Abstract
Objective
Coronavirus disease 2019 (COVID‐19) continues to spread, and younger patients are also being critically affected. This study analyzed obesity as an independent risk factor for mortality in hospitalized patients younger than 50.
Methods
This study retrospectively analyzed data of patients with COVID‐19 who were hospitalized to a large academic hospital system in New York City between March 1, 2020, and May 17, 2020. Data included demographics, comorbidities, BMI, and smoking status. Obesity groups included the following: BMI of 30 to < 40 kg/m2 and BMI ≥ 40 kg/m2. Multivariable logistic regression models identified variables independently associated with mortality in patients younger and older than 50.
Results
Overall, 3,406 patients were included; 572 (17.0%) patients were younger than 50. In the younger age group, 60 (10.5%) patients died. In the older age group, 1,076 (38.0%) patients died. For the younger population, BMI ≥ 40 was independently associated with mortality (adjusted odds ratio 5.1; 95% CI: 2.3‐11.1). For the older population, BMI ≥ 40 was also independently associated with mortality to a lesser extent (adjusted odds ratio 1.6; 95% CI: 1.2‐2.3).
Conclusions
This study demonstrates that hospitalized patients younger than 50 with severe obesity are more likely to die of COVID‐19. This is particularly relevant in the Western world, where obesity rates are high.
Study Importance.
What is already known?
-
►
Obesity was found to be more frequent among patients with coronavirus disease 2019 (COVID‐19) and was associated with increased need for invasive mechanical ventilation.
-
►
Obesity has been shown to be a risk factor for hospitalization in patients with COVID‐19 younger than 60.
What does this study add?
-
►
This multicenter US study shows that severe obesity (BMI ≥ 40) is strongly and independently associated with COVID‐19 mortality in hospitalized patients younger than 50.
How might these results change the direction of research or the focus of clinical practice?
-
►
Younger patients with BMI ≥ 40 are at a fivefold higher risk for mortality from COVID‐19.
-
►
These findings can have a significant impact on the triaging and evaluation of patients in similar settings.
-
►
The study highlights the central role of severe obesity in the pathogenesis of COVID‐19 mortality in the younger population. This is especially important in the United States and the Western world, where obesity rates are high. Further research should aim to understand the mechanisms that explain why obesity is associated with increased COVID‐19 mortality.
Introduction
The coronavirus disease 2019 (COVID‐19) is a pandemic viral disease caused by severe acute respiratory syndrome coronavirus 2. According to the Centers for Disease Control and Prevention, as of May 19, 2020, 1.48 million patients have tested positive for COVID‐19 in the United States. Of those, 89,407 (6.0%) patients have died (1). As COVID‐19 continues to spread, the younger population is also being critically affected. Comorbidities such as coronary artery disease (CAD), hypertension, and diabetes mellitus (DM) have been identified as risk factors for hospitalization and mortality (2, 3). Obesity was also shown as an important risk factor for severe COVID‐19 (4, 5). In previous publications, the median age of patients with COVID‐19 was approximately 60 years; thus, the risk factors highlighted are predominantly in the older population (2, 6, 7). Identifying comorbidities linked to poor outcome in the young is crucial.
This study analyzed obesity as an independent risk factor for mortality in hospitalized patients younger than 50.
Methods
We retrospectively analyzed data of patients with COVID‐19 hospitalized to a large academic hospital system in New York City. Data came from 5 hospital campuses serving different geographic populations (Mount Sinai Hospital, Mount Sinai Brooklyn, Mount Sinai Queens, Mount Sinai Morningside, and Mount Sinai West). The study time frame was between March 1, 2020, and May 17, 2020. The Mount Sinai Institutional Review Board approved this study.
We identified all adult patients who were positive for COVID‐19 by nasopharyngeal swab polymerase chain reaction test and were admitted to the hospital. Patients who were discharged or had died during the study period were included. Patients who were still hospitalized at the time of analysis and patients with missing BMI data were excluded.
Data were extracted from electronic medical records and included age, sex, comorbidities, BMI, and smoking status. BMI is calculated according to the weight and height measured on admission to the hospital. Obesity was defined as BMI ≥ 30 kg/m2. Obesity groups included the following: BMI of 30 to < 40 and BMI ≥ 40. Smoking status was defined as either past or present smoking.
The analysis was performed with Python (version 3.6.5, 64 bits). P < 0.05 was considered statistically significant.
The primary outcome was in‐hospital mortality. Calculations were performed separately for patients aged 50 years and younger and patients older than 50 years of age.
Univariate analysis separately compared comorbidities associated with mortality in the younger and older groups. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using the Mann‐Whitney U test. Multivariable logistic regression models identified variables independently associated with mortality in patients younger and older than 50. Models were adjusted for demographics and comorbidities (Table 2). Adjusted odds ratios (aOR), 95% CI, and P values were calculated for the variables in the models. A multivariable analysis for intubation and mechanical ventilation as a secondary outcome was also performed. The covariates in this model were the same as those in the mortality model.
TABLE 2.
Multivariable analysis evaluating variables independently associated with mortality in the 2 age groups
| Age ≤ 50 years, multivariate analysis, aOR (95% CI); P a | Age > 50 years, multivariate analysis, aOR (95% CI); P a | |
|---|---|---|
| Age decile | 3.0 (1.9‐4.8); < 0.001 | 1.7 (1.6‐1.8); < 0.001 |
| Male sex | 1.9 (0.9‐3.9); 0.081 | 1.4 (1.2‐1.6); < 0.001 |
| CAD | 0.6 (0.2‐2.1); 0.418 | 1.3 (1.1‐1.6); 0.006 |
| CHF | 4.0 (1.6‐10.4); 0.004 | 1.0 (0.8‐1.3); 0.954 |
| HTN | 0.5 (0.2‐1.1); 0.088 | 1.1 (0.9‐1.3); 0.571 |
| DM | 1.3 (0.7‐2.6); 0.442 | 1.4 (1.2‐1.7); < 0.001 |
| Hyperlipidemia | 0.8 (0.3‐2.1); 0.710 | 1.0 (0.8‐1.2); 0.898 |
| CKD | 3.3 (1.4‐7.7); 0.006 | 1.7 (1.4‐2.1); < 0.001 |
| Cancer | 2.5 (1.0‐6.5); 0.052 | 1.0 (0.8‐1.2); 0.936 |
| Smoking | 1.7 (0.8‐3.8); 0.162 | 1.0 (0.8‐1.2); 0.950 |
| BMI | ||
| < 30 | Reference | Reference |
| 30‐40 | 1.1 (0.5‐2.3); 0.755 | 1.1 (0.9‐1.3); 0.421 |
| ≥ 40 | 5.1 (2.3‐11.1); < 0.001 | 1.6 (1.2‐2.3); 0.004 |
| Race | ||
| Other | Reference | Reference |
| African American | 0.7 (0.3‐1.5); 0.352 | 0.8 (0.6‐1.0); 0.043 |
| White | 0.4 (0.1‐1.1); 0.069 | 1.1 (0.9‐1.3); 0.454 |
The multivariable models were adjusted for age decile, male sex, CAD, CHF, HTN, DM, hyperlipidemia, CKD, history of cancer, smoking (past or present), BMI 30‐40, BMI ≥ 40, and race.
aOR, adjusted odds ratio; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension.
Results
Overall, 4,705 hospitalized patients with COVID‐19 were identified. We excluded 1,047 patients who were still hospitalized during the study period and 252 patients with missing BMI data. The final cohort included 3,406 patients; 572 patients were younger than 50 years and 2,834 patients were older than 50 years. Characteristics of the survivors and nonsurvivors are presented in Table 1.
TABLE 1.
Characteristics of study cohort stratified by mortality and age
| Age ≤ 50 years | Age > 50 years | |||||
|---|---|---|---|---|---|---|
| Survivors (n = 512; 89.5%) | Nonsurvivors (n = 60; 10.5%) | P value | Survivors (n = 1,758; 62.0%) | Nonsurvivors (n = 1,076; 38.0%) | P value | |
| Age, median (IQR), y | 40.0 (34.0‐46.0) | 46.5 (42.8‐49.0) | < 0.001 | 68.0 (60.0‐77.0) | 76.0 (67.0‐84.0) | < 0.001 |
| Male sex, n (%) | 352 (68.8) | 45 (75.0) | 0.376 | 949 (54.0) | 615 (57.2) | 0.102 |
| CAD, n (%) | 26 (5.1) | 6 (10.0) | 0.132 | 358 (20.4) | 354 (32.9) | < 0.001 |
| CHF, n (%) | 25 (4.9) | 10 (16.7) | 0.002 | 254 (14.4) | 224 (20.8) | < 0.001 |
| HTN, n (%) | 151 (29.5) | 24 (40.0) | 0.104 | 1,260 (71.7) | 864 (80.3) | < 0.001 |
| DM, n (%) | 129 (25.2) | 24 (40.0) | 0.020 | 839 (47.7) | 607 (56.4) | < 0.001 |
| Hyperlipidemia, n (%) | 63 (12.3) | 11 (18.3) | 0.220 | 704 (40.0) | 502 (46.7) | < 0.001 |
| CKD, n (%) | 53 (10.4) | 17 (28.3) | < 0.001 | 298 (17.0) | 299 (27.8) | < 0.001 |
| Cancer, n (%) | 30 (5.9) | 9 (15.0) | 0.014 | 290 (16.5) | 201 (18.7) | 0.138 |
| Smoking, n (%) | 63 (12.3) | 13 (21.7) | 0.067 | 429 (24.4) | 288 (26.8) | 0.168 |
| BMI, median (IQR) | 29.4 (25.4‐36.4) | 31.5 (26.8‐43.0) | 0.012 | 27.5 (24.0‐31.9) | 27.3 (23.4‐31.6) | 0.041 |
| BMI, n (%) | ||||||
| < 30 | 272 (53.1) | 25 (41.7) | 0.102 | 1,151 (65.5) | 727 (67.6) | 0.269 |
| 30‐40 | 171 (33.4) | 16 (26.7) | 0.313 | 496 (28.2) | 274 (25.5) | 0.117 |
| ≥ 40 | 69 (13.5) | 19 (31.7) | < 0.001 | 111 (6.3) | 75 (7.0) | 0.532 |
| Race, n (%) | ||||||
| African American | 125 (24.4) | 17 (28.3) | 0.528 | 491 (27.9) | 247 (23.0) | 0.004 |
| White | 90 (17.6) | 5 (8.3) | 0.097 | 419 (23.8) | 332 (30.9) | < 0.001 |
| Other | 297 (58.0) | 38 (63.3) | 0.489 | 848 (48.2) | 497 (46.2) | 0.296 |
| Intubation and mechanical ventilation, n (%) | 26 (5.1) | 53 (88.3) | < 0.001 | 101 (5.7) | 629 (58.5) | < 0.001 |
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range.
In the younger age group, 60 (10.5%) patients died. In the older age group, 1,076 (38.0%) patients died.
For the younger group, 383 of 512 (74.8%) survivors and 55 of 60 (91.7%) nonsurvivors had at least 1 comorbidity. For the older group, 1,637 of 1,758 (93.1%) survivors and 1,051 of 1,076 (97.7%) nonsurvivors had at least 1 comorbidity.
In univariate analysis, for the younger group, BMI ≥ 40 was significantly associated with mortality (P < 0.001). For the older population, CAD (P < 0.001), congestive heart failure (P < 0.001), hypertension (P < 0.001), DM (P < 0.001), hyperlipidemia (P < 0.001), and chronic kidney disease (CKD) (P < 0.001) were associated with increased mortality. Figure 1 presents a box plot for BMI values in the younger and older groups, stratified by mortality status.
Figure 1.
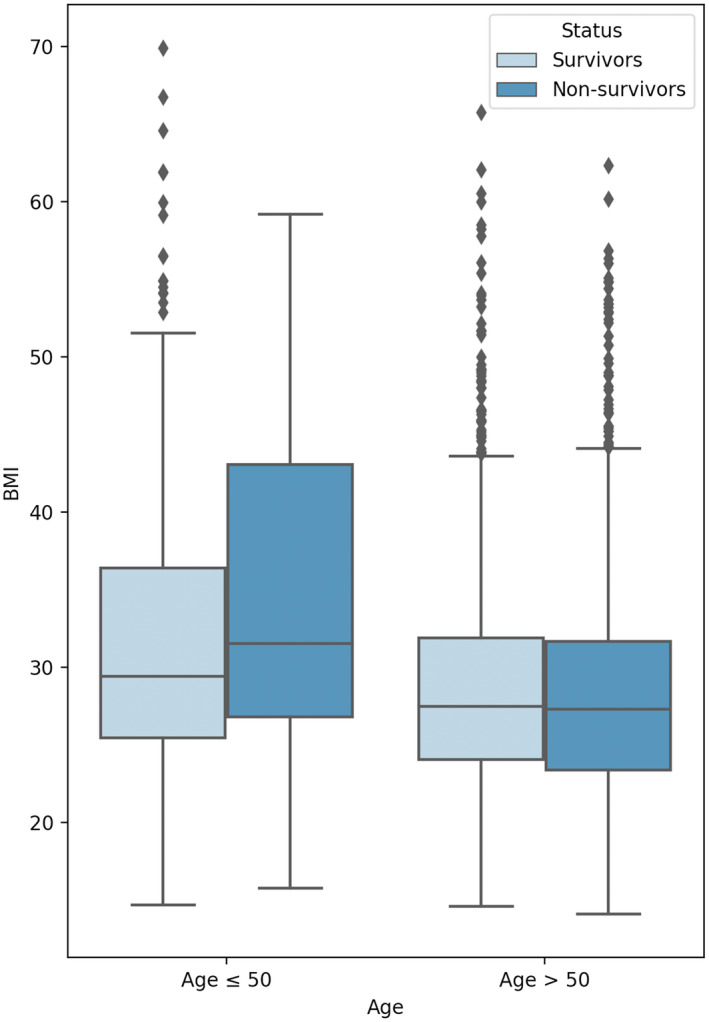
Box plot comparing BMI between patients who survived or died, stratified by age. [Color figure can be viewed at wileyonlinelibrary.com]
In the never‐smoking group, BMI ≥ 40 was significantly associated with mortality in the younger age group (19.2% mortality with severe obesity vs. 7.8% mortality without severe obesity; P = 0.019) but not in the older age group (39.6% mortality with severe obesity vs. 37.1% mortality without severe obesity; P = 0.254). The median BMI for African American patients was 28.8 (IQR 24.0‐35.6), and the median BMI for white patients was 27.3 (interquartile range 23.0‐31.6).
In multivariable analysis, for the younger population, BMI ≥ 40 was independently associated with mortality (aOR 5.1; 95% CI: 2.3‐11.1) (Table 2). Other risk factors independently associated with mortality in the young group included congestive heart failure (aOR 4.0; 95% CI: 1.6‐10.4) and CKD (aOR 3.3; 95% CI: 1.4‐7.7).
For the older population, BMI ≥ 40 (aOR 1.6; 95% CI: 1.2‐2.3) was also independently associated with mortality. Other risk factors independently associated with mortality in the older population included CAD (aOR 1.4; 95% CI: 1.2‐1.6), DM (aOR 1.4; 95% CI: 1.2‐1.7), and CKD (aOR 1.7; 95% CI: 1.4‐2.1).
For the secondary outcome, intubation and mechanical ventilation status was independently associated with BMI ≥ 40, both in the young age group (aOR 4.1; 95% CI: 2.1‐8.2) and in the older age group (aOR 1.5; 95% CI: 1.1‐2.1) (Supporting Information Table S1).
Discussion
Our study highlights that the comorbidity profile associated with COVID‐19 mortality is different for younger patients. Severe obesity (BMI ≥ 40) was found to be strongly and independently associated with mortality in hospitalized patients younger than 50. Severe obesity was also associated with intubation and mechanical ventilation. As in previous research, other than obesity, CAD, DM, and CKD were independently associated with mortality in the older population.
Several recent studies have highlighted obesity as a risk factor for COVID‐19. These studies have shown that obesity is more frequent among patients with COVID‐19 and is associated with increased need for invasive mechanical ventilation (8, 9). Another study identified obesity as a risk factor for hospitalization in patients younger than 60 (4). Our results identified that younger patients with BMI greater than 40 are 5 times more likely to die. This is of concern because the prevalence of obesity among US young adults is approximately 40% (10). Thus, although the younger population is considered at a lower risk for COVID‐19 mortality, young patients with severe obesity are an important high‐risk population.
The link between obesity and COVID‐19 severity has not been established yet. Several mechanisms that may explain the obesity role in the pathogenesis of the disease have been suggested. Patients with obesity are associated with impaired immune response and abnormal secretion of pro‐inflammatory cytokines, such as interleukin 6 and tumor necrosis factor α (11). Furthermore, obesity results in physiological lung alteration, such as decreased functional residual capacity and hypoxemia (12). Finally, angiotensin‐converting enzyme 2 is expressed in adipose tissue, and angiotensin‐converting enzyme 2 was also shown to have high affinity to the COVID‐19 virus (13).
Interestingly, in our study, the effects of obesity on COVID‐19 are independent of obesity‐related comorbidities, such as diabetes and cardiovascular disease. Ryan et al. suggested that the burden of increased adipose tissue in patients with severe obesity plays a key role in the pathogenicity of COVID‐19 (14). They proposed that adipose tissue is a pro‐immunogenic and richly vascularized organ with the ability to augment the pro‐inflammatory response to viral infection. Adipose tissue has the potential to prolong viral shedding in an environment that is already inflamed with local cytokine amplification (14).
This study was limited by its retrospective nature and by the small number of patients younger than 50. This resulted in relatively wide CIs for the younger population. Nonetheless, severe obesity was found to be strongly and independently associated with mortality in this population. Moreover, the association between obesity and mortality is intricate and may involve other covariates that might have not been accounted for in our study. Additionally, in our study, African American patients had a slightly lower adjusted risk for death. Ethnicity data were incomplete in our study and, thus, were not included in the calculations. The intricate correlations between race, ethnicity, marital status, and socioeconomic status and COVID‐19 should be further studied. Finally, because this is an ongoing pandemic, patients who remained hospitalized were not included in the study, and this might have biased the results as well as the mortality rates.
Our study demonstrates that hospitalized patients younger than 50 with severe obesity are more likely to die of COVID‐19. This is particularly relevant in the Western world, where obesity rates are high.
Disclosure
The authors declared no conflict of interest.
Supporting information
Table S1
References
- 1. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19): cases in the U.S. Published 2020. Accessed May 4, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/cases‐in‐us.html
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen R, Liang W, Jiang M, et al; Medical Treatment Expert Group for COVID‐19 . Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 2020;158:97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID‐19 hospital admission. Clin Infect Dis 2020;71:896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity (Silver Spring) 2020;28:1200‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. doi:10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015‐2016. NCHS Data Brief, no. 288. National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 11. Coppack SW. Pro‐inflammatory cytokines and adipose tissue. Proc Nutr Soc 2001;60:349‐356. [DOI] [PubMed] [Google Scholar]
- 12. Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 2006;13:203‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kassir R. Risk of COVID‐19 for patients with obesity. Obes Rev 2020;21:e13034. doi:10.1111/obr.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020;28:1191‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


