagnosed with severe COVID‐19 symptoms (18 males, 7 females, average age 66 years) in Guangzhou Eighth People’s Hospital between January 2020 and February 2020 were enrolled in the study with informed consent. Diagnosis of COVID‐19 infection, pneumonia, and clinical classification were based on the new coronavirus pneumonia diagnosis and treatment plan (trial version 6) issued by the National Health Committee of the People's Republic of China. The clinical classifications are based on the following manifestations: (a) mild/moderate: fever, respiratory tract symptoms and pneumonia on chest CT scan, respiratory rate >30 breaths/min, or mean oxygen saturation <93%; (b) severe: one of the following: respiratory distress with respiratory rate >30 breaths/min, peripheral capillary oxygen saturation ≤93%, the ratio of the partial pressure of oxygen (Pao 2) to the fraction of inspired oxygen (Fio 2) <300, respiratory failure requiring mechanical ventilation, shock, admission to intensive care unit required for combined organ failure, pulmonary pathological progression. All patients were confirmed by the test on upper respiratory throat swab samples using the standard COVID‐19 test kit. Individuals with fever but negative for the SARS‐CoV‐2 test were recruited as controls.
Sample collection
Two to four millilitres of peripheral venous blood was withdrawn from each patient into K3 EDTA blood collection tubes. The complete blood cell count, lymphocyte count and neutrophil count were determined on an XN‐A1 automatic blood analyser (Sysmex, Kobe, Japan). The serum CRP levels were tested using a specific protein analyser PA‐990. The reagents, calibrators and quality controls were from the same vendor.
Flow cytometry assay
To measure lymphocytes and T‐cell subsets, 100 µl of whole blood was incubated in 900 µl of Tris–NH4Cl buffer (ThermoFisher Scientific, Waltham, MA) at room temperature for 5 min to lyse erythrocytes. After two washes with phosphate‐buffered saline (PBS), cells were incubated with BD Multitest 6‐Color TBNK Reagent or BD Multitest CD3/CD8/CD45/CD4 (BD Biosciences, Frankland, NJ) following the vendor’s instructions. The BD Trucount™ Absolute Counting Tubes, which contain a known number of fluorescent beads, were used for quantifying leucocyte populations. After another two washes with PBS, cells were resuspended in 500 µl of PBS.
To evaluate regulatory T (Treg) cells, erythrocytes were lysed as described above. The blood samples were then incubated with fluorescein isothiocyanate‐conjugated CD4 antibody, Violet 450‐conjugated CD127 antibody, Peridinin chlorophyll protein‐Cy5·5‐conjugated CD45 antibody and allophycocyanin‐conjugated CD25 antibody (5 µg/ml each, all from BD Biosciences) for 15 min on ice. After another two washes with PBS, cells were resuspended in 500 µl of PBS.
Cell samples were analysed on a BD FACSCanto Plus flow cytometer. Among all collected events, single events were gated between FSC‐A and FSC‐H. Cell debris was excluded and intact cells were then gated from single events based on FSC‐A and SSC. Each cell population was then detected based on the antibody staining. The data were assessed using the BD facsdiva™ software.
To analyse serum cytokines, the whole blood was centrifuged at 500 g for 5 min at 4°. The serum was carefully harvested and stored at −80° before analysis. Multiple serum cytokines were quantified using the Human Th1/Th2 Cytokine Kit II (Guangzhou Weimi Bio‐Tech, Guangzhou, China) following the manufacturer’s manual. The principle of the cytokine tests was the same as the BD™ Cytometric Bead Array. The data were evaluated using the FCAP array Software v3.0.
Statistics
The data were indicated as means ± standard deviation and measured by graphpad prism 6.0. The non‐parametric Kruskal–Wallis test or one‐way analysis of variance with post‐hoc Tukey HSD test was applied to compare the mean values or mean ranks among different groups, depending on the presence or absence of Gaussian distribution.
Results
Lymphocytes are decreased in patients with COVID‐19
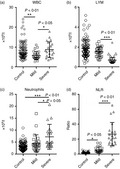
Venous blood was collected from each patient at inpatient admission. These patients were suffering mild or severe COVID‐19 symptoms and were not treated before blood collection. All cases were confirmed later by nucleic acid tests. To assess the general immune status of patients with COVID‐19, we first quantified the absolute number of whole white blood cells (WBC) and immune cell populations in the blood samples of patients at inpatient admission. As indicated in Fig. 1(a), the WBC number was significantly decreased in patients with mild symptoms compared with the control group. However, the WBC number was not remarkably changed in patients with severe disease. The total lymphocyte number was decreased in patients with severe disease but not in those with mild disease, suggesting lymphopenia in the severe disease (Fig. 1b). Neutrophils were increased in patients with severe disease but not in those with mild disease (Fig. 1c). The neutrophil‐to‐lymphocyte ratio (NLR), consistent with the change in lymphocytes, was moderately increased in patients with mild disease and profoundly increased in patients with severe disease, in comparison to the control group (Fig. 1d). Notably, patients with severe disease had a higher NLR than those with mild disease, suggesting that NLR was associated with the disease severity (Fig. 1d).
Figure 1.
The number of white blood cells (WBC) in patients with coronavirus disease 2019 (COVID‐19) at hospital admission. (a–c) Absolute cell number of WBCs, lymphocytes (LYM), and neutrophils. (d) Neutrophil‐to‐lymphocyte ratio. *P < 0·05; **P < 0·01; ***P < 0·001. One‐way analysis of variance for comparing the mean difference
T‐cell number is reduced in COVID‐19
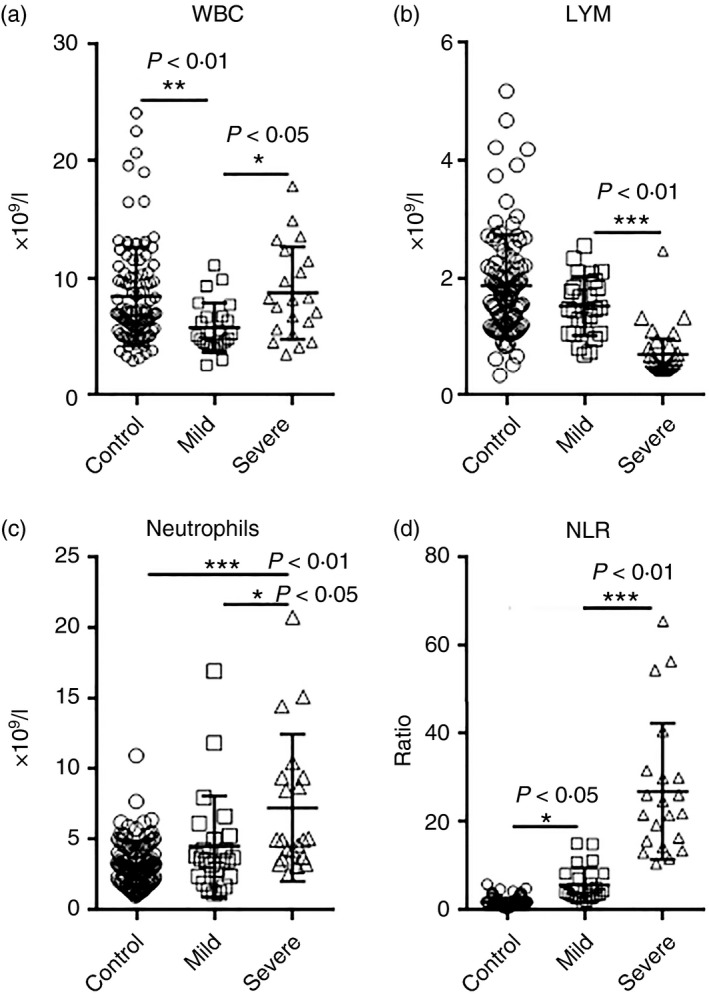
To analyse the adaptive immune cell populations, we conducted flow cytometry analysis on CD45+ CD3+ total T cells, CD3+ CD4+ T helper cells and CD3+ CD8+ cytotoxic T cells (Fig. 2a). We found a similar decrease in CD45+ lymphocytes in patients with COVID‐19 (Fig. 2b). Total T‐cell number was significantly decreased in patients with COVID‐19, but no significant difference was observed between mild and severe disease (Fig. 2c). Similarly, CD4+ T helper cells and CD8+ cytotoxic T cells were diminished in patients with COVID‐19 compared with the control group, but no significant difference was found between patients with mild and severe disease (Fig. 2d,e). The ratio between CD4+ T cells and CD8+ T cells was not changed (Fig. 2f). Analysis of the frequencies of total T cells, CD4+ T cells and CD8+ T cells in lymphocytes revealed that only the frequency of CD4+ T cells was significantly reduced in patients with severe disease (Fig. 2g–i).
Figure 2.
The number of blood T cells in patients with coronavirus disease 2019 (COVID‐19) at hospital admission. (a) Representative flow cytometry dot plots showing the gating strategy for total T cells, CD4+ T cells and CD8+ T cells. C: control; M: mild; S: severe. (b–e) The number of CD45+ lymphocytes, total T cells, CD4+ T cells and CD8+ T cells. (f) The ratio between CD4+ T cells and CD8+ T cells. (g–i) Frequencies of indicated cells in lymphocytes. *P < 0·05; **P < 0·01; ***P < 0·001. Kruskal–Wallis test for comparing the median difference
B cells and natural killer cells are decreased in patients with COVID‐19
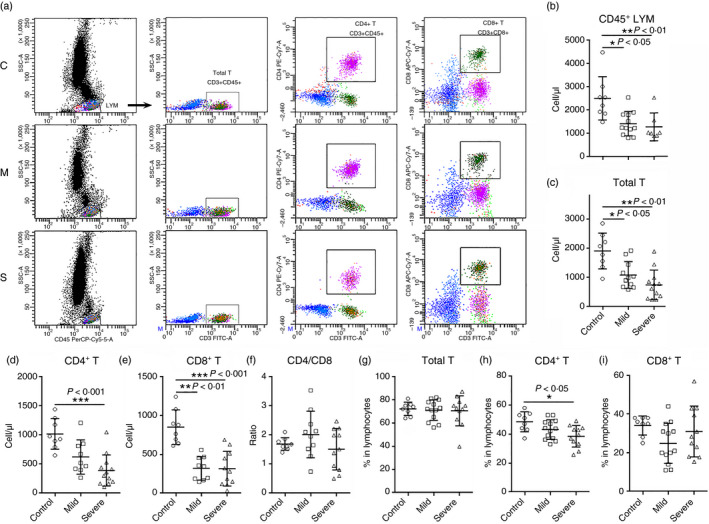
Other immune cells including CD3− CD19+ B cells, CD3− CD16+ CD56+ natural killer (NK) cells and CD3+ CD16+ CD56+ natural killer T (NKT) cells were also evaluated in blood samples (Fig. 3a). We found that in comparison with the control group, there was a trend of decrease in B‐cell number in patients with mild disease, and a notable reduction in B cells in patients with severe disease (Fig. 3b). A similar decrease was also seen in NK cells (Fig. 3c). No remarkable change in the NKT cell number was observed (Fig. 3d). Analysis of the frequencies of these cells in lymphocytes indicated that the frequency of B cells was significantly increased in patients with severe disease (Fig. 3e), but no profound change was found in the frequencies of NK cells and NKT cells (Fig. 3f,g).
Figure 3.
The number of blood B cells, natural killer (NK) cells and natural killer T (NKT) cells in patients with coronavirus disease 2019 (COVID‐19) at hospital admission. (a) Representative flow cytometry dot plots showing the gating strategy for B cells, NK cells and NKT cells. (b–d) The number of B cells (b), NK cells (c) and NKT cells (d). (e–g) Frequencies of indicated cells in lymphocytes. *P < 0·05; **P < 0·01. Kruskal–Wallis test for comparing the median difference
Treg cells are increased in patients with mild COVID‐19
Regulatory T cells are an important T‐cell subset for immune tolerance and anti‐inflammatory reaction. In our study, we measured the number of CD3+ CD4+ CD25+ CD127low T cells that was suggestive of the Treg‐enriched population (Fig. 4a). We found that the number of these cells was mildly increased in patients with mild disease compared with controls (Fig. 4b). There was only a trend towards an increase in the number of this population in patients with severe disease (Fig. 4b). However, the frequency of these suggestive Treg cells in lymphocytes was remarkably elevated in both mild and severe disease (Fig. 4c). CD25 expression was enhanced in Treg cells of patients with severe disease, as demonstrated by their higher mean fluorescence (Fig. 4d). Because CD25 is an IL‐2 receptor subunit crucial for the survival, proliferation and immunosuppressive activity of Treg cells, 6 , 7 the higher CD25 expression might indicate promoted Treg activity and function. However, Treg activity in patients with severe disease should be investigated in the future.
Figure 4.
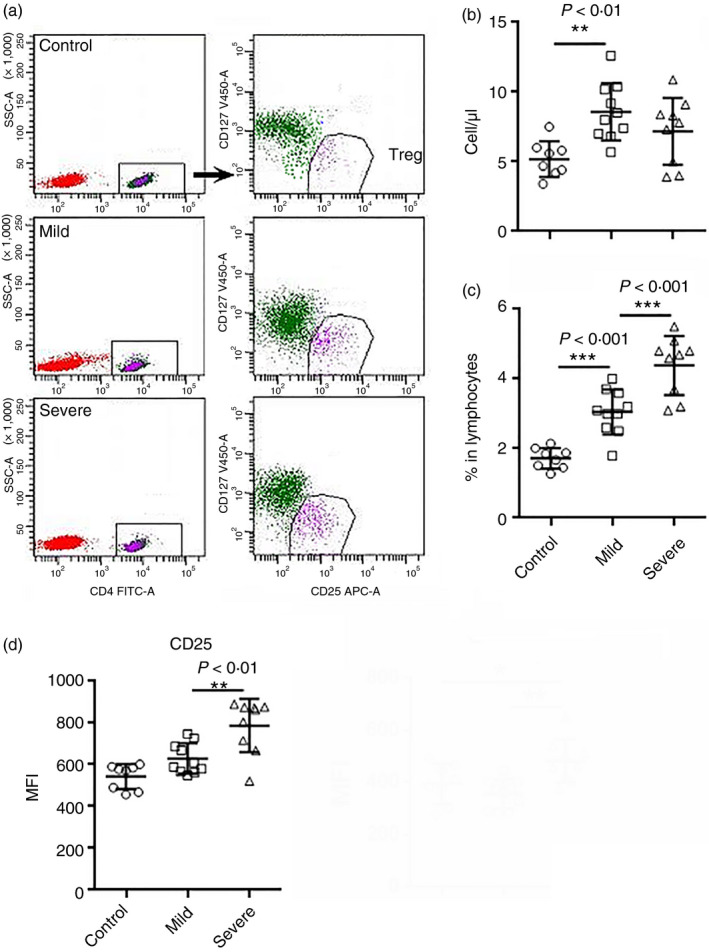
The number of blood regulatory T (Treg) cells in patients with coronavirus disease 2019 (COVID‐19) at hospital admission. (a) Representative flow cytometry dot plots showing the gating strategy for Treg cells. (b) The number of blood Treg cells. (c) Treg proportion in lymphocytes. (d) Mean fluorescence of CD25 on Treg cells. *P < 0·05; **P < 0·01; ***P < 0·001. Kruskal–Wallis test for comparing the median difference
IL‐6, IL‐10 and CRP reflect the severity of SARS‐CoV‐2 infection
Cytokines are crucial biomarkers of the progression of various inflammatory disorders including pneumonia. We characterized the expression of T helper type 1 cytokines [IL‐2 and interferon‐γ (IFN‐γ)], T helper type 2 cytokines (IL‐4 and IL‐10) and pro‐inflammatory cytokines (IL‐6 and TNF‐α) in the blood samples of patients at inpatient admission (Fig. 5a). The concentrations of IL‐2, IL‐4, TNF‐α and IFN‐γ were under their detection thresholds in all three groups (data not shown). As demonstrated in Fig. 5(b), IL‐6 was significantly increased in patients with severe disease but not in those with mild disease. Similarly, IL‐10 was significantly increased in severe disease but not in mild (Fig. 5c). CRP, a plasma protein synthesized by the liver as a sensitive marker of inflammation, was remarkably elevated in patients with severe disease compared with mild disease (Fig. 5d). However, we did not observe a significant correlation between either IL‐6 and IL‐10 or IL‐6 and CRP (data not shown).
Figure 5.
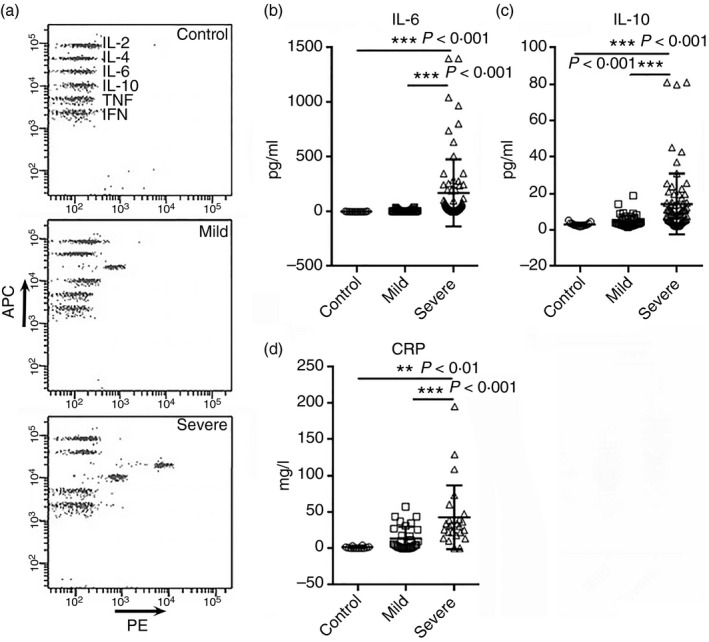
Cytokine and C‐reactive protein (CRP) levels in patients with coronavirus disease 2019 (COVID‐19). (a) Representative flow cytometry dot plots showing the cytokine expression in patients. (b, c) Concentrations of serum interleukin‐6 (IL‐6) and IL‐10 in patients at hospital admission (d) Serum CRP levels in patients. **P < 0·01; ***P < 0·001. One‐way analysis of variance for comparing the mean difference
Discussion
The outbreak of COVID‐19 poses an urgent demand for understanding the role of immunity in the progression of viral infection and subsequent pneumonia. As a new coronavirus, SARS‐CoV‐2 is highly contagious, probably owing to virus spread through asymptomatic infected individuals. 8 Until recently, very few publications characterized the comprehensive changes in the innate and adaptive immunity in SARS‐CoV‐2 infected people, although some non‐peer‐reviewed sources such as Medrxiv have released some information.
In the present clinical study, we analysed almost all immune cell types in both mild and severe COVID‐19. We found lymphopenia consistent with former reports. 4 , 9 , 10 Neutrophils, however, were increased in patients with severe disease. It is noteworthy that the lymphopenia seemed to arise from a comprehensive reduction of all lymphocyte populations, including CD4+ and CD8+ T cells, B cells and NK cells. The decrease in blood lymphocytes might be the result of the recruitment of reactive lymphocytes in the lungs. It will be helpful to investigate infiltrating cells in the lungs to test if the infiltrates are predominantly lymphocytes rather than granulocytes. Another possibility is that lymphocytes are more sensitive than granulocytes to the disturbed homeostasis and then underwent significant cell death. Whatever the case, lymphopenia is an indicator of severe COVID‐19.
Conventional blood T cells, especially CD8+ T cells, were reduced even in patients with mild COVID‐19. This phenomenon might suggest the recruitment of reactive anti‐viral CD8+ T cells into the infected tissues. According to reported T‐cell responses in SARS‐CoV infection, a CD8+ T‐cell reaction was more frequent and robust than the CD4+ T‐cell reaction. 11 It is, therefore, possible that this is also the case in SARS‐CoV‐2. Indeed, the latest case report shows signs of excessive T‐cell activation in COVID‐19, as evidenced by high proportions of an HLA‐DR (CD4 3·47%) and CD38 (CD8 39·4%) double‐positive T‐cell fraction. 5 Unpublished data indicate highly expanded clonal CD8+ T cells in the lung microenvironment of patients with mild COVID‐19, suggesting a robust adaptive immune response connected to better control of COVID‐19 (https://www.medrxiv.org/content/10.1101/2020.02.23.20026690v1.full.pdf). However, B‐cell and NK cell numbers were also remarkably reduced in patients with severe disease, suggesting a profound suppression of lymphocytes. Interestingly, Treg cells, which play a crucial role in immunoregulation and anti‐inflammatory responses, 12 were elevated in patients with mild disease, suggesting possible immunosuppression. In patients with severe disease, the Treg cells number was comparable to that in control individuals. However, the Treg frequency in lymphocytes was remarkably increased in both mild and severe COVID‐19, suggesting that unlike conventional CD4+ T cells, Treg cells were not lost in patients. Instead, their number or proportion was enlarged in patients with COVID‐19, probably indicating an active anti‐inflammatory response. Indeed, CD25 expression was increased in the Treg cells of patients with severe disease, further suggesting a promoted Treg immunoregulatory activity. However, Treg activity in COVID‐19 patients remains elusive and demands further investigations. Besides, although the reduction of lymphocytes is clear, the changes in the functions of lymphocyte populations remain unknown. In the future, it will be necessary to test the functions of distinct immune cell populations, so as to tell whether the innate or adaptive immunity is activated or impaired in COVID‐19 (Fig. 5).
Recent research reveals a fatal cytokine storm in critical COVID‐19 patients. Increased serum IL‐6, IP‐10, MCP‐1, MIP‐1A and TNF‐α were observed in COVID‐19 patients. 10 Several cytokine levels were also evaluated in our study. We found that serum IL‐6 and IL‐10 were significantly up‐regulated in patients with severe disease. Hence, serum IL‐6 and IL‐10 could be used to assess the severity of COVID‐19.
Cytokines are produced by both innate immune cells and adaptive immune cells. Interleukin‐6 is a well‐known pro‐inflammatory cytokine but it can also act as an anti‐inflammatory mediator. 13 The cellular sources of IL‐6 include myeloid cells, T cells, smooth muscle cells, endothelial cells, etc. 14 In patients with COVID‐19, the dramatic increase in IL‐6 probably originated from activated macrophages, consistent with another report 3 and similar to the IL‐6 up‐regulation in patients with SARS. 15 Interleukin‐10 acts as an anti‐inflammatory cytokine deriving from alternatively activated macrophages, T helper type 2 cells, Treg cells, etc. 16 The significant increase of IL‐10 in patients with severe disease might be negative feedback on the systemic and local inflammation. Surprisingly, IFN‐γ remained at a low level in COVID‐19 patients, because it is a crucial anti‐viral cytokine produced by both CD4+ T cells, CD8+ T cells, NK cells and macrophages, 17 , 18 and it has been reported to participate in the cytokine storm in SARS. 19 Perhaps serum IFN‐γ remains unchanged whereas IFN‐γ in the lung tissue is elevated. More investigations are needed to test this hypothesis. Moreover, IFN‐γ can down‐regulate the expression of the SARS coronavirus receptor angiotensin‐converting enzyme 2 in a laboratory study. 20 SARS‐CoV‐2 seems to bind to the same receptor to infect target cells. 21 Hence, the potential effect of IFN‐γ against SARS‐CoV‐2 infection needs further research.
In conclusion, our study shows that the comprehensive decrease of lymphocytes and the elevation of IL‐6, IL‐10 and CRP are reliable indicators of severe COVID‐19.
Disclosures
The authors declare no conflicts of interest.
Funding
The study is supported by the Guangzhou Science and Technology Programme (Grant number: 202008010008).
Acknowledgements
YS designed the study and wrote the paper. MT and RZ collected samples and performed most immune cell detection. YL and XD conducted cytokine assays. FL did the statistical analysis, and KL evaluated the Treg cells.
References
- 1. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020; 38:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Vetter P, Eckerle I, Kaiser L. Covid‐19: a puzzle with many missing pieces. BMJ 2020; 368:m627. [DOI] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T et al IL‐2 induces in vivo suppression by CD4+CD25+Foxp3+ regulatory T cells. Eur J Immunol 2008; 38:1643–53. [DOI] [PubMed] [Google Scholar]
- 7. Letourneau S, Krieg C, Pantaleo G, Boyman O. IL‐2‐ and CD25‐dependent immunoregulatory mechanisms in the homeostasis of T‐cell subsets. J Allergy Clin Immunol 2009; 123:758–62. [DOI] [PubMed] [Google Scholar]
- 8. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C et al Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG et al A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li CK, Wu H, Yan H, Ma S, Wang L, Zhang M et al T cell responses to whole SARS coronavirus in humans. J Immunol 2008; 181:5490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov 2018; 17:823–44. [DOI] [PubMed] [Google Scholar]
- 13. Scheller J, Chalaris A, Schmidt‐Arras D, Rose‐John S. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta 2011; 1813:878–88. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014; 6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang W, Ye L, Li B, Gao B, Zeng Y, Kong L et al Up‐regulation of IL‐6 and TNF‐α induced by SARS‐coronavirus spike protein in murine macrophages via NF‐κB pathway. Virus Res 2007; 128:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL‐10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29:71–109. [DOI] [PubMed] [Google Scholar]
- 17. Kang S, Brown HM, Hwang S. Direct antiviral mechanisms of interferon‐γ . Immune Netw 2018; 18:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thale C, Kiderlen AF. Sources of interferon‐γ (IFN‐γ) in early immune response to Listeria monocytogenes . Immunobiology 2005; 210:673–83. [DOI] [PubMed] [Google Scholar]
- 19. Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC et al An interferon‐γ‐related cytokine storm in SARS patients. J Med Virol 2005; 75:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Lang A, Osterhaus AD, Haagmans BL. Interferon‐γ and interleukin‐4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology 2006; 353:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol 2020; 5:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


