Abstract
At the end of December 2019, a novel acute respiratory syndrome coronavirus 2 (SARS‐CoV2) appeared as the third unheard of outbreak of human coronavirus infection in the 21st century. First, in Wuhan, China, the novel SARS‐CoV2 was named by the World Health Organization (WHO), as 2019‐nCOV (COVID‐19), and spread extremely all over the world. SARS‐CoV2 is transmitted to individuals by human‐to‐human transmission leading to severe viral pneumonia and respiratory system injury. SARS‐CoV2 elicits infections from the common cold to severe conditions accompanied by lung injury, acute respiratory distress syndrome, and other organ destruction. There is a possibility of virus transmission from asymptomatic cases as active carriers, in addition to symptomatic ones, which is a crucial crisis of COVID‐19 that should be considered. Hence, paying more attention to the accurate and immediate diagnosis of suspected and infected cases can be a great help in preventing the rapid spread of the virus, improving the disease prognosis, and controlling the pandemic. In this review, we provide a comprehensive and up‐to‐date overview of the different types of Clinical and Para‐clinical diagnostic methods and their practical features, which can help understand better the applications and capacities of various diagnostic approaches for COVID‐19 infected cases.
Keywords: COVID‐19, diagnosis, molecular assays, radiological findings, serological assays
At the end of December 2019, a novel acute respiratory syndrome Coronavirus 2 (SARS‐CoV2) named COVID‐19 emerged in Wuhan, China. Coronavirus becoming a critical challenge in global public health due to rapid development and extreme spread by active carriers in human‐to‐human transmission. Accordingly, there is a critical requirement for early diagnosis of COVID‐19 infected or suspected cases for applying appropriate care and treatment. In this review, we comprehensively present the clinical and para‐clinical diagnostic approaches to COVID‐19 and their various applications, advantages, and disadvantages aspects, which would be useful for expanding perspectives and understanding more about diagnostic methods.
1. INTRODUCTION
First, in Wuhan, China, SARS‐CoV2 arose as a new viral infection, and is currently named Coronavirus Disease 2019 (COVID‐19). New SARS‐CoV2, along with the Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) and SARS‐CoV are different strains of coronaviruses belonging to the β‐coronavirus cluster (P. Zhou et al., 2020). SARS‐CoV2, the chief pathogen of the human respiratory system, is the third zoonotic coronavirus disease and the third major medical crisis with a different genome from SARS‐CoV. SARS‐CoV2 is mostly transmitted by respiratory system droplets, gastric tract, and close human interaction, and is located in the nasal mucosa, mouth, and lungs of exposed individuals. Of note, middle‐aged and elderly individuals, as well as patients with chronic or autoimmune underlying diseases, are most susceptible to be infection by SARS‐CoV2. Incredibly, coronavirus has become a critical challenge in global public health due to rapid development and extreme spread by active carriers in human‐to‐human transmission (Riou & Althaus, 2020). Clinically, COVID‐19 brings about very severe respiratory infections and lethal sickness the same as SARS and MERS. Besides respiratory system injury, COVID‐19 hurts various organs, including the kidney, liver, gastrointestinal, and neurologic systems (Yin & Wunderink, 2018). Structurally, the coronavirus is characterized as an enveloped, nonsegmented, and single‐stranded RNA virus. The structure is composed of an Envelope (E), Nucleocapsid (N), Membrane (M), and Spike (S) proteins. Both E and M proteins have a central role in virus assembly and release of the virus (Schoeman & Fielding, 2019; Sheikh, Al‐Taher, Al‐Nazawi, Al‐Mubarak, & Kandeel, 2020). The S protein, an immense multipurpose viral transmembrane protein, induces immune responses by mediating the virus attachment to host receptors (F. Li, 2016). The N protein, a multifunctional protein, is identified as a viral RNA silencing suppressor. The N protein has a substantial role in viral transcription and replication and is involved in packaging the encapsidated genome into virions. Regarding the overexpression and high immunogenicity of N protein, it can be considered as a potential diagnostic target for SARS‐CoV2 detection (Hurst, Koetzner, & Masters, 2009).
After exposure, the virus enters into target cells and acts by binding to its receptor, the so‐called angiotensin‐converting enzyme 2 (ACE2). ACE2 is present on type I and II alveolar epithelial cells of healthy lung tissue. Also, it has been reported that organ failure can occur all over the body if organ cells express ACE2. The interaction between SARS‐CoV2 and ACE2 leads to overexpression of ACE2 resulting in alveolar cell detriment, interstitial and alveolar edema, respiratory system failure, and ARDS (Y. Zhao et al., 2020). Among various laboratory abnormalities, lymphopenia with or without leukocyte irregularities is commonly observed as a major para‐clinical criterion of COVID‐19 infected patients (L. Zhou & Liu, 2020). According to SARS‐CoV2 immunopathogenesis, lymphopenia is characterized by a decrease in lymphocytes, particularly CD8+ T cells and a slight increase in neutrophils (Wan et al., 2020).
Moreover, it has been found that SARS‐CoV2 can indirectly infect immune cells, mostly T cells, and macrophages, and elicits their destruction. Increased frequency of infected immune cells, lymphopenia, and high levels of inflammatory cytokines are considered as the most related elements to COVID‐19 immunopathogenesis (J. Liu et al., 2020). In this regard, elevated levels of pro‐ and inflammatory cytokines, including tumor necrosis factor‐α (TNF‐α), granulocyte colony‐stimulating factor (G‐CSF), macrophage inflammatory protein (MIP‐1A), monocyte chemoattractant protein‐1 (MCP‐1), interferon gamma‐induced protein‐10 (IP‐10), interleukin‐1 (IL‐1), IL‐2, IL‐6, IL‐7, and IL‐10 have been documented in COVID‐19 patients with severe conditions, which generate the cytokine storm. It seems that the cytokine storm is the primary phenomenon of virus pathogenesis leading to inflammation, lung injury, ARDS, and other organ failures (Wan et al., 2020).
Hence, there is a critical need for the detection of the infected or suspected cases as soon as possible to apply the appropriate treatments for COVID‐19 and prevent the spread of virus. For this reason, in this review, we will focus on the importance of diagnostic techniques by describing the different clinical and para‐clinical diagnostic approaches, related assays for both groups, advantages and disadvantages, technical comparison, and Food and Drug Administration (FDA) approved rapid tests (Figure 1). The current review provides a comprehensive overview of COVID‐19 diagnostic methods due to the importance of diagnosis in preventing the SARS‐CoV2 spread and controlling the infection.
Figure 1.
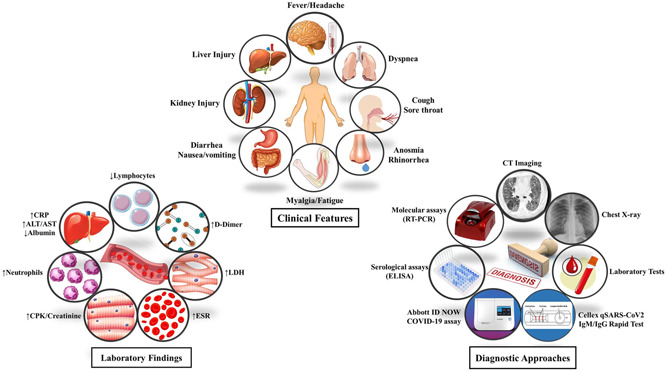
Clinical features, laboratory findings, and diagnostic approaches of COVID‐19 at a glance (Designed by Esmaeilzadeh et al.). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase
2. IMPORTANCE OF DIAGNOSIS
The importance of different diagnostic methods of COVID‐19 has been emphasized in many studies. Several studies have been designed or are underway to investigate the efficacy of COVID‐19 diagnostic methods, and have been listed in Table 1. Overall, COVID‐19 diagnostic methods are categorized into clinical diagnosis (physical examination, clinical features, and the radiological findings) and para‐clinical diagnosis. Para‐clinical diagnostic approaches include molecular assays or nucleic acid amplification tests (NAATs), viral sequencing, serological assays, and viral culture. Real‐time polymerase chain reaction (RT‐PCR) assays, particularly real‐time reverse‐transcriptase PCR (rRT‐PCR), are the most applicable method among NAATs. The RT‐PCR is usually utilized to detect the SARS‐CoV2 virus, qualitatively. Virus sequencing by Sanger sequencing and next‐generation sequencing (NGS) methods has applications when the results of the molecular assays are unreliable. Serological assays have been developed based on detecting specific immunoglobulin M (IgM) and IgG antibodies or viral antigens. Recently, the use of serological tests to rule out or confirm the infection, specifically in negative NAATs has been suggested due to the ease of use, quick presentation of results, as well as acceptable specificity and sensitivity. Contrarily, viral culture does not need to be used for detection due to the research aspect of culture. However, it can be applied to determine the contagiousness of the infection, the presence of the virus at different surfaces, and study the efficacy of various treatments on cultured cells. In the following, we have described in detail the different types of diagnostic methods in the two general clinical and para‐clinical categories.
Table 1.
Studies of COVID‐19 diagnostic methods
| Registration cCode | Study status | Study type | Diagnostic test |
|---|---|---|---|
| NCT04284046 | Completed | Observational | CT score |
| NCT04320017 | Recruiting | Observational | Electrocardiogram transthoracic echocardiography |
| NCT04313946 | Recruiting | Observational | Scanning chest X‐rays |
| NCT04318314 | Recruiting | Observational | COPAN swabbing blood sample collection |
| NCT04245631 | Recruiting | Observational | RT‐RAA assay |
| NCT04322513 | Recruiting | Observational | Biomarkers expression |
| NCT04322279 | Recruiting | Observational | IgM/IgG serology assay whole exome sequencing |
| NCT04329507 | Not yet recruiting | Observational | GC‐IMS assay |
| NCT04311398 | Not yet recruiting | Observational | New QIAstat‐Dx fully automatic multiple PCR detection platform |
| NCT04320511 | Not yet recruiting | Observational | CT‐V |
| NCT04324866 | Not yet recruiting | Observational | Nasopharyngeal swab for the molecular diagnosis |
| NCT04322487 | Not yet recruiting | Observational | Lung ultrasound |
| NCT04326387 | Not yet recruiting | Observational | Point of care Isothermal‐PCR Viral RNA Amplication for virus detection reverse‐transcription PCR chest X‐ray and CT scan detection |
| NCT04281693 | Not yet recruiting | Interventional | Screening strategy (RNA detection) |
| NCT04316728 | Not yet recruiting | Interventional | VivaDiag™ lgM/IgG Rapid Test |
| NCT04318431 | Not yet recruiting | Interventional | Rhinopharyngeal swab‐PCR |
Abbreviations: CT, computed tomography; RT‐RAA, real‐time reverse‐transcription recombinase aided amplification; GC‐IMS, gas chromatography‐ion mobility spectrometry; RNA, ribonucleic acid; PCR, polymerase chain reaction.
3. SAMPLE COLLECTION
Sample collection is an important procedure that should be done correctly to achieve accurate diagnostic results. With regard to the transmission of the SARS‐CoV2 through the respiratory tract, fecal‐oral, and body fluids, anal swabs, oral swabs, and blood samples are different methods of sample collection for diagnosis of the novel coronavirus (W. Zhang et al., 2020). A recent study has analyzed repeated sample collection from positive cases, which showed that 15 infected patients still had a virus after days of receiving treatments. They further reported that they might have more positive oral swabs on the first day of sampling, and more positive anal swabs on the late period of sampling. Notably, prolonged positive stool samples are not correlated with the severity of the disease, and some positive rectal swabs may have no gastrointestinal symptoms. Accordingly, the COVID‐19 patients could not be discharged based on negative oral swabs, while they could transmit the virus from the fecal‐oral route. According to their reports, eight patients (53.3%) had positive oral swabs, four patients (26.7%) had positive anal swabs, six patients (40%) had positive blood samples, and three patients (20%) had positive serum samples. Two patients had positive results in both anal and oral swabs, but none of the patients with positive blood samples had positive swab results. The above results indicated that patients may have negative swabs results, while they are still in viremic condition. Additionally, infected patients may have a negative oral swab, whereas they have positive anal swabs or positive blood samples (Wu et al., 2020; Zhang et al., 2020). Also, they evaluated the levels of IgM and IgG antibodies in samples on Day 0 and Day 5. The positive rate of IgM shifted from 50% to 81%, and the positive rate of IgG shifted from 81% to 100%. On the basis of a high detection rate of antibody titers, it is beneficial to assess both serological and molecular tests in suspected patients.
One of the latest studies has analyzed 1,070 samples collected from different sites of 205 infected patients. The specimens were collected from the pharynx, urine, feces, sputum, blood, and nasal. Fibrobronchoscope brush biopsies and bronchoalveolar lavages were taken from patients in severe conditions or those under mechanical ventilation. Results have shown that the highest positive rate for the virus belongs to bronchoalveolar lavage specimens (93%). Also, the positive rate of the other specimens was as follows: sputum (72%), nasal swabs (63%), fibrobronchoscope brush biopsy (46%), pharyngeal swabs (32%), feces (29%), and blood samples (1%). None of the urine samples showed positive results. As a result, sampling from different sites of the suspected patients increases the sensitivity rate of diagnosis and decreases the false‐negative results (W. Wang, Kang, Liu, & Tong, 2020). Another study evaluated the viral load of serial specimens from multiple sites of two patients in Beijing. Their reports indicated that the viral load in sputum and throat swabs elevated on 5–6 days. The sputum specimens showed a higher viral load compared with the other clinical specimens. Moreover, two patients had positive RT‐PCR test results a day before the onset of clinical symptoms. Hence, infected people can transmit the virus to others before the manifestation of their symptoms (Pan, Zhang, Yang, Poon, & Wang, 2020). A study has reported a suspected patient with three negative oropharyngeal swabs for COVID‐19 before admission to a hospital in Beijing. The test results were positive for influenza. Accordingly, 5 days after admission in the hospital, his positive test result for novel coronavirus happened by alveolar lavage specimen. Hence, we should consider that the suspected patients with negative test results for novel coronavirus may be diagnosed, eventually (Y. Han & Yang, 2019).
On the basis of the research, oropharyngeal and nasopharyngeal swabs are common upper respiratory tract samples for diagnosing the novel coronavirus. Nevertheless, we should consider that this kind of sample collection would increase the transmission rate of the virus from patients to healthcare workers. Furthermore, this invasive sample collection method may traumatize the respiratory tracts and cause bleeding, especially in patients with thrombocytopenia (J. F.‐W. Chan, Yuan, et al., 2020). However, the mentioned methods are not appropriate for viral load serial monitoring. The sputum is a noninvasive specimen from the lower respiratory tract; however, a case series study demonstrated that only 28% of the suspected patients could produce sputum for the diagnosis process (C. Huang et al., 2020). Additionally, saliva specimens are noninvasive sample collection methods that would not increase the risk of transmission to healthcare workers. Studies have represented that saliva specimens and nasopharyngeal swabs have a >90% concordance rate. Indeed, some viruses may be identifed in saliva but not in nasopharyngeal samples (K. K. W. To et al., 2019).
To et al. investigated the novel coronavirus in saliva specimens from 12 laboratory‐confirmed patients in Hong Kong. As a result, 11 of 12 patients (91.7%) had positive results in their initial saliva specimens. The average viral load of the samples was 3.3 × 106 copies/ml (9.9 × 102− 1.2 × 108 copies/ml). The serial saliva samples of the patients demonstrated a declining route of viral load. Moreover, the positive viral culture in saliva showed the existence of the live virus in saliva. This study introduced the saliva sample collection, as a noninvasive and cost‐effective procedure for the diagnosis and viral load monitoring of the novel coronavirus. Patients themselves can make this kind of sample collection. Interestingly, the possibility of nosocomial infection will be decreased by administrating this sample collection type. On the contrary, human‐to‐human transmission can easily occur through saliva, so it is essential to identify the strategies to prevent the transmission of virus to dentists and healthcare workers (Sabino‐Silva, Jardim, & Siqueira, 2020; To et al., 2020). Considerably, it is required to note that the primary handling of the specimens should be done under biosafety level‐3 (BSL‐3) conditions.
4. CLINICAL DIAGNOSIS
4.1. Physical examination
According to recent literature, the severity of the novel coronavirus has been divided into three subsets; mild, moderate, and severe. The patients in the mild category almost always show the signs and symptoms of an upper respiratory viral infection such as mild fever, sore throat, dry cough, nasal congestion, headache, fatigue, and myalgia. The clinical symptoms of a serious disease like dyspnea is absent in these patients. Probably, the cases may have a gastrointestinal manifestation (diarrhea, nausea, and vomiting). In the moderate group, patients exhibit respiratory symptoms such as shortness of breath and cough. Likewise, children may have tachypnea at this level. The patients in the severe group show fever, severe dyspnea, respiratory distress, reduction in the saturation of the oxygen (SpO2 < 90% on room air), and tachypnea (almost > 30 beats/min). At this stage, the diagnosis of the disease would be clinical, but laboratory tests, RT‐PCR results, and radiological findings can assist the clinician to achieve the final diagnosis (Cascella, Rajnik, Cuomo, Dulebohn, & Di Napoli, 2020). Another study showed the diagnostic criteria in suspected children. At first, we should consider the exposure contact with the COVID‐19 infected patients. It is essential to know the past medical illnesses and drug histories. The substantial issues include immunocompromised or immune deficiency states, usage of long‐term immunosuppressants, severe malnutrition, congenital heart disease, anomalies of the respiratory tract, and broncho‐pulmonary hypoplasia. On the basis of their reports, the stratification of suspected children would be as follows:
-
1.
Silent infection (asymptomatic patients): At this level, patients have laboratory‐confirmed COVID‐19, whereas they do not manifest any clinical symptoms.
-
2.
Acute respiratory tract infection: The patients have only cough, fever, sore throat, fatigue, myalgia, and nasal congestion. The radiological images do not display the involvement of the lung in pneumonia.
-
3.
Mild pneumonia: At this level, radiological images evidence pneumonia. Patients may have a fever and involvement of the respiratory tract. These patients are not in the severe condition of the disease.
-
4.
Severe pneumonia: The signs and symptoms of these patients are as follows:
-
1.
Decrease in the saturation level of oxygen (SpO2 < 92%)
-
2.
Increased respiratory rate (>70 times/min (<1 year), >50 times/min (>1 year))
-
3.
Hypoxia, nasal flaring, and cyanosis.
-
4.
Changes in mental status and consciousness
-
5.
Anorexia, and the existence of dehydration signs.
-
1.
-
5.
Critical cases: These patients need to receive ICU care. The complications of the critical cases include shock (A respiratory failure that requires mechanical ventilation), and other organ failures (Shen et al., 2020).
4.2. Clinical features
The Centers for disease control (CDC) have reported that the newly prevalent virus has mild to severe clinical manifestations in many cases and sometimes leads to death (CDC). In the suspected patients, symptoms almost appear as fever, cough, fatigue, pneumonia, and respiratory distress; whereass some symptoms such as rhinorrhea, diarrhea, hemoptysis, headache, and phlegm‐producing cough are rare in these patients (Adhikari et al., 2020). From the recent literature, COVID‐19 clinical features have some similarities to the manifestations of the other coronaviruses such as MERS‐CoV and SARS‐CoV. The common symptoms of the disease prove that the targeted cells for the virus are located in the lower respiratory tract. However, few patients indicate involvement of upper respiratory tract symptoms (for instance, sore throat, sneezing, and rhinorrhea). Gastrointestinal symptoms (diarrhea) are infrequent in COVID‐19 patients, while 20–25% of the patient with SARS‐CoV and MERS‐CoV had diarrhea. One of the recent studies reported that the mortality rate of COVID‐19 is approximately 2% and is lower than those of the other coronaviruses (MERS; mortality rate, 30%) and (SARS; mortality rate >40% in aged patients) (Chang et al., 2020). C. Huang et al. (2020) analyzed the clinical features of the 41 COVID‐19 patients. The common clinical features that they reported were fever (98%), cough (76%), fatigue (44%), the production of sputum (28%), headache (8%), hemoptysis (8%), and diarrhea (3%). On the basis of their reports, 55% of the patients showed dyspnea during their hospitalization. They anticipated 7 days as the median time from the onset of initial symptoms to the first admission in hospitals. Also, they predicted 8‐9 days for the median time from the onset of initial symptoms to the development of dyspnea and acute respiratory distress syndrome (ARDS). Eventually, mechanical ventilation and intensive care unit (ICU) admission happened at around day 10. The mortality rate of this cohort study was 15% (6/41). X. Yang et al. (2020) analyzed 52 critically‐ill COVID‐19 patients. The most common clinical manifestations were fever (98%), cough (77%), and dyspnea (63.5%). The median time to confirm with imaging findings was 5 days, and to ICU admission was about 9 days. Most of the patients in the study had end‐organ damage such as ARDS (67%), liver damage (29%), acute kidney injury (29%), cardiac injury (23%), and pneumothorax (2%). The mortality rate of the patients was 61.5%. The median duration from ICU admission to death was 1–2 weeks. In comparison, the nonsurvivors were older and had concomitant past medical histories. The nonsurvivor patients were more predisposed to develop ARDS and to receive invasive/noninvasive mechanical ventilation. Accordingly, older patients (>65 years) who have ARDS and concomitant medical illnesses are highly susceptible to death by COVID‐19.
A recent single‐center case‐series study has examined 138 hospitalized COVID‐19 patients at the Zhongnan Hospital of Wuhan. The average age was 56% and 54.3% were male. The most common clinical manifestations were fever (98.6%), fatigue (69.6%), and cough (59.4%). Some complications, such as ARDS (61.1%), arrhythmia (44.4%), and shock (30.6%), caused the transference of 26.1% of the patients to the ICU. In comparison, the ICU admitted older patients had concomitant illnesses who were more likely to have had anorexia and dyspnea. On the basis of the final reports, the hospital‐related transmission was estimated in 41% of the patients. Moreover, 26% of patients were transferred to the ICU, and the mortality rate was 4.3% (D. Wang et al., 2020).
The seventh Australian epidemiological report for COVID‐19 evaluated 295 infected cases. The average age of the patients was 47, and most of the patients were aged 50–59 and 60–69 years. Also, the death cases were older than 70. Only 53% of the patients had clinical symptoms. The characteristics included fever (69%), cough (54%), sore throat (46%), shortness of breath (35%), diarrhea (31%), pneumonia, ARDS, and joint pain (1%). The median interval to recovery from the disease is different in different age groups, estimated from 27 days in 20–29 years old patients to 36 days in patients older than 70 years old. Also, the severity of the disease has a direct correlation with the time interval to recovery (COVID‐19, 2020).
Zhao et al. evaluated the clinical manifestations of 19 COVID‐19 cases of pneumonia and 15 non‐COVID‐19 cases of pneumonia. According to the reports, the median time from onset of the clinical symptoms to hospital admission was 5 days in COVID‐19 patients and 4 days in non‐COVID‐19 patients. The most common clinical symptoms were fever and cough in both groups. Likewise, all the patients showed evidence of pulmonary infection on their computed tomography (CT) images. In short, there are no significant differences in regard to clinical features and imaging abnormalities between COVID‐19 and non‐COVID‐19 patients. So, laboratory tests and RT‐PCR results would be helpful to differentiate the COVID‐19 patients (D. Zhao et al., 2020). Z. Hu et al. (2020) evaluated 24 RT‐PCR confirmed COVID‐19 patients in Nanjing. The mentioned cases had not shown any clinical manifestations. Close monitoring of the patients showed that five patients (20.8%) showed clinical symptoms (cough, fever, etc.). The younger cases did not develop clinical characteristics. The reports of the study demonstrated that the mean communicable time interval (from the first positive nucleic acid test result to the first negative result) is approximately 9.5 days. On the basis of the reports, a substantial proportion of suspected cases are asymptomatic. Thus, close monitoring of these cases by RT‐PCR, laboratory tests, and radiological modalities would be required to control the spread rate of virus. A recent study has demonstrated that initial symptoms of COVID‐19 infection.
The most complicated symptom of this highly‐pathogenic virus is respiratory distress. Also, some of the patients revealed neurological signs such as nausea, vomiting (approximately 1%), and headache (approximately 8%). These signs prove that the virus can invade the central nervous as well. Furthermore, studies demonstrated that coronaviruses could spread from chemoreceptors and mechanoreceptors in the lung to the medullary cardiorespiratory center through synapse‐connected routes. It seems that the infection of the nervous system could be a reason for the respiratory failure of the coronavirus.
According to research, the average time interval from the onset of initial symptom to dyspnea is 5 days, that to admission in hospital is 7 days, and that to receive intensive care is 8 days. This latency time interval is enough for the virus to penetrate the nervous system and destroy the medullary neurons (Y. C. Li, Bai, & Hashikawa, 2020). Both the neuroinflammatory process and hypoxia in the brain would lead to neurological and psychiatric symptoms of the virus (Steardo, Steardo, & Zorec, 2020). Another clinical symptom of the novel coronavirus is affecting olfactory function. The 2019‐nCoV requires ACE2 and transmembrane protease, serine 2 (TMPRSS2) receptors to enter cells and destroy them. Olfactory stem cells and olfactory epithelial support cells express both mentioned receptors on their cell surface. So anosmia and olfactory dysfunction are new clinical aspects of the COVID‐19 (Brann, Tsukahara, Weinreb, Logan, & Datta, 2020). A recent case series study evaluated the clinical manifestations of 38 COVID‐19 patients in Hubei province. One‐third of the patients had ocular symptoms such as chemosis, conjunctival hyperemia, and epiphora. They showed that the patients in severe conditions of COVID‐19 may have ocular abnormalities (P. Wu et al., 2020).
Taken together, the most typical clinical characteristics of the novel coronavirus include fever, dry cough, and fatigue. Notably, the less common symptoms in suspected patients (headache, nausea, and vomiting, anosmia, cognitive impairments, sore throat, etc.) should be considered. Moreover, some patients may not indicate any clinical manifestations at the onset of their illness. So, it is essential to monitor highly suspect patients with RT‐PCR, laboratory tests, and radiological images. The clinical characteristics of the other studies have been represented in Table 2.
Table 2.
Clinical features of the novel coronavirus (COVID‐19)
| Study | Patients number | Mean age | Fever | Cough | Fatigue myalgia | Headache | Gastrointestinal symptoms | Others | References |
|---|---|---|---|---|---|---|---|---|---|
| Chang et al. | 13 | 34 | 92.3% | 46.3% | – | 23.1% | – | Upper airway congestion (61.5%) | (Chang 2020 #9) |
| Chung et al. | 21 | 51 | 67% | 43% | 14% | 14% | Nausea (5%) | – | (Chung, 2020 #10) |
| Chen et al. | 29 | 56 | 96.5% | 72.4% | 41.4% | 6.9% | Diarrhea (13.8%) | Sputum production (72.4%) Dyspnea (58.6%) | L. Chen et al. (2020) |
| Zhang et al. | 9 | 36 | 88.8% | 55.5% | 44.4% | – | – | Sore throat (44.4%) Nasal congestion (11.1%) | M. Zhang et al. (2020) |
| Kui et al. | 137 | 57 | 81.8% | 48.2% | 32.1% | 9.5% | Diarrhea (8%) | Palpitation (Less common) Hemoptysis (5.1%) Dyspnea (19%) Sputum production (4.4%) | Kui et al. (2020) |
| Chen et al | 99 | 55.5 | 83% | 82% | 11% | 8% | Diarrhea (2%) Nausea/vomiting (1%) | Dyspnea (31.3%) Confusion (9%) Sore throat (5%) Rinorrhea (4%) Chest pain (2%) | Chu et al. (2020) |
| Gaun et al. | 1,324 | 47 | 87.9% | 67.7% | – | – | – | Sever pneumonia (15.7%) | Guan et al. (2020) |
| Liu et al | 24 | 43 | 79.17% | 25% | 25% | 16.7% | Anorexia (less common) | Dizziness (16.67%) Dyspnea (8.3%) | (Liu, 2020 #1) |
| Feng et al. | 15 | – | 33.3% | 6.7% | – | – | – | – | (Feng, 2020 #14) |
| Pan et al. | 21 | 40.9 | 85.7% | 57.1% | 52.4% | – | – | Sore throat (19%) Sputim production (28.6%) | (Pan, 2020 #15) |
4.3. Radiological findings
COVID‐19 (SARS‐COV2), as well as other coronaviruses such as SARS‐CoV and MERS‐CoV, can lead to coronaviral pneumonia and ARDS. According to recent reports, some diagnostic radiological imaging has been discovered in coronaviral pneumonia. These findings demonstrate the disruption of pulmonary parenchyma, interstitial inflammation, and consolidations. The CT scan is a preliminary diagnostic approach in COVID‐19 suspected patients with viral pneumonia. The typical CT findings include involvement of the lower respiratory tract, bilateral and peripheral pure ground‐glass opacities (GGOs), or combined with consolidations, mainly in the subpleural site in the lower lobes, and crazy‐paving patterns (thickening the interlobular septa and intralobular lines accompanied by GGOs). Generally, bilateral GGOs and consolidations in the chest imaging of the patients with clinical symptoms would confirm the diagnosis of the COVID‐19. However, normal imaging would not exclude the diagnosis of the COVID‐19, completely (Kanne, 2020). It is worth noting that the related CT lesions increase within the first 10 weeks, especially 9–13 days after infection onset. After that, a plateau phase happens, and reduction in lesions occurs, gradually (Kanne, Little, Chung, Elicker, & Ketai, 2020). Pan, Ye, et al. (2020) evaluated the imaging findings of 21 RT‐PCR confirmed COVID‐19 patients by serial CT scan with a 4 days’ interval. On the basis of their reports, four stages of the lung involvement are described based on CT findings of the COVID‐19 recovered patients as follows:
-
1.
Early‐stage (0–4 days): At this stage, some patients may have no CT findings. However, the next pulmonary CTs would demonstrate changes. The main abnormalities in this stage are GGOs, which are allocated unilateral/bilateral subpleural in the lower lobes.
-
2.
Progressive stage (5–8 days): At this stage, the GGOs are distributed multi‐focally in other lobes. Also, consolidations and crazy‐paving patterns are identified in this stage.
-
3.
Peak stage (9–13 days): At this stage, the peak involvement of the lungs occurs. The CT findings of this stage are diffuse GGOs and consolidations, residual pulmonary bands, and crazy‐paving patterns.
-
4.
Absorption stage (≥14 days): At this stage, the absorption and healing process starts. It seems that the viral infection is controlled. The crazy‐paving pattern and consolidations do not exist anymore. However, diffuse GGOs may be present as a characteristic of absorbed consolidations.
These stages demonstrate that the severity of the lung lesions happens in the first 10 days of CT infection. The improvement of the CT findings initiates at least 14 days after the initial symptoms. Huang et al. reported on the clinical, epidemiological, laboratory, and imaging results of 41 COVID‐19 confirmed Chinese patients. The reports showed the lung involvement of all patients on admission. Accordingly, 40 out of 41 patients (98%) had bilateral pulmonary involvement in imaging. The chest CT images of the ICU‐admitted patients showed bilateral lobular consolidations. However, the chest CT images of the patients out of ICU showed bilateral GGOs. It is worth noting that the consolidations were resolved in later chest CTs, but the GGOs remained (C. Huang et al., 2020). Song et al. (2020) investigated the clinical symptoms, laboratory results, and chest CT findings in 51 suspected patients. According to their reports, 77% of patients had only GGOs in their imaging, 55% had consolidations alone in their CT, and 59% had GGO and consolidations, simultaneously. Moreover, they reported that patients older than 50 years exhibited more consolidated lung abnormalities. Among those patients, 86% showed bilateral pulmonary involvement. Also, 86% of patients had peripheral lung involvement. Furthermore, the chest CT follow‐up illustrated a 54% improvement and a 31% progression, in contrast. As a consequence, a case with clinical symptoms, combined with positive exposure contact, which had bilateral and peripheral GGO and/or consolidations on CT, would be highly suspect for having COVID‐19 lung involvement. Chung and Bernheim (2020) reported the initial CT findings of 21 COVID‐19 patients from three hospitals of three provinces in China. Three patients (14%) had a normal CT scan. Of the 18 patients, the affected lobes were seen as follows: one affected lobe in one (5%), two affected lobes in two (10%), three affected lobes in three (14%), four affected lobes in four (19%), and five affected lobes in eight (38%). The most involved lobe in the initial CT included the right lower lobe in 76%, the left upper lobe in 67%, the left lower lobe in 67%, the right upper lobe in 67%, and the right middle lobe in 57%. Of the 18 patients with lung involvement, 16 had bilateral opacities, and 2 had unilateral lung opacities (the right lung was involved in both groups). Moreover, GGOs (57%), consolidations (29%), a linear abnormality (14%), the crazy‐paving pattern (19%), and the peripheral distribution of viral pneumonia (21%) were found in patients. Also, they have reported that none of the patients' CT had lymphadenopathy, pleural effusion, cavitation, pulmonary emphysema, fibrosis, and pulmonary nodules.
Bernheim et al. (2020) evaluated the typical CT findings of 121 suspected patients, which were related to the time between the onset of the clinical symptoms and the first screening CT scan. As a result, 20/36 patients (56%) who had undergone CT imaging 0–2 days after the onset of clinical symptoms, had normal CT. They reported that bilateral lung involvement was seen in 10/36 (28%) early patients, 25/33 (76%) intermediate patients (taking CT 3–5 days after the onset of the clinical symptoms), and 22/25 (88%) late patients (taking CT 6–12 days after the onset of the clinical symptoms). It seems that the unilateral/bilateral peripheral lung involvement in COVID‐19 patients occurs long time after the onset of the symptoms.
W. Yang et al. (2020) evaluated the clinical and imaging findings of 149 patients. The imaging findings reports demonstrate that lung involvement is more localized in peripheral (35.9%) than central (2.15%) regions. Likewise, the lesions are more in a patchy shape (39.35%) than oval (6.6%). Moreover, the results showed that 17 of the 149 patients had normal initial CT findings. The chest CT findings of 5 patients turned positive in 7 days, but the imaging manifestations of the residual 12 patients remained negative. It seems that the absence of imaging findings on CT in COVID‐19 suspected patients could not exclude a patient from the diagnosis.
Ng et al. (2020) analyzed the radiological findings in the chest radiograph (CXR) and CT of the 21 COVID‐19 confirmed patients from four previous publications. Two patients had normal CT findings. The predominant imaging abnormality in 19 residual patients was GGO. All the GGOs and occasional consolidations were in peripheral locations of the lungs, except from one patient that had ground‐glass involvement in the perihilar site. Also, some changes such as pleural effusion, pericardial effusion, enlargement of mediastinal and/or hilar lymph nodes, and cavitation were absent in the radiological findings of the patients. Also, they reported that the most involved lobe was the left lower lobe (81%), the left upper lobe and the right lower lobe were the same (76%), and the involvement of the right middle lobe was less than the others (48%). They further reported the CXR findings of five patients, which had CT. Two CXR were normal, despite the imaging abnormalities on their CT. The remaining three CXRs demonstrated vague lung involvement, while their respective CTs had shown peripheral lesions. Yoon et al. (2020) evaluated the imaging findings of CXR and CT of the nine COVID‐19 Korean patients. Their reports showed that the CT manifestations of COVID‐19 pneumonia include bilateral and peripheral pure GGO sometimes mixed with occasional consolidations. They also reported the obscure radiological findings on CXRs of the patients. Accordingly, CT would be more sensitive for COVID‐19 investigation compared with CXR. Therefore, clinicians and radiologists need to become acquainted with the radiological findings of COVID‐19 pneumonia based on CT images to control the outbreak of the disease successfully.
Additionally, we should consider that the imaging manifestation of the COVID‐19 is similar to other viral pneumonia, bacterial pneumonia, and some other lesions. Sometimes, there are challenges in differentiating COVID‐19 from other underlying lung abnormalities by imaging manifestations alone. So, clinical symptoms, laboratory screening tests, and the contact history with confirmed patients accompanied by CT would result in the final diagnosis (Dai & Zhang, 2020). Besides this, from one report of the published literature, high‐resolution CT has been suggested to differentiate COVID‐19 lung involvement from other differential diagnoses (L. Chen et al., 2020). Eventually, the final diagnosis of the positive imaging findings on CT, laboratory tests, clinical symptoms should be verified by positive RT‐PCR or gene sequencing studies. With regard to attention to the CT manifestations of the disease, radiologists have a pivotal role in the early and rapid detection of suspected cases. Thus, a chest CT scan is an accessible method that can create a quick imaging diagnosis in patients with clinical symptoms. Hence, the chest CT scan should be performed as soon as possible to detect most COVID‐19 patients at an early stage. This would accelerate the isolation process of COVID‐19 patients and also benefit the general population's health state.
5. PARA‐CLINICAL DIAGNOSIS
5.1. RT‐PCR
RT‐PCR or quantitative‐PCR (Q‐PCR) is a molecular technique to investigate gene expression using the PCR product. Generally, RT‐PCR assesses the presence or absence of a target gene or its amount based on an increased intensity of fluorescence within the target sequence amplification. Subsequently, amplified specific regions are validated by NGS or Sanger sequencing technique.
Specific primers, along with standardized laboratory protocols for RT‐PCR, have been developed after the SARS‐CoV2 genome discovery. Accordingly, RT‐PCR assays are gold standard molecular tests for the final detection of SARS‐CoV2 using respiratory secretions. Various RT‐PCR assays have been introduced to effectively diagnose COVID‐19 cases, which include qRT‐PCR, rRT‐PCR, and reverse‐transcription‐insulated isothermal polymerase chain reaction (RT‐iiPCR). Among these, rRT‐PCR is identified as a potent and standard screening diagnostic method for early detection of SARS‐CoV2 worldwide by detecting the E, N, and RNA‐dependent RNA polymerase (RdRp) genes (Chan, 2020 #1; Corman, 2020). The rRT‐PCR assay is performed to detect the SARS‐CoV2 RNA in different clinical specimens, including blood, sputum, bronchoalveolar lavage fluid (BALF), biopsies, pharyngeal and nasal swabs, feces, and fibrobronchoscope brush biopsies.
First, in January 2020 (V. M. Corman et al., 2020), RT‐PCR was used to assay the E, N, and RdRp genes of the SARS‐CoV‐2 virus. Interestingly, measuring the RdRp gene revealed the high analytical sensitivity containing the 3.8 RNA copies/reaction at a 95% detection probability.
In a study conducted by J. F.‐W. Chan, Yip, et al. (2020), three real‐time RT‐PCR assays were performed to detect the RdRp/Hel, a nonstructural gene, along with S and N genes as structural proteins of COVID‐19. As a result, in SARS‐CoV2 RNA detection, significant sensitivity was observed assessing the COVID‐19‐RdRp/Hel compared with RdRp‐P2 in both respiratory‐ and non‐respiratory tract clinical specimens. Moreover, COVID‐19‐RdRp/Hel assay indicated the considerable sensitivity for detecting the SARS‐CoV2 RNA in plasma and saliva specimens, throat swab, and nasopharyngeal aspirate/swab compared with RdRp‐P2 assay. The low viral loads of SARS‐CoV2, SARS‐CoV, and MERS‐CoV in nasopharyngeal aspirate/swabs or throat swabs may lead to false‐negative results, which would be reduced using the COVID‐19‐RdRp/Hel assay.
Chu et al. (2020) designed two monoplex real‐time RT‐PCR assays to target the ORF1b and N genes of SARS‐CoV2. In this study, two COVID‐19 suspected patients from Beijing were enrolled. Sputum and throat swab samples were collected from patient one and patient two, respectively for RNA extraction. Due to the limited information about the genetic diversity of COVID‐19 in humans and animals and viral sequence, two RT‐PCR assays were conducted to react with different coronaviruses in the subgenus Sarbecovirus. They selected the ORF1b and N genes as highly conserved targets. SARS coronavirus nucleic acids wereconsidered as positive controls. As a result, the respiratory specimens of two infected patients showed positive results after detectionby two RT‐PCR assays, in which the N gene assay revealed significant sensitivity 10 times more than the ORF1b gene in positive specimens. Overall, the findings of this study suggest using the N‐RT‐PCR as a screening assay and the ORF1b‐RT‐PCR as a confirmatory assay.
5.1.1. RT‐qPCR test in multi‐sample pools
From decades ago, pooling diagnostic tests have been developed to optimize detection time, save on reagents, and quickly detect large numbers of suspicious or contaminated cases in infectious disease. Beneficially, intricate equipment and additional training are not required in the use of these tests (Nguyen, Bish, & Aprahamian, 2018). Pooling has been evidenced for RT‐qPCR, which would allow to RT‐PCR to detect the low‐concentration RNA with further optimization (Arnold et al., 2013). In this context, Yelin et al. (2020) conducted a study to investigate the efficacy of standard RT‐qPCR in distinguishing a single positive sample within a pool of negative samples using five positive samples and 67 negative samples. First, they solely confirmed the RNA positive samples, then combined them with a large number of negative samples, which were previously confirmed. Interestingly, they could detect the positive samples in pools of up to 32 samples, which could be detected in even up to 64 samples with extra PCR cycles. A 10% false‐negative rate was estimated using this technique. Promisingly, this diagnostic pooling test could be used in clinical testing laboratories for detecting the COVID‐19 infected cases. Furthermore, it can lead to broadening the screening capacities and community detection of suspicious or infected individuals.
5.1.2. Closed‐tube Penn‐RAMP
Despite the benefits of the rRT‐PCR technique, there are some limitations, such as requiring expensive instruments, trained experts, long procedures, high false‐negative results, and importantly, high risk of disease spread. To solve these restrictions, the Jinzhao Song research team proposed using an alternative rapid test with some advantages, such as low cost, easy usage, high sensitivity, and the possibility of use at home (El‐Tholoth, Bau, & Song, 2020). In this study, a novel closed‐tube COVID‐19 assay (Penn‐RAMP) comprised of a two‐stage isothermal double stranded DNA amplification method was presented to amplify and detect the nucleic acid of the pathogen. This diagnostic procedure was designed to simplify the detection of positive cases in a single tube reaction using both recombinase polymerase amplification (RPA) and loop‐mediated isothermal amplification (LAMP) techniques in a single tube.
5.1.3. RT‐PCR assay in recovered patients
It should be noted that there is a possibility of a positive RT‐PCR result in COVID‐19 recovered patients. With this point of view, a study evaluated four treated COVID‐19 patients from Wuhan, China, by rRT‐PCR to confirm whether they could return to work. The patients' recovery was confirmed by rehabilitation criteria, including normal temperature over 3 days, relieved respiratory difficulties, and confirmed CT imaging based on recovered acute exudative lesions. Finally, confirmation of two separated negative RT‐PCR results led to hospital discharge and discontinuation of quarantine. Accordingly, RT‐PCR assays were carried out on throat swabs of patients using BioGerm kits, 5–13 days after hospital discharge, and discontinuation of quarantine, all of which were positive (Lan et al., 2020). Surprisingly, the results revealed that a proportion of rehabilitated patients can be virus carriers and should continue the quarantine protocol for about 5 days more. Also, the RT‐PCR assay should be reevaluated to confirm the complete remission of treated patients.
5.1.4. RT‐PCR advantages and disadvantages
Several advantages of RT‐PCR assays make them potent and effective diagnostic techniques for COVID‐19 infection. The RT‐PCR assay is identified as a practical diagnostic approach used to confirm the COVID‐19 positive cases (Roberts et al., 2015). It has the capability of precise and reproducible determination of infected cases. Also, it can be employed to quantify the presence or absence of viral RNA and viral load in patients' specimens. In plasma therapy, selection and confirmation of COVID‐19 recovered patients, as appropriate donors are performed by two sequential negative SARS‐CoV2 nucleic acid identifications using the RT‐PCR. To discharge the COVID‐19 improved patients from the hospital, RNA‐based diagnosis should be determined (Lan et al., 2020). To decrease the turnaround time, using point‐of‐care tests would be significantly useful. In this regard, an Xpert® Xpress SARS‐CoV2 diagnostic test has been developed by Cepheid based on qualitative rRT‐PCR to detect E, N, RdRp, and ORF1a SARS‐CoV2 antigens within 45 min (PH).
Despite the multiple benefits of RT‐PCR assays, some disadvantages may decrease the efficacy of RNA‐based techniques. Briefly, prolonged turnaround time (TAT), technical and individual errors, the importance of sampling quality, different sources of reagents, multiple steps of detection procedure, and viral load variation in different stages of infection may affect the diagnosis accuracy and restrict the RT‐PCR usefulness (PH). The prolonged procedure of assay may not be suitable for emergency early detection. Undesirably, high false‐negative results are common unwanted disadvantages of RT‐PCR assays, which reduce confidence in using this approach. In addition to false‐negative results, cross‐reaction of false‐positive results may also diminish diagnosis efficacy. Requiring complicated and advanced material, equipment, and laboratories, as well as experienced experts are the other limitations of RNA‐based techniques.
High false‐negative rate (FNR)
According to a report, in one SARS‐CoV2 infected patient with both RT‐qPCR and NGS, the positive results were not confirmedby RT‐qPCR testing within 3 weeks before obtaining the BALF (Wang et al., 2020). This data suggests the high FNR or inconsistency of RT‐PCR assays, which significantly affects the efficacy of the detection of positives cases. For example, variation in viral RNA sequences may affect the primers in the detection of the ORF1b and N genes, which leads to false‐negative results. Additionally, various infection conditions in patients, the natural history of the disease, viral load alteration in different anatomic sites, and diverse sampling processes with different accuraciesare the other reasons related to the high FNR. Urgent measures should be usedto optimize appropriate and accurate diagnostic tests as well as the standard operating procedure (SOP). For example, the recommendation of using the lower respiratory tract specimens (sputum and BALF) and the nasopharyngeal swab would reduce the FNR (Y. Wang et al., 2020). Moreover, paying more attention to related parameters ofthe RT‐PCR assay, including sample reagents, transportation, and laboratory SOP, would decrease the high FNR. For instance, trisol is a key reagent that should be considered due to the application in protecting the stability of RNA samples and inactivation of viruses. As a suggestion, according to previously published studies, combinational diagnosis based on using the clinical features, laboratory tests, CT imaging, RT‐PCR, and NGS testing may be more beneficial foraccurate diagnosis of infected patients.
5.2. Laboratory screening tests
Laboratory screening tests (laboratory findings) based on assessing the biological and chemical factors in blood would be helpful for better determination of COVID‐19 infected patients, although they do not have higher specificity and sensitivity (Table 3). For this purpose, the complete blood count, as well as blood biochemistry measurement, should be carried out for each patient. According to published results, the different common laboratory abnormalities which have been found in SARS‐CoV2 cases are as follows: low count of white cells (leukopenia; lower than 1,000), a low percentage of lymphocytes (lymphopenia), decreased level of albumin (hypoalbuminemia), increased levels of C‐reactive protein (CRP), and erythrocyte sedimentation rate (ESR; C. Huang et al., 2020). Moreover, there are elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in infected patients due to the abnormal function of the liver. Increased levels of lactate dehydrogenase (LDH) and creatine phosphokinase (CPK) due to myocardial zymogram abnormality have been reported, as well. Moreover, a higher level of D‐dimer was also detected in patients with severe conditions (Bangash, Patel, & Parekh, 2020; F. Zhou et al., 2020). The procalcitonin level is mostly normal in COVID‐19 patients, but a higher‐level was reported in bacterial co‐infection.
Table 3.
The laboratory findings of the novel coronavirus (COVID‐19)
| Study | PN | Lymphopenia | Leukopenia | Leukocytosis | LDH | ESR | CRP | Others | References |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al. | 24 | 8.3% | 20.8% | – | – | High (25%) | High (50%) | – | {Liu, 2020 #1} |
| Wang et al. | 34 | 2.9% | 2.9% | 14.7% | High (29.4%) | High (2.9%) | High (14.7%) | – | {Wang, 2020 #2} |
| Kui et al. | 137 | 72.3% | 37.2% | 19% | – | – | High (38.9%) | – | Kui et al. (2020) |
| Wang, et al. | 138 | 70.3% | – | – | High (39.9%) | – | ‐ | – | D. Wang et al. (2020) |
| Chen et al. | 29 | 69% | 20.7% | 20.7% | – | – | High (93.1%) | High AST (24.1%) | L. Chen et al. (2020) |
| High ALT (17.2%) | |||||||||
| High LDH (69%) | |||||||||
| High creatinine (6.9%) | |||||||||
| High bilirubin (3.4%) | |||||||||
| Hypoalbuminemia (51.7%) | |||||||||
| Huang et al. | 41 | 63.4% | 24.4% | 29.3% | High (70.7%) | – | – | High AST (36.6%) | Chaolin Huang et al. (2020) |
| High creatinine (9.8%) | |||||||||
| High CK (31.7%) | |||||||||
| High Troponin I (12.2%) | |||||||||
| High D‐dimer/PT in ICU Patients | |||||||||
| High procalcitonin (7.31%) | |||||||||
| Chen et al. | 99 | 35.4% | 9.1% | 24.2% | High (75.8%) | High (85%) | High (63.6%) | High AST (35.4%) | Chu et al. (2020) |
| High ALT (28.3%) | |||||||||
| High Creatinine (3%) | |||||||||
| High CK (13.1%) | |||||||||
| High bilirubin (18.2%) | |||||||||
| Hypoalbuminemia (98%) | |||||||||
| Zhang et al. | 9 | 22.2% | – | 11.1% | – | – | High (55.6%) | – | M. Zhang et al. (2020) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CK, creatine kinase; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; ICU, Intensive Care Unit; LDH, lactate dehydrogenase; PN, patients number; PT, prothrombin time.
Increased levels of pro‐ and inflammatory cytokines in the serum of infected COVID‐19 patients also were reported in several studies, which is considered as the main reason for the cytokine storm (C. Huang et al., 2020). In addition to the laboratory findings as mentioned above, detecting the lymphocyte subsets and their levels in the blood samples of patients would provide valuable information for better diagnosis of infection. In a study, Wan et al. (2020) indicated the reduced levels of T‐cell subsets. The reduction rates of CD4+ T cells were 52.90% and 95.24% in the mild and severe group of patients, respectively. Moreover, CD8+ T cells were responsible for 28.40% and 61.90% reduction rates in the mild and severe groups, respectively. These data suggest that T lymphocytes were significantly reduced in COVID‐19 infected patients. Also, B cells indicated a 25.49% and 28.57% reduction ratio in the mild and severe groups, respectively. Notably, the limited activity of natural killer (NK) cells in infected cases was evidenced in the mild and severe group of patients with a 34.31% and 47.62% reduction ratio, respectively. Furthermore, evaluation of proinflammatory cytokines, including TNF‐α, IL1B, IL7, IL8, IL9, IL10, MIP1A, MIP1B, MCP1, IP10, GMCSF, interleukin‐1 receptor antagonist (IL1RA), interferon‐γ, granulocyte colony‐stimulating factor (GCSF), fibroblast growth factor (FGF), vascular endothelial growth factor, platelet‐derived growth factor in plasma of ICU and non‐ICU patients showed increased amounts compared with the healthy individuals. Moreover, ICU patients indicated greater levels of TNF‐α, IP10, MIP1A, MCP1, GCSF, IL2, IL7, and IL10 than non‐ICU patients. The higher levels of the mentioned cytokines were correlated with the cytokine storm, pulmonary inflammation, and lung injury.
In a systematic review and meta‐analysis study (Pormohammad et al., 2020), investigating the available laboratory data among 2,361 SARS‐CoV2 patients, the results demonstrated 13.3% leukocytosis, 26% leukopenia, and 62.5% lymphopenia. Furthermore, among 2,200 patients, increased levels of platelets (thrombocytosis) and CRP were reported in 91% and 81% of patients, respectively. Also, a case report study by W. Han et al. (2020) reported the clinical diagnosis and alteration of clinical parameters in a 47‐year‐old man infected by COVID‐19 from Wuweian. The findings revealed an increased level of CRP (84 mg/L), reduced levels of lymphocytes (Lymphopenia), and a slight increase in neutrophil numbers, fibrinogen, and lactic dehydrogenase (230 U/L).
Another systematic review and meta‐analysis investigated the clinical, laboratory, and imaging features of COVID‐19 patients in 18 studies. Accordingly, the findings reported lymphopenia (43.1%), higher levels of CRP (58.3%) and ESR (41.8%), a decreased level of albumin (75.8%), and an increased level of LDH (57%; Rodriguez‐Morales et al., 2020). Y. Liu et al. (2020) investigated the correlation between clinical and biochemical indexes and viral loads and lung injury in 12 2019‐nCoV infected patients from Shenzhen, China. The Murray score is considered as an index of the severity of acute lung injury in ARDS, a greater score indicates a higher severity in ARDS patients. The study findings revealed the positive correlation between the CRP and LDH levels with Murray scores; however, a negative correlation was observed between the Albumin and lymphocyte counts with Murray scores. On the basis of these results, a significant relationship can be predicted between severe acute lung injury in COVID‐19 patients and high levels of CRP and LDH, as well as decreased levels of albumin (hypoalbuminemia), lymphocyte counts (lymphopenia), and the percentage of lymphocytes and neutrophils. Consequently, despite the nonspecific findings of blood count and parameters in diagnosing COVID‐19 infected patients, these laboratory findings would be helpful in early detection of infected cases in combination with highly specific and sensitive approaches, such as CT imaging and RT‐PCR assay.
5.3. Serological assays
Serological assays are used for diagnostic identification of antibodies in the serum and other body fluids. Antibodies, which can be detected by serological tests, are found in immune responses against microorganisms and foreign or one's own proteins. Due to the presence of some limitations related to commercial reagents of serological assays, they are not commonly applied for diagnosing coronavirus infections. However, serological tests have a vital role in obtaining the epidemiology comprehensive, infection burden, asymptomatic cases, and for early detection of SARS‐CoV2, SARS‐CoV, and MERS‐CoV in infected patients (Ksiazek et al., 2003). Serological tests are required for their urgent practical utility, determination of infection and mortality rates in a population, qualitative and quantitative definition of the anti‐SARS‐CoV2 immune responses, distinguishing of the infected cases, and potentially immune individuals. Moreover, these assays may be beneficial in diagnosing the disease in the early stages of infection and in those who did not have a positive viral RNA result.
According to previous studies on SARS‐CoV and MERS‐CoV, antibodies can be detected 2 weeks following the initiation of the infection, and last for about 12 years in 80–100% of patients (Corman et al., 2016; Guo et al., 2020). Furthermore, it was reported that there could be a relationship between infection severity and antibody responses (Ko et al., 2017). Encouragingly, serological assays, as supplementary diagnostic approaches, would help SARS‐CoV2 identification while rapid antigen tests or molecular assays like RT‐PCR are not available or stable for detecting infected patients. Enzyme‐linked immunosorbent assay (ELISA), colloidal gold‐immunochromatographic assay (GICA), and lateral flow assays (LFAs) are some examples of serological tests that have been developed to detect COVID‐19 infected cases based on the identification of antibodies against SARS‐CoV2 antigens. Among the SARS‐CoV2 proteins, Spike (S), Nucleocapsid (N), and receptor‐binding domain (RBD) are potential target antigens that IgM and IgG antibodies are produced against. Accordingly, ELISA assay kits and other serological techniques have been developed to detect antibodies based on the aforementioned antigens.
5.3.1. The ELISA
Following the exposure to SARS‐CoV2, the host immune system releases the IgM and IgG antibodies against the virus. First, IgM appears in the initial exposure to response to SARS‐CoV2 antigens and is involved in the primary immune responses; however, IgG is also engaged in secondary immune responses. IgM is the first produced antibody with low concentration, low affinity, and limited maintenance time, which is disappeared at the end of the infection. Contrarily, IgG is later secreted with high concentration, affinity, and maintenance time, and also persists after infection recovery. Accordingly, IgM detection indicates acute infection, and IgG is a diagnostic index of previous, middle, or late infection. Thereby, developing ELISA tests for detecting these anti‐SARS‐CoV2 antibodies would help early detection of positive cases at least a few weeks after infection onset. Interestingly, a large number of suspected cases can be identified in a short time with simple facilities and acceptable sensitivity/specificity rates, as well as a low rate of false‐negative results.
In one study, W. Zhang et al. (2020) investigated the dynamic alterations of viral presence in both oral and anal swabs derived from COVID‐19 patients who received medical treatments for about 10 days. They evaluated the presence of both viral antibody and viral nucleotide levels based on a previously established method. Both IgM and IgG in all 39 SARS‐CoV2 patients were detected 5 days after infection onset, which was undetectable on Day 0. As a result, the positive rate of IgM was enhanced from 50% to 81%, and IgG reached from 81% to 100%.
Xiang et al. (2020) conducted the ELISA test on 63 COVID‐19 infected patients admitted to Jinyintan Hospital in Wuhan, Hubei. Serum samples of patients were assessed by IgM capture ELISA and IgG indirect ELISA. The study findings revealed that IgM was positive in 28 of 63 samples with 64.3% accuracy, 44.4% sensitivity, and 100% specificity. Also, the sample detection of 52 cases indicates a positive IgG result with 82.54% sensitivity, 100% specificity, and 88.8% accuracy. Moreover, 87.3% sensitivity was obtained using the combinational detection of IgM and IgG.
Another study by Liu, Liu, Wang, and Zheng (2020) was performed to analyze the diagnostic values of IgM and IgG antibodies against the N protein of SARS‐CoV2 in 238 hospital admitted patients with confirmed or suspected SARS‐COVID‐19 infection. Surprisingly, the ELISA findings indicated that suspected cases were positively contaminated by SARS‐CoV2. They also detected the IgM and IgG in 194 cases, with a 81.5% positive rate, which was considerably more than the RT‐PCR assay (64.3%). Additionally, it was found that ELISA detected suspected patients who had negative RT‐PCR results with a 78.8% positive rate for IgM and IgG.
W. Liu et al. (2020) measured the produced IgM, and IgG antibodies against nucleocapsid protein (rN) and spike protein (rS) of SARS‐CoV2 in 214 COVID‐19 confirmed patients using the ELISA. Successfully, rN‐based IgM and IgG were detected in 68.2% and 70.1% of patients, respectively. Besides this, rS‐based IgM and IgG were discovered in 77.1% and 74.3% of patients, respectively. Findings also reported that positive rates of IgM/IgG were 80.4% and 82.2% for rN‐ and rS‐based detections, respectively. Furthermore, it was found that rS‐based detection of IgM had a higher sensitivity than rN‐based detection.
In an investigation by Amanat et al. (2020), ELISA was developed using recombinant antigens originating from the spike protein (SP) of SARS‐CoV2. They showed that screening and identification of COVID‐19 seroconverters were possible as early as 3 days following the symptom onset with high sensitivity and specificity. As expected, no reactivity was observed in individuals who were not exposed to SARS‐CoV2 and were completely naive for SP. By reliance on these details, exposed/immune and naive people can be easily distinguished. In a related study by Stadlbauer et al. (2020), a detailed protocol was provided for the expression of antigens derived from the spike protein that can be used as a substrate for setting up ELISA and other immunological assays. They claimed that the presented protocol was adapted to local needs, including research, diagnostic, and clinical laboratories. With respect to the applications of ELISA in detecting the anti‐SARS‐CoV2 IgM/IgG antibodies based on the findings mentioned above, it is considered as an applicable serological assay for the detection of COVID‐19 with high sensitivity and specificity.
5.3.2. Magnetic chemiluminescence enzyme immunoassay (MCLIA)
In one study, Long et al. (2020) designed a study to investigate the profile of the acute antibody responses through a cross‐section analysis of 285 patients and a follow‐up study of 63 patients. They assessed the application of the antibody assay, as a facilitated diagnostic approach, to identify the COVID‐19 infection in both suspicious and asymptomatic close contact cases. A MCLIA kit was used to detect the anti‐SARS‐CoV2 IgM and IgG in the plasma samples based on double‐antibodies sandwich immunoassay using an automated magnetic chemiluminescence analyzer. Accordingly, 17–19 days following the onset of symptoms, the positive rate of IgG was 100%; whereas, after 20–22 days, the IgM had an around 94.1% positive rate. They also found that the IgM and IgG titers were gradually increased within 3 weeks after onset of the symptoms; however, IgM indicated a slight decrease after more than 3 weeks. Moreover, it was understood that in patients with a severe condition, IgG and IgM titers were more remarkable than those with the non‐severe condition. On the basis of the cross‐section analysis and the follow‐up study, the findings demonstrated that IgG and IgM seroconversion were detected in all COVID‐19 confirmed cases, 20 days after onset of the symptoms. As a consequence, they found that the elimination of SARS‐CoV2 infection could be confirmed due to the under detectable IgM and IgG in patients 20 days after symptom initiation and 23 days after exposure.
5.3.3. Colloidal gold‐immunochromatographic assay (GICA)
Another potent and useful diagnostic serologic immunoassay is the GICA, which is beneficial for the early detection of COVID‐19 patients. The GICA technique is based on the specific antigen–antibody immunoreactions, which is completed within 30 min without requiring intricate equipment and procedures. In this context, Xiang et al. (2020) figured out the anti‐SARS‐CoV2 IgM and IgG antibodies using the rapid GICA kits in the serum samples of 91 patients. Results unraveled that IgM and IgG were positive in 52 and 74 patients, respectively. In detail, 57.1% sensitivity, 100% specificity, and 69% accuracy were reported for the IgM detecting test. Also, 81.3% sensitivity, 100% specificity, and 86.5% accuracy was obtained for IgG detection. Interestingly, the combined detection of IgM and IgG accounted for 82.4% sensitivity.
In another investigation, Pan, Li, et al. (2020) applied the GICA assay to detect the IgM and IgG in infected patients confirmed with RT‐PCR. As a result, IgM and IgG have a relatively low positive rate in the early stages of the disease but gradually increased within the disease progression. Accordingly, the IgM positive rate in the early, middle, and late stages of the disease, was 11.1%, 78.6%, and 74.2%, respectively. Moreover, the positive rate of IgG in the early, middle, and late stages of the disease, was 3.6%, 57.1%, and 96.8%, respectively. Noteworthily, combined detection of IgM and IgG by GICA demonstrated the 92.9% positive rate in the middle stage of the disease.
As for advantages, the GICA assay has shown high sensitivity, particularly 7 days after infection onset, in detecting patients with a negative nuclear‐acid diagnostic. It is completed within 15 min without requiring specialist equipment and procedures. The GICA assay can be utilized with high adoption in areas with limited diagnostic capacity. On the basis of this, it is recommended to use the GICA as a supplementary diagnostic approach. It is known as a sensitive complementary and early detection assay. Despite the mentioned advantages, GICA provides only qualitative findings.
5.3.4. LFAs
The LFA, a qualitative immunoassay, is another potent serological technology used to detect the COVID‐19 infected cases with a rapid, simple, and low‐cost procedure. LFA acts based on a paper‐based platform for quantifying multiple analytes in a sample within 5–30 min. Accordingly, it can be employed to detect the presence or absence of the anti‐SARS‐CoV2 IgM and IgG antibodies in blood samples of patients within 15 min, simultaneously. Moreover, due to good adoption, LFA can be widely used in remote regions, developing countries, and small laboratories (Koczula & Gallotta, 2016). Wang et al. (Z. Li et al., 2020) designed a study to detect the IgM and IgG antibodies in blood samples of 397 clinically positive and 128 clinically negative COVID‐19 patients using the IgM/IgG combined antibody test (Point of care LFA Kit). As a result, they reported 88.66% sensitivity and 90.63% specificity for overall testing. Additionally, they found that both IgM and IgG antibodies were positive in 64.48% of patients; however, false positive and false negative results were also observed using this technique.
5.3.5. Rapid antigen tests
Nucleic acid‐based molecular tests and antibody assays have been applied for detecting the SARS‐CoV2 infection. However, a viral antigen has not been developed to identify the infection. Viral antigens derived from virus proteins are known as specific indications of the virus, which appear before the antibodies. Therefore, diagnosing viral antigens would facilitate the rapid screening and diagnosis of infection to prevent virus outbreak and increase survival of patients. To recognize the human coronaviruses diseases (HCoVs), utilizing the rapid antigen tests provides an infection diagnosis by a short time, low cost, and simple equipment procedure. Unfortunately, the low sensitivity of the antigen rapid test limits the broader usage, which has been evidenced in the detection of the influenza viruses (Y. Chen et al., 2016; Sastre et al., 2011). Interestingly, to enhance the efficacy of antigen rapid test to detect the SARS‐CoV2 and other respiratory viruses, colloidal gold‐labeled IgG can be employed as a diagnostic element.
According to a study conducted for an antigen rapid test, the findings demonstrated the high sensitivity of detecting the N antigen derived from SARS‐CoV in the early stages of the infection, which was a useful early diagnostic approach for SARS‐CoV (Che et al., 2004; Di et al., 2005). In a pre‐peer reviewed study (Diao et al., 2020), a cohort of 239 participants with suspected SARS‐CoV2 infection was included to detect the viral antigen. Accordingly, the nucleocapsid protein (NP) of SARS‐CoV2 was evaluated in nasopharyngeal swab and urine sample within 10 minutes using a fluorescence immunochromatographic assay. In this method, the nucleic acid test and the nasopharyngeal swab nucleic acid test were considered as the reference standards for detecting the NP in nasopharyngeal swab and urine samples, respectively. As a result, in 141 of 208 patients with positive COVID‐19 nucleic acid results, the N antigen was detected with 68% sensitivity. Likewise, in 31 patients with negative nucleic acid tests, the N protein was negative with 100% specificity. The findings also revealed 72% accuracy (total coincidence rate) of this antigen rapid test. In addition, they found that 73.6% of patients were diagnosed through the detection of N protein in the urine samples. Consequently, the results of this study indicated 94% and 78% sensitivity for N antigen detection in the first 5 days and 6–10 days after infection onset, respectively. As a consequence, antigen rapid tests can be employed as early, rapid, accurate, and simple diagnostic approaches for COVID‐19 infection.
5.3.6. Immunoassay techniques
Immunoassay techniques have been developed for the quantitative, semiquantitative, or qualitative detection of the analytes. Immunoassays have multiple functional aspects, including clinical diagnosis, environmental monitoring, food testing, and biopharmaceutical analysis. The direct fluorescent antibody (DFA) test, the microneutralization (MN) assay, NP detection assay, immunofluorescence assay (IFA), semiconductor quantum dots (QDs), and protein chip are some examples of immunoassays carried out to detect the concentration of the analytes based on antigen–antibody reactions (Chan et al., 2013; Roh & Jo, 2011). Accordingly, immunoassays can detect related antigens or antibodies of coronavirus and determine the antigen–antibody interactions. Despite the benefits of these simple and rapid immunoassay techniques, low sensitivity and specificity have limited their usage. However, they can be applied as rapid and available diagnostic methods to assist in the early detection of COVID‐19.
5.3.7. Pseudotyped‐lentiviral‐vector‐based neutralization assay
Neutralizing antibodies (NAbs) are vital immune factors produced against viral diseases, which have principal roles in virus clearance, protection, and recovery of infected patients. According to previously published results, the detection of NAbs is considered as a gold standard evaluation of antiviral vaccines against polio, influenza, and smallpox infections and plasma therapy in Ebola, influenza, and SARS‐CoV infections (Van Griensven et al., 2016; Wong, Dai, Wu, & Sung, 2003; B. Zhou, Zhong, & Guan, 2007). To develop vaccines or transfusion of convalescent plasma for prophylaxis or treating COVID‐19 patients, SARS‐CoV2‐specific NAbs can be detected in the plasma of recovered patients using the pseudotyped‐lentiviral‐vector‐based neutralization assay, a sensitive and reproducible test (Casadevall & Pirofski, 2020). In one study, F. Wu et al. (2020) measured the anti‐SARS‐CoV2 NAbs in the plasma of 175 adult COVID‐19 recovered patients with common or mild conditions performing the pseudovirus neutralization assay to investigate the correlation between clinical features of infected patients and NAbs levels. As for results, medium‐low (ID50: 500‐999), medium‐high (ID50: 1000–2500), and high (ID50 > 2500) levels of NAb titers were found in around 17%, 39%, and 14% studied cases, respectively. However, 30% of them have decreased levels of NAb titers (ID50 < 500) and ten of them have NAb titers below the limit of the detection (ID50 < 40). Moreover, they showed that there is a positive correlation between anti‐SARS‐CoV2 NAbs and CRP level; whereas no significant association was observed between NAbs titers and lymphocyte count in older COVID‐19 recovered patients. Consequently, evaluating the NAbs against SARS‐CoV2 would provide an understanding of whether they could protect the patients from disease progression and help develop the immune‐based treatments like vaccines and plasma infusion.
6. COMPARISON BETWEEN CT IMAGING ASSAY AND RT‐PCR ASSAY
According to the low viral load of the virus and the high false‐negative rate of the RT‐PCR results at the initial stages of the disease, it is required to screen the suspected patients with CT images. Fang et al. analyzed the sensitivity rate of COVID‐19 lung involvement on chest CT and detection of the virus nucleic acid by RT‐PCR. They reported that the sensitivity of the CT (98%) is higher than RT‐PCR (71%; p < .001). The reasons for the low effectiveness of the RT‐PCR approach may be as follows; inapplicable clinical sampling, rudimentary improvement of nucleic acid detection by RT‐PCR, differences in the detection rate of various companies, and low viral load. This study has supported the idea of using the chest CT in the early stages to screen suspected patients, especially those who have negative RT‐PCR results (Fang et al., 2020). Xie et al. (2020) followed five COVID‐19 suspected patients with initial negative RT‐PCR test results. The radiological findings on CT represented GGO and/or consolidations. They isolated the mentioned patients. Eventually, repeated swab tests and CT scans led to the COVID‐19 confirmed state. So, it was beneficial to examine the highly suspicious patients with repeated swab tests, especially while they had initial negative RT‐PCR results. Ai et al. (2020) compared the two CT and RT‐PCR methods in 1,014 patients with clinical symptoms. As a result, 601/1,014 patients (59%) had positive RT‐PCR results for COVID‐19, and 888/1,014 patients (88%) had positive imaging findings on CT. Their reports have demonstrated the higher sensitivity rate of CT diagnosis compares to initial RT‐PCR. The serial analysis of RT‐PCR and CT scans proved that 60–93% of the patients had serial positive imaging manifestations for COVID‐19 on CT before the RT‐PCR tests became positive. Also, the follow‐up CT scans show a recovery process of 42% in the patients before the RT‐PCR results became negative.
7. COMPARISON BETWEEN ELISA ASSAY AND RT‐PCR ASSAY
Early and accurate detection of positive infected cases is a critical issue in managing the spread of COVID‐19. RT‐PCR assays are required for confirming the infection; however, considering the related limitations, serological tests may lead to better diagnosis of positive cases. For this reason, detecting the serum specific antibodies of SARS‐CoV2 by ELISA technique is recommended due to the capability of tracing immune responses against the virus, short time procedure, and requiring simple facilities. Serological tests have potent applications in early diagnosis of infection. The advantages of serological assays based on antibody detection include less false‐negative results, indicating past infections, determining infection stage, and estimating time since exposure. This means that antibody tests can be used to determine whether a patient with no symptoms has previously had COVID‐19 and could thus be immune‐protected to it. As IgM can be detected in 3 days after infection onset in most infections, it confirms the advantage of early detection of the serological tests compared with RT‐PCR. Also, the importance of specimen quality for the serological assays is less stringent compared with RT‐PCR assays. Importantly, false‐negative results derived from sampling quality are fewer in using antibodies for serological diagnosis (Xiao, Wu, & Liu, 2020). On the basis of this, it has been suggested that using serological assays provides the facility of early diagnosis of positive cases even with an improper sample collection and unsatisfied molecular examinations.
As another benefit, a broad scale of infection diagnosis is achieved using the ELISA technique that detects the antibodies by automatic devices. Moreover, ELISA provides the feasibility of identifying a large number of infected cases in a quick turnaround time. The other limitations of RNA‐based assays that led to the critical necessity to use ELISA include requiring upscale and expensive lab facilities, restrictive biosafety levels, low or middle detection sensitivity and specificity, different sampling locations, and technical experts. Another essential issue is the ease of using serological tests in the clinical laboratory of any hospital, as opposed to the molecular approach that requires a specific and well‐equipped laboratory. Notably, reporting on the very mild or asymptomatically infected cases, as a large population without viral RNA‐based testing, is another critical aspect that should be considered. Hence, detection of the specific IgG antibody, as a large‐scale seroepidemiological study, would help understand the true scale of human‐to‐human transmission and report the accurate rate of COVID‐19 infected cases (Xiao et al., 2020).
8. FDA‐APPROVAL DIAGNOSTIC RAPID TESTS
As the SARS‐CoV2 leads to serious‐life‐threatening conditions like severe respiratory disorders, the importance of early detection of suspected individuals has been highlighted from the virus outbreak until now. In this regard, the FDA emergency has approved some potent SARS‐CoV2 diagnostic techniques such as IgM/IgG diagnostic rapid tests and the new Abbott ID NOW COVID‐19 test for emergency use.
8.1. Cellex qSARS‐CoV‐2 IgG/IgM rapid test
Currently, encouragingly, FDA approved the rapid qualitative test for the detection of the anti‐SARS‐CoV2 IgM and IgG antibodies in serum, plasma, and venipuncture whole blood specimens of COVID‐19 suspected cases. The Cellex qSARS‐CoV2 IgG/IgM Rapid acts based on a lateral chromatographic flow immunoassay intended for the qualitative detection and differentiation of anti‐SARS‐CoV2 IgM and IgG antibodies (U.S. Food & Drug Administration, 2020). Promisingly, these types of applicable diagnostic tests would contribute to the early detection of infected or suspected cases and prevent the spread of the virus.
8.2. New Abbott ID NOW COVID‐19
Recently, the new Abbott ID NOW COVID‐19 test has received emergency use authorization (EUA) from the FDA to detect the novel coronavirus (SARS‐CoV2), as the fastest available molecular point‐of‐care test. The Abbott ID NOW test is identified as a rapid, isothermal, and instrument‐based, system that can be used for the qualitative identification of infections. Interestingly, this approach has a unique isothermal nucleic acid amplification technology that provides molecular results in a short time, which lets clinicians make evidence‐based clinical decisions during a patient visit. Accordingly, it has been reported that the Abbott ID NOW assay presents positive results in as few as 5 min and negative results within 13 min, based on the rRT‐PCR assay. Moreover, for the detection of Influenza A&B, Strep A and respiratory syncytial virus (RSV), ID NOW is the pioneer molecular point‐of‐care platform (Abbott, 2020).
9. CONCLUDING REMARKS
With regardto the current outbreak of the novel coronavirus (2019‐nCoV) worldwide, arisen from Wuhan, China, it is critically required to identifypractical strategies for precise diagnostic methods and appropriate treatment choices. The rapid human‐to‐human transmission of SARS‐CoV2, even by asymptomatic carriers has led to an intensive health disaster. The most common clinical manifestations of the disease are fever, cough, and dyspnea. Some complications such as ARDS, acute kidney injury, and liver damage may also occur during the process of the disease, which can cause death, subsequently. Additionally, the elevated levels of pro‐ and inflammatory cytokines elicit the cytokine storm phenomenon, which has proved to be a prominent reason for respiratory system destruction.
The typical laboratory abnormalities in COVID‐19 patients include lymphopenia, hypoalbuminemia, and increased levels of CRP, ESR, LDH, AST, ALT, and D‐dimer. Although the characteristics of clinical features and abnormal laboratory finding results can guide healthcare workers toward a primary diagnosis, the final detection of the COVID‐19 patients is confirmed by spiral lung CT scans, molecular assays, and serological tests. Mostly, bilateral and peripheral GGOs and/or occasional consolidations have been observed in radiological findings of lung involvements. Indisputably, initial chest CT could be performed as a potential diagnostic modality in symptomatic cases, especially at the early stages of the disease. Of note, it should be considered that some suspected patients may have no CT findings; therefore, repeated CT imaging and other diagnostic techniques would assist in ruling out or confirming the suspected individuals. The other applicable confirmation methods are RNA‐based molecular assays, among which the rRT‐PCR test is a gold‐standard approach. Despite the rapid and definite diagnosis of the infected cases, high‐FNR, lengthy procedure, and the necessity of intricate equipment restricts the utilization of the RT‐PCR assays compared with CT imaging. Recently, serological techniques have been developed as another quick diagnostic test based on detecting SARS‐CoV2‐specific IgM/IgG antibodies with high sensitivity and specificity. Besides this, it is worth noting that the accurate process of sample collection, sampling clinical specimens from multiple sites, and different detection rates of positive specimens should be considered to achieve the most accurate test results for COVID‐19 diagnosis. In conclusion, the profound comprehension of various application aspects of the diagnostic approaches, their advantages and disadvantages, as well as their appropriate administration could lead to taking efficacious steps forward to prevent the virus outbreak, manage the current pandemic state, contribute to infected patients’ recovery, and subsequently promote public health.
CONFLICT OF INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
S. T. and E. K. H. contributed to data gathering, writing the primary draft of the manuscript, and designing figures and tables. A. E. contributed to the hypothesis, correspondence, scientific and structural editing, and verifying the manuscript before submission.
ACKNOWLEDGMENTS
The authors would like to dedicate this study to healthcare workers, which are struggling with the novel coronavirus.
Tahmasebi S, Khosh E, Esmaeilzadeh A. The outlook for diagnostic purposes of the 2019‐novel coronavirus disease. J Cell Physiol. 2020;235:9211–9229. 10.1002/jcp.29804
Safa Tahmasebi and Elnaz Khosh contributed equally to this study.
DATA AVAILABILITY STATEMENT
n/a
REFERENCES
- Abbott . (2020). Abbott launches molecular point‐of‐care test to detect novel coronavirus in as little as five minutes. Retrieved from https://abbott.mediaroom.com/2020‐03‐27‐Abbott‐Launches‐Molecular‐Point‐of‐Care‐Test‐to‐Detect‐Novel‐Coronavirus‐in‐as‐Little‐as‐Five‐Minutes
- Adhikari, S. P. , Meng, S. , Wu, Y. J. , Mao, Y. P. , Ye, R. X. , Wang, Q. Z. , & Zhou, H. (2020). Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: A scoping review. Infectious Diseases of Poverty, 9(1), 29. 10.1186/s40249-020-00646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai, T. , Yang, Z. , Hou, H. , Zhan, C. , Chen, C. , Lv, W. , … Xia, L. (2020). Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: A report of 1014 cases. Radiology, 200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat, F. , Nguyen, T. , Chromikova, V. , Strohmeier, S. , Stadlbauer, D. , Javier, A. , … Krammer, F. (2020). A serological assay to detect SARS‐CoV‐2 seroconversion in humans. MedRxiv, 10.1101/2020.03.17.20037713 [DOI] [PMC free article] [PubMed]
- Arnold, M. , Slomka, M. , Coward, V. , Mahmood, S. , Raleigh, P. , & Brown, I. (2013). Evaluation of the pooling of swabs for real‐time PCR detection of low titre shedding of low pathogenicity avian influenza in turkeys. Epidemiology & Infection, 141(6), 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangash, M. N. , Patel, J. , & Parekh, D. (2020). COVID‐19 and the liver: Little cause for concern. The Lancet Gastroenterology & Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim, A. , Mei, X. , Huang, M. , Yang, Y. , Fayad, Z. A. , Zhang, N. , … Chung, M. (2020). Chest CT findings in coronavirus disease‐19 (COVID‐19): Relationship to duration of infection. Radiology, 200463. 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann, D. , Tsukahara, T. , Weinreb, C. , Logan, D. W. , & Datta, S. R. (2020). Non‐neural expression of SARS‐CoV‐2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID‐19 patients. bioRxiv, 10.1101/2020.03.25.009084 [DOI] [PMC free article] [PubMed]
- Casadevall, A. , & Pirofski, L. A. (2020). The convalescent sera option for containing COVID‐19. The Journal of Clinical Investigation, 130(4), 1545–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella, M. , Rajnik, M. , Cuomo, A. , Dulebohn, S. C. , & Di Napoli, R. (2020). Features, evaluation and treatment coronavirus (COVID‐19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [PubMed]
- Centers for Disease Control and Prevention (CDC) . (2020). 2019 Novel coronavirus, Wuhan, China. Retrieved from https://www.cdc.gov/coronavirus/2019‐ncov/publications.html
- Chan, J. F.‐W. , Yip, C. C.‐Y. , To, K. K.‐W. , Tang, T. H.‐C. , Wong, S. C.‐Y. , Leung, K.‐H. , & Tsoi, H.‐W. (2020). Improved molecular diagnosis of COVID‐19 by the novel, highly sensitive and specific COVID‐19‐RdRp/Hel real‐time reverse transcription‐polymerase chain reaction assay validated in vitro and with clinical specimens. Journal of Clinical Microbiology, 58, e00310‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. F.‐W. , Yuan, S. , Kok, K.‐H. , To, K. K.‐W. , Chu, H. , Yang, J. , & Poon, R. W.‐S. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: A study of a family cluster. The Lancet, 395(10223), 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, K. H. , Chan, J. F. , Tse, H. , Chen, H. , Lau, C. C. , Cai, J. P. , & Yuen, K. Y. (2013). Cross‐reactive antibodies in convalescent SARS patients' sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. Journal of Infection, 67(2), 130–140. 10.1016/j.jinf.2013.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, D. , Lin, M. , Wei, L. , Xie, L. , Zhu, G. , Dela Cruz, C. S. , & Sharma, L. (2020). Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. Journal of the American Medical Association, 323(11), 1092–1093. 10.1001/jama.2020.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, X. Y. , Hao, W. , Wang, Y. , Di, B. , Yin, K. , Xu, Y. C. , & Yuen, K. Y. (2004). Nucleocapsid protein as early diagnostic marker for SARS. Emerging Infectious Diseases, 10(11), 1947–1949. 10.3201/eid1011.040516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Liu, H. , Liu, W. , Liu, J. , Liu, K. , Shang, J. , & Wei, S. (2020). Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi, 43, E005. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Chan, K.‐H. , Hong, C. , Kang, Y. , Ge, S. , Chen, H. , & Wernery, U. (2016). A highly specific rapid antigen detection assay for on‐site diagnosis of MERS. Journal of Infection, 73(1), 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D. K. , Pan, Y. , Cheng, S. M. , Hui, K. P. , Krishnan, P. , Liu, Y. , & Wang, Q. (2020). Molecular diagnosis of a novel coronavirus (2019‐nCoV) causing an outbreak of pneumonia. Clinical Chemistry, 66(4), 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, M. , & Bernheim, A. (2020). CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology, 295(1), 202–207. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman, V. M. , Albarrak, A. M. , Omrani, A. S. , Albarrak, M. M. , Farah, M. E. , Almasri, M. , & Assiri, A. M. (2016). Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clinical Infectious Diseases, 62(4), 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman, V. M. , Landt, O. , Kaiser, M. , Molenkamp, R. , Meijer, A. , Chu, D. K. W. , & Drosten, C. (2020). Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveillance, 25(3), 2000045. 10.2807/1560-7917.es.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID‐19 . (2020). Australia: Epidemiology report 7 (reporting week ending 19:00 AEDT 14 March 2020). Communicable Diseases Intelligence, 44, 10.33321/cdi.2020.44.23 [DOI] [PubMed] [Google Scholar]
- Dai, W. C. , & Zhang, H. W. (2020). CT imaging and differential diagnosis of COVID‐19. Canadian Association of Radiologists Journal, 71(2), 195–200. 10.1177/0846537120913033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, B. , Hao, W. , Gao, Y. , Wang, M. , Wang, Y. D. , Qiu, L. W. , & Che, X. Y. (2005). Monoclonal antibody‐based antigen capture enzyme‐linked immunosorbent assay reveals high sensitivity of the nucleocapsid protein in acute‐phase sera of severe acute respiratory syndrome patients. Clinical and Diagnostic Laboratory Immunology, 12(1), 135–140. 10.1128/cdli.12.1.135-140.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, B. , Wen, K. , Chen, J. , Liu, Y. , Yuan, Z. , Han, C. , … Wu, Y. (2020). Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. MedRxiv, 10.1101/2020.03.07.20032524 [DOI]
- El‐Tholoth, M. , Bau, H. H. , & Song, J. (2020). A single and two‐stage, closed‐tube, molecular test for the 2019 novel coronavirus (COVID‐19) at home, clinic, and points of entry.
- Fang, Y. , Zhang, H. , Xie, J. , Lin, M. , Ying, L. , Pang, P. , & Ji, W. (2020). Sensitivity of chest CT for COVID‐19: Comparison to RT‐PCR. Radiology, 200432. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. J. , Ni, Z. Y. , Hu, Y. , Liang, W. H. , Ou, C. Q. , He, J. X. , … Zhong, N. S. (2020). Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv.
- Guo, X. , Guo, Z. , Duan, C. , Wang, G. , Lu, Y. , Li, M. , & Lu, J. (2020). Long‐term persistence of IgG antibodies in SARS‐CoV infected healthcare workers. MedRxiv.
- Han, W. , Quan, B. , Guo, Y. , Zhang, J. , Lu, Y. , Feng, G. , & Jiao, N. (2020). The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. Journal of Medical Virology, 92, 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , & Yang, H. (2019). The transmission and diagnosis of 2019, novel coronavirus infection disease (COVID‐19): A Chinese perspective. Journal of Medical Virology, 92, 639–644. 10.1002/jmv.25749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Z. , Song, C. , Xu, C. , Jin, G. , Chen, Y. , Xu, X. , & Shen, H. (2020). Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Science China: Life Sciences, 63, 706–711. 10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , & Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, K. R. , Koetzner, C. A. , & Masters, P. S. (2009). Identification of in vivo‐interacting domains of the murine coronavirus nucleocapsid protein. Journal of Virology, 83(14), 7221–7234. 10.1128/jvi.00440-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne, J. P. (2020). Chest CT findings in 2019 novel coronavirus (2019‐nCoV) Infections from Wuhan, China: Key points for the radiologist. Radiology, 295(1), 16–17. 10.1148/radiol.2020200241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne, J. P. , Little, B. P. , Chung, J. H. , Elicker, B. M. , & Ketai, L. H. (2020). Essentials for radiologists on COVID‐19: An update‐radiology scientific expert panel. 200527. 10.1148/radiol.2020200527 [DOI] [PMC free article] [PubMed]
- Ko, J. H. , Muller, M. A. , Seok, H. , Park, G. E. , Lee, J. Y. , Cho, S. Y. , & Peck, K. R. (2017). Serologic responses of 42 MERS‐coronavirus‐infected patients according to the disease severity. Diagnostic Microbiology and Infectious Disease, 89(2), 106–111. 10.1016/j.diagmicrobio.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczula, K. M. , & Gallotta, A. (2016). Lateral flow assays. Essays in Biochemistry, 60(1), 111–120. 10.1042/ebc20150012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek, T. G. , Erdman, D. , Goldsmith, C. S. , Zaki, S. R. , Peret, T. , Emery, S. , & Lim, W. (2003). A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine, 348(20), 1953–1966. [DOI] [PubMed] [Google Scholar]
- Kui, L. , Fang, Y.‐Y. , Deng, Y. , Liu, W. , Wang, M.‐F. , Ma, J.‐P. , & Li, C.‐H. (2020). Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chinese Medical Journal, 133(9), 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, L. , Xu, D. , Ye, G. , Xia, C. , Wang, S. , Li, Y. , & Xu, H. (2020). Positive RT‐PCR test results in patients recovered from COVID‐19. Journal of the American Medical Association, 323, 1502. 10.1001/jama.2020.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. (2016). Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology, 3(1), 237–261. 10.1146/annurev-virology-110615-042301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. C. , Bai, W. Z. , & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Yi, Y. , Luo, X. , Xiong, N. , Liu, Y. , Li, S. , & Chen, W. (2020). Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. Journal of Medical Virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Liu, Y. , Xiang, P. , Pu, L. , Xiong, H. , Li, C. , … Wang, X. (2020). Neutrophil‐to‐lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv, 10.1101/2020.02.10.20021584 [DOI] [PMC free article] [PubMed]
- Liu, L. , Liu, W. , Wang, S. , & Zheng, S. (2020). A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 238 admitted hospital patients. MedRxiv, 10.1101/2020.03.06.20031856 [DOI] [PMC free article] [PubMed]
- Liu, W. , Liu, L. , Kou, G. , Zheng, Y. , Ding, Y. , Ni, W. , … Zheng, S. (2020). Evaluation of nucleocapsid and spike protein‐based ELISAs for detecting antibodies against SARS‐CoV‐2. Journal of Clinical Microbiology, 10.1101/2020.03.16.20035014 [DOI] [PMC free article] [PubMed]
- Liu, Y. , Yang, Y. , Zhang, C. , Huang, F. , Wang, F. , Yuan, J. , & Feng, C. (2020). Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences, 63(3), 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Q. X. , Deng, H. J. , Chen, J. , Hu, J. , Liu, B. Z. , Liao, P. , … Huang, A. L. (2020). Antibody responses to SARS‐CoV‐2 in COVID‐19 patients: The perspective application of serological tests in clinical practice. MedRxiv, 10.1101/2020.03.18.20038018 [DOI]
- Ng, M.‐Y. , Lee, E. Y. , Yang, J. , Yang, F. , Li, X. , Wang, H. , & Kuo, M. D. (2020). Imaging profile of the COVID‐19 infection: Radiologic findings and literature review. Radiology: Cardiothoracic Imaging, 2(1), e200034. 10.1148/ryct.2020200034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, N. T. , Bish, E. K. , & Aprahamian, H. (2018). Sequential prevalence estimation with pooling and continuous test outcomes. Statistics in Medicine, 37(15), 2391–2426. [DOI] [PubMed] [Google Scholar]
- Pan, F. , Ye, T. , Sun, P. , Gui, S. , Liang, B. , Li, L. , … Zheng, C. (2020). Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology, 200370. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y. , Li, X. , Yang, G. , Fan, J. , Tang, Y. , Zhao, J. , … Li, Y. (2020). Serological immunochromatographic approach in diagnosis with SARS‐CoV‐2 infected COVID‐19 patients. Journal of Infection, 10.1101/2020.03.13.20035428 [DOI] [PMC free article] [PubMed]
- Pan, Y. , Zhang, D. , Yang, P. , Poon, L. L. M. , & Wang, Q. (2020). Viral load of SARS‐CoV‐2 in clinical samples. The Lancet Infectious Diseases, 20(4), 411–412. 10.1016/s1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pormohammad, A. , Ghorbani, S. , Baradaran, B. , Khatam, A. , Turner, R. , Mansournia, M. A. , & Bahr, N. C. (2020). Clinical characteristics, laboratory findings, radiographic signs and outcomes of 52,251 patients with confirmed COVID‐19 infection: A systematic review and meta‐analysis. [DOI] [PMC free article] [PubMed]
- Riou, J. , & Althaus, C. L. (2020). Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐nCoV), December 2019 to January 2020. Euro Surveillance, 25(4), 2000058. 10.2807/1560-7917.es.2020.25.4.2000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, T. C. , Hart, J. R. , Kaikkonen, M. U. , Weinberg, M. S. , Vogt, P. K. , & Morris, K. V. (2015). Quantification of nascent transcription by bromouridine immunocapture nuclear run‐on RT‐qPCR. Nature Protocols, 10(8), 1198–1211. 10.1038/nprot.2015.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Morales, A. J. , Cardona‐Ospina, J. A. , Gutiérrez‐Ocampo, E. , Villamizar‐Peña, R. , Holguin‐Rivera, Y. , Escalera‐Antezana, J. P. , & Henao‐Martinez, A. F. (2020). Clinical, laboratory and imaging features of COVID‐19: A systematic review and meta‐analysis. Travel Medicine and Infectious Disease, 34, 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh, C. , & Jo, S. K. (2011). Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots‐conjugated RNA aptamer on chip. Journal of Chemical Technology & Biotechnology, 86(12), 1475–1479. 10.1002/jctb.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino‐Silva, R. , Jardim, A. C. G. , & Siqueira, W. L. (2020). Coronavirus COVID‐19 impacts to dentistry and potential salivary diagnosis. Clinical Oral Investigations, 24(4), 1619–1621. 10.1007/s00784-020-03248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre, P. , Dijkman, R. , Camunas, A. , Ruiz, T. , Jebbink, M. F. , van der Hoek, L. , & Rueda, P. (2011). Differentiation between human coronaviruses NL63 and 229E using a novel double‐antibody sandwich enzyme‐linked immunosorbent assay based on specific monoclonal antibodies. Clinical and Vaccine Immunology, 18(1), 113–118. 10.1128/cvi.00355-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman, D. , & Fielding, B. C. (2019). Coronavirus envelope protein: Current knowledge. Journal of Virology, 16(1), 69. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, A. , Al‐Taher, A. , Al‐Nazawi, M. , Al‐Mubarak, A. I. , & Kandeel, M. (2020). Analysis of preferred codon usage in the coronavirus N genes and their implications for genome evolution and vaccine design. Journal of Virological Methods, 277, 113806. 10.1016/j.jviromet.2019.113806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, K. , Yang, Y. , Wang, T. , Zhao, D. , Jiang, Y. , Jin, R. , & Gao, L. (2020). Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: Experts' consensus statement. World Journal of Pediatrics, 10.1007/s12519-020-00343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, F. , Shi, N. , Shan, F. , Zhang, Z. , Shen, J. , Lu, H. , & Shi, Y. (2020). Emerging 2019 novel Ccoronavirus (2019‐nCoV) pneumonia. Radiology, 295(1), 210–217. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer, D. , Amanat, F. , Chromikova, V. , Jiang, K. , Strohmeier, S. , Asthagiri Arunkumar, G. , … Krammer, F. (2020). A detailed protocol for a serological assay to detect SARS‐CoV‐2 seroconversion in humans: antigen production and test setup. [DOI] [PMC free article] [PubMed]
- Steardo, L. , Steardo, L., Jr. , & Zorec, R. (2020). Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID‐19. Acta Physiologica (Oxford, England), e13473. 10.1111/apha.13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. , Tsang, O. T. , Chik‐Yan Yip, C. , Chan, K. H. , Wu, T. C. , Chan, J. M. C. , & Yuen, K. Y. (2020). Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases, 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. W. , Yip, C. C. Y. , Lai, C. Y. W. , Wong, C. K. H. , Ho, D. T. Y. , Pang, P. K. P. , & Yuen, K. Y. (2019). Saliva as a diagnostic specimen for testing respiratory virus by a point‐of‐care molecular assay: A diagnostic validity study. Clinical Microbiology and Infection, 25(3), 372–378. 10.1016/j.cmi.2018.06.009 [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration . (2020). qSARS‐CoV‐2 IgG/IgM rapid test—Letter of authorization. Retrieved from https://www.fda.gov/media/136622/download
- Van Griensven, J. , Edwards, T. , de Lamballerie, X. , Semple, M. G. , Gallian, P. , Baize, S. , & Antierens, A. (2016). Evaluation of convalescent plasma for Ebola virus disease in Guinea. New England Journal of Medicine, 374(1), 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, S. , Yi, Q. , Fan, S. , Lv, J. , Zhang, X. , Guo, L. , … Chen, Y. (2020). Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv, 10.1101/2020.02.10.20021832 [DOI]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , & Xiong, Y. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association, 323, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Xu, Y. , Gao, R. , Lu, R. , Han, K. , Wu, G. , & Tan, W. (2020). Detection of SARS‐CoV‐2 in different types of clinical specimens. Journal of the American Medical Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Kang, H. , Liu, X. , & Tong, Z. (2020). Combination of RT‐qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. Journal of Medical Virology, 92, 538–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, V. , Dai, D. , Wu, A. , & Sung, J. (2003). Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Medical Journal, 9(3), 199–201. [PubMed] [Google Scholar]
- Wu, F. , Wang, A. , Liu, M. , Wang, Q. , Chen, J. , Xia, S. , & Lu, L. (2020). Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. MedRxiv. [Google Scholar]
- Wu, P. , Duan, F. , Luo, C. , Liu, Q. , Qu, X. , Liang, L. , & Wu, K. (2020). Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID‐19) in Hubei Province, China. JAMA Ophthalmol, 10.1001/jamaophthalmol.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Guo, C. , Tang, L. , Hong, Z. , Zhou, J. , Dong, X. , & Huang, X. (2020). Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol, 5, 434–435. 10.1016/s2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, J. , Yan, M. , Li, H. , Liu, T. , Lin, C. , Huang, S. , & Shen, C. (2020). Evaluation of enzyme‐linked immunoassay and colloidal gold‐immunochromatographic assay kit for detection of novel coronavirus (SARS‐Cov‐2) causing an outbreak of pneumonia (COVID‐19). MedRxiv, 10.1101/2020.02.27.20028787 [DOI]
- Xiao, S.‐Y. , Wu, Y. , & Liu, H. (2020). Evolving status of the 2019 novel coronavirus infection: Proposal of conventional serologic assays for disease diagnosis and infection monitoring. Journal of Medical Virology, 92(5), 464–467. 10.1002/jmv.25702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X. , Zhong, Z. , Zhao, W. , Zheng, C. , Wang, F. , & Liu, J. (2020). Chest CT for typical 2019‐nCoV pneumonia: Relationship to negative RT‐PCR testing. Radiology, 200343. 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Cao, Q. , Qin, L. , Wang, X. , Cheng, Z. , Pan, A. , & Yan, F. (2020). Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): A multi‐center study in Wenzhou city, Zhejiang, China. Journal of Infection, 80(4), 388–393. 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Yu, Y. , Xu, J. , Shu, H. , Liu, H. , Wu, Y. , & Yu, T. (2020). Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: A single‐centered, retrospective, observational study. The Lancet Respiratory Medicine, 8, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin, I. , Aharony, N. , Shaer‐Tamar, E. , Argoetti, A. , Messer, E. , Berenbaum, D. , & Hashimshony, T. (2020). Evaluation of COVID‐19 RT‐qPCR test in multi‐sample pools. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y. , & Wunderink, R. G. (2018). MERS, SARS and other coronaviruses as causes of pneumonia. Respirology (Carlton, Vic.), 23(2), 130–137. 10.1111/resp.13196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, S. H. , Lee, K. H. , Kim, J. Y. , Lee, Y. K. , Ko, H. , Kim, K. H. , & Kim, Y. H. (2020). Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID‐19): Analysis of nine patients treated in Korea. Korean Journal of Radiology, 21(4), 494–500. 10.3348/kjr.2020.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Wang, X. , Chen, Y. , Zhao, K. , Cai, Y. , An, C. , & Mu, X. (2020). Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua jie he he hu xi za zhi, 43(3), 215–218. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Du, R. H. , Li, B. , Zheng, X. S. , Yang, X. L. , Hu, B. , & Shi, Z. L. (2020). Molecular and serological investigation of 2019‐nCoV infected patients: Implication of multiple shedding routes. Emerging Microbes & Infections, 9(1), 386–389. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, D. , Yao, F. , Wang, L. , Zheng, L. , Gao, Y. , Ye, J. , & Gao, R. (2020). A comparative study on the clinical features of COVID‐19 pneumonia to other pneumonias. Clinical Infectious Diseases, 10.1093/cid/ciaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Zhao, Z. , Wang, Y. , Zhou, Y. , Ma, Y. , & Zuo, W. (2020). Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. bioRxiv, 10.1101/2020.01.26.919985 [DOI] [PMC free article] [PubMed]
- Zhou, B. , Zhong, N. , & Guan, Y. (2007). Treatment with convalescent plasma for influenza A (H5N1) infection. New England Journal of Medicine, 357(14), 1450–1451. [DOI] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , & Gu, X. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. The Lancet, 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , & Liu, H. G. (2020). [Early detection and disease assessment of patients with novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi, 43(3), 167–170. 10.3760/cma.j.issn.1001-0939.2020.03.003 [DOI] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , & Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
n/a


