Abstract
SARS‐CoV‐2 is highly infectious, and infection by this virus results in COVID‐19, manifesting predominantly symptoms in the lower respiratory system. Detection of viral genomic materials by RT‐PCR is the gold standard for diagnosis. Suspected COVID‐19 patients who had a documented history of exposure and exhibited symptoms, but did not have positive PCR test results, were generally self‐quarantined with prescriptions aiming to help attenuate their symptoms. These prescriptions are however neither specific nor highly effective for COVID‐19 treatment. Given the rapidly growing pandemic and the overwhelmed medical system, the number of self‐quarantined patients is increasing. There is an urgent need of alternative medicine to help patients relieve symptoms during self‐quarantine, and to potentially help increase their chances of survival and recovery from the infection. We report here a case of severe COVID‐19 that never had a positive PCR test result during disease progression but was confirmed with antibody test post recovery. This patient was self‐quarantined and received diammonium glycyrrhizinate (DG), a steroid‐like molecule, in combination with vitamin C as alternative medicine. This patient went through severe COVID‐19 but eventually recovered upon the implementation of this treatment regimen, suggesting potential therapeutic effects of DG as alternative medicine to help relieve COVID‐19 symptoms.
Keywords: alternative medicine, antibody test positive, COVID‐19, PCR test negative, prolonged symptoms
Highlights
We report here a case of severe COVID‐19, in which diammonium glycyrrhizinate and vitamin C were used as alternative medicine to help relieve COVID‐19 symptoms.
Abbreviations
- COVID‐19
coronavirus disease 2019
- DG
diammonium glycyrrhizinate
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- VC
vitamin C
1. BACKGROUND
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is highly infectious, 1 even when the patients show no or minor symptoms. 2 Infection by SARS‐CoV‐2 causes a broad spectrum of diseases that affects multiple organs including the lung, nervous system, heart, digestive system, kidney and others. 3 The resultant coronavirus disease 2019 (COVID‐19) manifests predominantly symptoms in the lower respiratory system, typically presented as viral pneumonia. Detection of viral genomic materials is the gold standard for diagnosis. Standard operation procedures use pharyngeal swabbing samples from the upper respiratory system, which may miss the anatomic location of viral replication, leading to false negative diagnostic result. 4 The unexpected pandemic outbreak also resulted in deprivation of medical resources in the peak phase in areas that did not have sufficient preparation and/or resources. Suspected COVID‐19 patients who had documented history of exposure and exhibited symptoms, but did not have positive polymerase chain reaction (PCR) test results, were generally self‐quarantined. Some patients had a contact history with a confirmed COVID‐19 patient but did not have typical viral pneumonia symptoms or fever, and so did not get selected for PCR testing. Given the rapidly growing pandemic and the overwhelmed medical system, a number of patients with typical COVID‐19 symptoms could not be hospitalized, even if they were tested positive by PCR. The combination of the false negative rate of PCR testing, the biased pre‐screening procedure for patients to get tested, and hospital space limitation results in an increasing number of nonhospitalized COVID‐19 patients. These patients are generally self‐quarantined with prescriptions for alternative medicine to help ameliorate their symptoms. Most of the prescribed alternative medicines are, however, neither specific nor highly effective for COVID‐19 treatment. There is an urgent need of alternative medicine to help patients relieve symptoms during self‐quarantine, and possibly to help increase their chances of survival and recovery from the infection.
The outbreak of COVID‐19 in Wuhan, China, in January 2020, was the first strike on public health system by SARS‐CoV‐2. 5 Patients infected with COVID‐19 had a high chance of developing severe pneumonia and acute respiratory distress syndrome (16%‐17%) and the mortality rate was high (4.3%‐11%). 3 , 5 , 6 , 7 Hospitalization became the only possible way for severe COVID‐19 patients to receive medical supports and treatment against symptoms. The unexpected outbreak with a rapidly growing patient number placed a significant demand of medical resources, and so hospitals in Wuhan prioritized hospitalization for severe patients with positive PCR test results until mid‐February. A number of patients in Wuhan had documented contact histories with confirmed COVID‐19 patients and also showed respiratory symptoms, however unfortunately, their upper respiratory system did not have viral loads that were high enough to pass the PCR test threshold, and so they did not have a positive PCR test result to qualify for hospitalization in January and early February in Wuhan. Our medical team tracked and helped treat a number of these nonhospitalized COVID‐19 patients.
Here, we report a case of nonhospitalized COVID‐19 that never had a positive PCR test result during disease progression and recovery. This patient was confirmed as a COVID‐19 case, by SARS‐CoV‐2‐specific antibody test post recovery. This severe COVID‐19 patient was self‐quarantined and received diammonium glycyrrhizinate (DG), a marketed Chinese traditional medicine with a steroid‐like effect, in combination with vitamin C (VC) as alternative medicine. Upon implementation of this treatment regimen, the patient's symptoms were significantly relieved and eventually recovered. In this article, we aim to report more details of this encouraging case and bring DG to the table for discussion about its potential as an alternative medicine to attenuate COVID‐19 symptoms in nonhospitalized patients. Of note, this case is not a clinical trial, and we decided to share this information with the hope of increasing treatment options for patients who cannot be “diagnosed” with PCR test, are turned down by hospitals and have to seek alternative medicine for COVID‐19 symptoms.
2. METHODS
2.1. Patient consent
We have received consent from the patient to report this case.
2.2. Tests in hospitals
Blood routine examination and SARS‐CoV‐2 reverse transcription PCR (RT‐PCR) and antibody tests were performed by Wuhan Xiehe Hospital and Wuhan 161 Military Hospital, in the city of Wuhan, China. The patient came to the clinic to seek medical help on Jan 25th, 2020. On February 2nd, the patient's fingertip oxygen saturation dropped below 93% (Table 1) and computed tomography (CT) scans showed typical ground‐glass opacity, indicating severe viral pneumonia condition. Oropharyngeal swab samples were tested negative for SARS‐CoV‐2 by RT‐PCR on January 28th, February 2nd, February 26th, February 27th, March 14th, March 15th, and April 3rd. By the end of February, patient's symptoms were relieved and gradually recovered, and on May 5th, the patient was tested positive with SARS‐CoV‐2‐specific IgG antibody.
Table 1.
Patient information, test results of IgM against common pathogens, and vital conditions
| Variable | ||
| Age, y | 62 | |
| Sex | Female | |
| Symptoms | ||
| Fever | Yes | |
| Dry cough | Yes | |
| Fatigue | Yes | |
| Dyspnea | Yes | |
| Headache | Yes | |
| IgM specific for pathogens, on January 29th, 2020 | ||
| Influenza A virus | Negative | |
| Influenza B virus | Negative | |
| Adenovirus | Negative | |
| Respiratory syncytial virus | Negative | |
| Parainfluenza virus | Negative | |
| Mumps virus | Negative | |
| Mycoplasma pneumoniae | Negative | |
| Chlamydia pneumoniae | Negative | |
| Vital signs, on February 2nd, 2020 | ||
| Temperature, ℃ | 39.2 | |
| Respiratory rate, breaths per min | 25 | |
| Heart rate (beats per min) | 97 | |
| SBP, mm Hg | 120 | |
| DBP, mm Hg | 80 | |
| Oxygen saturation | 93% | |
| Hematological and infection‐related indices | Value | Normal range |
| White blood cells, ×109/L | 6.14 | 3.5‐9.5 |
| Neutrophil, ×109/L | 4.21 | 1.8‐6.3 |
| Lymphocytes, ×109/L | 1.63 | 1.1‐3.2 |
| Eosinophil, ×109/L | 0.01 | 0.02‐0.52 |
| Basophil, ×109/L | 0 | 0.00‐0.06 |
| Erythrocyte, ×109/L | 4.3 | 3.8‐5.1 |
| Hemoglobin, g/L | 120 | 115‐150 |
| Platelets, ×109/L | 234 | 125‐300 |
| C‐reactive protein, mg/L | 50.02 | 0‐4 |
| D‐dimer, μg/mL | 0.1 | 0‐0.5 |
Note: Italic and bold values are significant parameters indicating a severe COVID‐19 condition.
2.3. Treatments during self‐quarantine
All medicines were prescribed by medical doctors in Wuhan Xiehe Hospital and Wuhan 161 Military Hospital, and taken as instructed.
3. RESULTS
3.1. Patient information and conditions before treatment with DG
On January 12th (symptom onset day 1), 2020, the patient, a 62‐year‐old female, started to feel persistent back pain and nausea, and additional symptoms including fatigue and vomiting developed 1 week post symptom onset. On January 25th (symptom onset day 14), she presented to an urgent care clinic in Wuhan, China, and later was discharged with Cephalexin prescribed (orally 250 mg 3× daily). Her clinical condition did not improve. While back pain, nausea, fatigue and vomiting persisted, the patient also developed persistent fevers (37.9℃‐39.3℃) starting on Jan 26th (day 15), and shortness of breath starting on January 28th (day 17), which was further exacerbated 2 days later (Figure 1B). Given the patient's ongoing fevers, Moxifloxacin (orally 400 mg 3× daily) and Acetaminophen (orally 1000 mg 2× daily) were prescribed on Jan 27th (symptom onset day 16), and Oseltamivir (orally 150 mg 3× daily) was prescribed on Jan 31st (day 20). All treatments failed to control the symptoms, and the patient developed a severe condition exhibiting shortness of breath (day 17). On Feb 2nd (day 22), the patient's fingertip oxygen saturation dropped to 93% (comparable to severe COVID‐19 patients) and routine blood examination showed elevated level of C‐reactive protein (50.02 in patient, vs normal ranges at 0‐4) (Table 1). CT scan revealed bilateral distribution of patchy shadows and ground‐glass opacity (Figure 1B, upper panel), typical hallmark of viral pneumonia. On January 29th, sera test for IgM antibodies against common respiratory viruses that may induce viral pneumonia, including influenza A, influenza B, adenovirus, respiratory syncytial virus, parainfluenza, and mumps, all of which returned negative results (Table 1). We also ruled out recent infections by mycoplasma pneumoniae and chlamydia pneumoniae, since IgM tests for these pathogens returned negative results as well (Table 1). Given the patient's history of contact with confirmed COVID‐19 patients and her typical COVID‐19 symptoms, the most possible infection at the time was SARS‐CoV‐2. However, the patient was tested negative by RT‐PCR for SARS‐CoV‐2 using samples collected via oropharyngeal swabbing, on January 28th and February 2nd (symptom onset days 17 and 22). It is possible that, due to the tissue tropism of the viral replication, this patient did not have a vibrant viral growth in her upper respiratory system, which led to negative test results in RT‐PCR. 8 During late January and early February, the protocol for COVID‐19 clinical diagnosis and patient admission used in China required a confirmation of infection by RT‐PCR positive result, and thus this patient was not eligible for hospitalization at that moment. This patient with suspected COVID‐19 was sent home for self‐quarantine. Our medical team continued to follow‐up and treat this patient during her quarantine at home.
Figure 1.
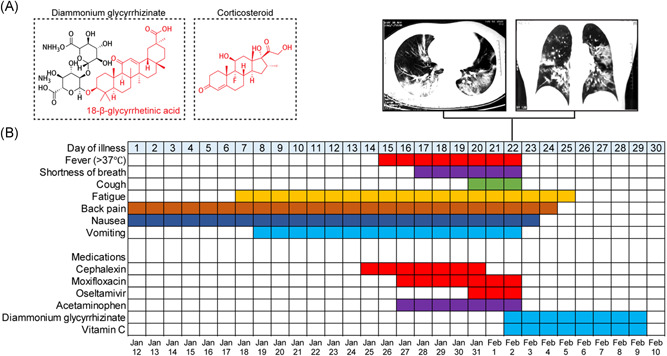
Diammonium glycyrrhizinate+vitamin C as a potential treatment to relieve COVID‐19 symptoms. A, Chemical structures of diammonium glycyrrhizinate and corticosteroid are shown. B, Symptoms and treatments of the patient outlined accordingly to days post symptom onset since Jan 12th, 2020
3.2. Alternative medicine treatment regimen using DG
Given that the medicines prescribed in the hospital failed to relieve the patient's symptoms and started to trigger side effects, and that the patient was not eligible for COVID‐19 treatment in the hospital, all prescribed medicines were discontinued. The patient was however in urgent need of medical intervention. After discussion with several physicians and professors who specialized in respiratory infectious diseases, the patient decided to take DG (Figure 1A; orally 150 mg 3× daily) as an alternative medicine (symptom onset day 22). VC (orally 200 mg 3× daily) was also empirically administered to reduce reactive oxygen species in inflammation and tissue injury. After 12 hours after the first dose of DG+VC, severe symptoms including fever, shortness of breath, and coughing were relieved. Since February 3rd (day 23) the patient's clinical condition had been improved overtime. In brief: on February 3rd, the patient's body temperature dropped below 37℃, vomiting stopped, and shortness of breath and dry cough were significantly relieved to the levels that the patient did not notice for reporting; on February 4th, the patient stopped reporting nausea; on February 5th, back pain was resolved; and on February 6th, the patient stopped feeling fatigued (Figure 1B). In about a week, all severe symptoms were resolved, and the patient's appetite improved to the level comparable to her regular appetite. After eight consecutive days with the DG+VC treatment regimen, on February 10th (day 30), the treatment was discontinued as the patient stopped reporting any discomforts.
We continued to monitor the patient's conditions. In follow‐up tests, she was tested negative for SARS‐CoV‐2 by RT‐PCR on February 26th, February 27th, March 14th, March 15th, and April 3rd. CT scans on February 26th indicated significantly improved lung condition regarding signs of inflammation, and CT scans on April 3rd indicated complete resolution of lung inflammation despite minor fibrosis. On May 5th, the patient was tested positive with SARS‐CoV‐2‐specific IgG antibody, confirming her history of COVID‐19.
4. DISCUSSION AND CONCLUSIONS
Thus far for COVID‐19, there are no pharmacological treatment regimens of proven efficacy. Immunopathology is a leading cause of morbidity and mortality in severe COVID‐19 patients. 9 There is an urgent need for treatment regimens that can manage inflammation and tissue damages associated with severe COVID‐19, to reduce mortality and buy time for the immune system to clear the virus from the patients. During the initial COVID‐19 outbreak in Wuhan, corticosteroid has been used in hospitals to suppress lung inflammation in COVID‐19 patients, 10 however, multiple side effects have been observed. Moreover, recent studies suggested limited clinical benefits of corticosteroid, yet the evidence for this remains largely circumstantial. 11 , 12
Here we report DG, a marketed Chinese traditional medicine with a steroid‐like effect, in combination with VC as a potential anti‐inflammatory treatment to relieve severe symptoms from COVID‐19. DG is the active ingredient in the traditional Chinese medicinal herb licorice. It is metabolized into glycyrrhetinic acid, which has a chemical structure similar to that of corticosteroid (Figure 1A) and thus functions as a glucocorticoid‐like drug, which may help provide immune regulation against cytokine storm and reduce inflammation, although might be less stringent than steroids. 13 DG has been used in the clinics for over 20 years in China and Japan as an antiviral and anti‐inflammatory drug against hepatitis with few reported side effects, likely via its function in modulating the levels of expression of IL‐6 and IL‐10. 14 Our previous results also supported its safety and protective effects against colitis using rat models. 15 In an LPS‐induced lung acute injury model in mice, DG exhibited potent anti‐inflammatory efficacy and attenuated lung injury. 16 Furthermore, DG has protective effects against Aβ1‐42‐induced neurotoxicity. 17 Last but not the least, it has been demonstrated that glycyrrhizic acid derivatives have antiviral activity against SARS‐CoV infection in Vero cells. 18 Our article here reports a case of nonhospital COVID‐19 that showed significantly positive responses to DG treatment. Although the exact functions of DG against SARS‐CoV‐2 infection and the associated immunopathology await further investigations, it is possible that the therapeutic effects of DG observed in this severe COVID‐19 patient were a combinatory result of the antiviral and anti‐inflammatory effects of DG in the respiratory and neurological systems. Given these encouraging pharmacological effects and the demonstrated safety, as well as the low cost and wide availability of DG and VC, we suggest that a combination of these might be a good candidate for alternative medicine against COVID‐19. Implementation of this treatment regimen might worth more attention, and further investigations in clinical trials can be considered to validate its effects in relieving severe symptoms and reducing mortality of COVID‐19 in a larger cohort. For suspected COVID‐19 patients who cannot be hospitalized and have no venues for COVID‐19 specific prescriptions, we suggest that the combination of DG and VC may be a promising candidate as an alternative medicine to help relieve severe symptoms of COVID‐19 during self‐quarantine. 8
CONFLICT OF INTERESTS
Weishan Huang receives research support from MegaRobo Technologies Corporation. The other authors claim no conflict of interests.
AUTHOR CONTRIBUTIONS
HD, WD, SY, and WH designed research and analyzed data; HD, WD, LD, and XY collected data; SY and WH wrote the manuscript.
ACKNOWLEDGMENTS
The authors thank the patient who volunteered to participate in this study and nurses and doctors who helped with tests, medical consulting, and discussions. Research related to signaling pathways regulating airway immunopathology and strategies of therapeutic development in the authors' laboratories is supported in part by grants from the National Institutes of Health (R56AI146226, R21AI137822, and P20GM130555‐6610).
Ding H, Deng W, Ding L, Ye X, Yin S, Huang W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID‐19 patients. J Med Virol. 2020;92:2200–2204. 10.1002/jmv.26064
Hong Ding and Wenjun Deng contributed equally to this study and both should be considered as first authors.
Contributor Information
Shanye Yin, Email: shanye_yin@dfci.harvard.edu.
Weishan Huang, Email: huang1@lsu.edu.
REFERENCES
- 1. Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID‐19 epidemic in China. Science. 2020;368(6491):638‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China: Life Sci. 2020;63(5):706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145‐151.32064853 [Google Scholar]
- 5. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu F, Huang W. COVID‐19 diagnostic process in mainland China: The math beyond pneumonia [published online ahead of print April 25, 2020]. J Allergy Clin Immunol. 10.1016/j.jaci.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID‐19: a systematic review and clinical perspective. Eur Respir J. 2020;55(5):2001009. 10.1183/13993003.01009-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Redeploying plant defences. Nat Plants. 2020;6(3):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng C, Wang H, Yao C, Zhang J, Tian Z. Diammonium glycyrrhizinate, a component of traditional Chinese medicine Gan‐Cao, prevents murine T‐cell‐mediated fulminant hepatitis in IL‐10‐ and IL‐6‐dependent manners. Int Immunopharmacol. 2007;7(10):1292‐1298. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Xiang J, Liu M, Wang S, Lee RJ, Ding H. Protective effects of glycyrrhizic acid by rectal treatment on a TNBS‐induced rat colitis model. J Pharm Pharmacol. 2011;63(3):439‐446. [DOI] [PubMed] [Google Scholar]
- 16. Shi JR, Mao LG, Jiang RA, Qian Y, Tang HF, Chen JQ. Monoammonium glycyrrhizinate inhibited the inflammation of LPS‐induced acute lung injury in mice. Int Immunopharmacol. 2010;10(10):1235‐1241. [DOI] [PubMed] [Google Scholar]
- 17. Zhu X, Chen C, Ye D, et al. Diammonium glycyrrhizinate upregulates PGC‐1alpha and protects against Abeta1‐42‐induced neurotoxicity. PLoS One. 2012;7(4):e35823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoever G, Baltina L, Michaelis M, et al. Antiviral activity of glycyrrhizic acid derivatives against SARS‐coronavirus. J Med Chem. 2005;48(4):1256‐1259. [DOI] [PubMed] [Google Scholar]


