Abstract
Coronavirus disease 2019 (COVID‐19) is responsible for at least 2 546 527 cases and 175 812 deaths as of April 21, 2020. Psoriasis and atopic dermatitis (AD) are common, chronic, inflammatory skin conditions, with immune dysregulation as a shared mechanism; therefore, mainstays of treatment include systemic immunomodulating therapies. It is unknown whether these therapies are associated with increased COVID‐19 susceptibility or worse outcomes in infected patients. In this review, we discuss overall infection risks of nonbiologic and biologic systemic medications for psoriasis and AD and provide therapeutic recommendations. In summary, in patients with active infection, systemic conventional medications, the Janus kinase inhibitor tofacitinib, and biologics for psoriasis should be temporarily held until there is more data; in uninfected patients switching to safer alternatives should be considered. Interleukin (IL)‐17, IL‐12/23, and IL‐23 inhibitors are associated with low infection risk, with IL‐17 and IL‐23 favored over IL‐12/23 inhibitors. Pivotal trials and postmarketing data also suggest that IL‐17 and IL‐23 blockers are safer than tumor necrosis factor alpha blockers. Apremilast, acitretin, and dupilumab have favorable safety data and may be safely initiated and continued in uninfected patients. Without definitive COVID‐19 data, these recommendations may be useful in guiding treatment of psoriasis and AD patients during the COVID‐19 pandemic.
Keywords: atopic dermatitis, biologics, COVID‐19, immunosuppression, psoriasis
Abbreviations
- AD
atopic dermatitis
- BADBIR
British Association of Dermatologists Biologic Interventions Register
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- FDA
Food and Drug Administration
- HR
hazard ratio
- IL
Interleukin
- JAK
Janus kinase
- RR
relative risk
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TNF‐α
tumor necrosis factor alpha
- URI
upper respiratory infection
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a novel human coronavirus, with 2 546 527 confirmed cases of coronavirus disease 2019 (COVID‐19) and 175 812 deaths worldwide (April 21, 2020). 1 It was declared a pandemic by the World Health Organization. An overall case fatality rate of 3.61% has been reported 2 ; however, inaccuracies may exist because those who are asymptomatic or suffer from mild disease may never receive confirmation.
Psoriasis and atopic dermatitis (AD) are common, chronic, inflammatory skin diseases, affecting 2% to 3% of the general population and 7% of adults in the United States, respectively. 3 , 4 Disease mechanisms are multifactorial, with immune dysregulation important for both conditions, and mainstays of treatment immune‐modulation. 5 Systemic therapy is preferred for psoriasis treatment in patients with body surface area >10%, involvement of sensitive areas or topical therapy failure. 6 Systemic treatment is recommended for AD patients with severe disease or recalcitrant to topical therapy. 7 Immunocompromised patients are highly vulnerable to infections, which is particularly concerning in the context of the COVID‐19 pandemic.
In this review, we summarize the current literature regarding overall infection risks with systemic immunomodulating agents for psoriasis and AD and provide evidence‐based treatment recommendations during the COVID‐19 pandemic.
2. NONBIOLOGIC SYSTEMIC THERAPIES
2.1. Systemic corticosteroids
Systemic corticosteroids are immunosuppressive medications used to treat AD flares, but very rarely psoriasis. They have been shown to increase infection risk. In a systematic review of 101 studies on AD children (n = 6817) treated with systemic corticosteroids ≥15 days, infection rate was 8.7%, with 21 associated deaths. 8 In a meta‐analysis of corticosteroid use in patients with influenza pneumonia (10 studies, n = 6548), compared with placebo, corticosteroids were associated with higher mortality, longer intensive care unit length of stay and a higher rate of secondary infection. 9 Therefore, oral corticosteroids should be avoided, weighing the risks of disease flare vs SARS‐CoV‐2 infection, to prevent COVID‐19 susceptibility. Before discontinuation, dose tapering may be considered to avoid a negative effect on respiratory symptoms.
2.2. Methotrexate, cyclosporine, and acitretin
Methotrexate and cyclosporine are among the most frequently used systemic medications for psoriasis and AD, with both associated with increased infection rates. There was a 58% higher overall infection risk with cyclosporine vs methotrexate in the BIOBADADERM Registry (Spanish Registry of Adverse Events for Biological Therapy in Dermatological Disease) including 2153 psoriasis patients. 10 In a head‐to‐head comparison of methotrexate (n = 50) vs cyclosporine (n = 47) in moderate‐to‐severe AD adults, infections rates were 32% and 24%, respectively. 11 While methotrexate and cyclosporine are associated with decreased infection rates and favored over treatment with systemic corticosteroids, 12 their impact on susceptibility to/severity of COVID‐19 is unknown and, if essential, precautions should be taken to avoid infection. Of interest, cyclosporine has anticoronavirus activity in vitro, but the effect in humans is unknown. 13
The systemic retinoid, acitretin, is anti‐inflammatory and inhibits cell differentiation; it is Food and Drug Administration (FDA)‐approved for psoriasis. 14 It does not suppress the immune system to the extent of the other conventional treatments for psoriasis. In an observational cohort study, there was no increased rate of overall serious infections among acitretin‐treated psoriasis patients vs methotrexate; acitretin increased risk of cellulitis compared to methotrexate (propensity score‐adjusted hazard ratio [HR], 1.76; 95% confidence interval [CI], 1.11‐2.80), possibly due to skin fragility and Staphylococcus aureus colonization. 15 Therefore, acitretin has not shown increased viral/respiratory infection risk and can be safely used during the pandemic. Retinoids have been shown to inhibit human herpesvirus eight replication, but their effect on SARS‐CoV‐2 remains to be established. 16
2.3. Azathioprine
Azathioprine is used off‐label in the United States for AD treatment in patients recalcitrant or who have contraindications to cyclosporine and methotrexate. In 12 AD children treated with azathioprine, there were no associated infections. 17 In a double‐blind, placebo‐controlled, crossover study of 37 AD adults treated with azathioprine, there were five cases of upper respiratory infections (URIs) (14%), two cases folliculitis (5%), and one report each impetigo (3%) and sore throat (3%). 18 In a retrospective analysis of 232 611 systemically treated adults with AD (6 months), there were increased risks of serious and opportunistic infections with azathioprine (relative risk (RR) = 1.89) and prednisone (RR = 1.78) compared with methotrexate, with a reduced risk with cyclosporine (RR = 0.87). 12 Therefore, azathioprine may increase susceptibility to infections, and if essential, exposure to COVID‐19 should be minimized.
2.4. Apremilast
Apremilast, an orally administered phosphodiesterase‐4 inhibitor is FDA approved for moderate‐to‐severe plaque psoriasis and has been used off‐label for AD. 19 , 20 , 21 Although it does modulate immunologic cascades, this pathway does not seem to significantly increase susceptibility to infection. In a pooled safety analysis of two randomized controlled trials (RCTs) involving psoriasis patients treated with apremilast (n = 1184), URIs and nasopharyngitis occurred in 19.2% and 16.6% of patients, respectively; serious infections (urinary tract infection n = 2; appendicitis n = 3; pneumonia = 2) occurred in 1.4%. 22 Furthermore, in an observational cohort study including systemically treated psoriasis patients, overall serious infections were decreased with apremilast vs methotrexate (HR, 0.50; 95% CI, 0.26‐0.94). Thus, apremilast seems to be a safe alternative for uninfected psoriasis patients during the pandemic, but specific COVID‐19 data are needed.
Data regarding infection risks of nonbiological therapies for psoriasis and AD are summarized in Table 1.
TABLE 1.
Studies on infection risk of nonbiological systemic therapies for psoriasis and atopic dermatitis
| Study, year/medication | Patient demographics | Medication, dosage | Indication | Outcome/type of infection, n (%) |
|---|---|---|---|---|
| Cyclosporine | ||||
| Garritsen et al 23 |
n = 267 Mean age = 35.50 y Male = 146 (55%) |
Mean maximum dose = 4.23 mg/kg/d | Atopic dermatitis | Infection leading to discontinuation: recurrent viral infection with herpes simplex: 1 (0%) |
| Schmitt et al 24 |
n = 17 Mean age = 30.1 y Female = 7 (41%) |
2.7‐4.0 mg/kg/d for 6 wk | Atopic dermatitis | Common cold: 4 (24.0%), infection of the skin: 4 (24.0%) |
| Goujon et al 11 |
n = 47 Mean age = 33 y Male = 31 (66%) |
2.5‐5 mg/kg divided in two doses daily | Atopic dermatitis | Nonskin infection: 10 (21.3%), skin infection: 5 (10.6%) |
| Methotrexate | ||||
| Garritsen et al 23 |
n = 37 Mean age = 43.89 y Male = 19 (51%) |
Mean maximum dose = 20.90 mg/wk | Atopic dermatitis | Infection leading to discontinuation: none reported |
| Goujon et al 11 |
n = 50 Mean age = 32 y Male = 28 (57%) |
15‐25 mg/wk | Atopic dermatitis | Nonskin infection: 6, skin infection: 6 |
| Baranauskaite et al 25 |
n = 54 Mean age = 42.3 y Male = 33 (61.1%) |
15‐20 mg/wk, for 16 wk | Psoriatic arthritis | Infection leading to discontinuation: none reported |
| Saurat et al 26 |
n = 110 Mean age = 41.6 y Male = 66.4% |
7.5 mg, increased as needed and as tolerated to 25 mg weekly | Psoriasis | Serious infection: 0, nonserious infection: 46 (41.8%), nasopharyngitis: 26 (23.6%), viral infection: 6 (5.5%) |
| Corticosteroids | ||||
| Garritsen et al 23 |
n = 24 Male = 14 (58%) Prednisone Mean age = 43.89 y Celestone Mean age = 36.40 |
Mean maximum dose: Prednisone: 23.0 mg/d Celestone: 1.50 mg/d |
Atopic dermatitis | Infection leading to discontinuation: unknown |
| Aljebab et al 8 |
n = 6817 Age range = 28 d to 18 y |
Prednisolone, dexamethasone, budesonide, methylprednisolone, deflazacort, betamethasone. For ≥15 d | Atopic dermatitis | Incidence rate: all infections: 8.7%, resulting in 21 deaths |
| Aljebab et al 27 |
n = 3200 Age range = 28 d to 18 y |
Prednisolone, dexamethasone, or betamethasone | Atopic dermatitis | Incidence rate: all infections: 0.9%, resulting in 1 death |
| Schmitt et al 24 |
n = 21 Mean age = 28.8 y Female = 10 (48%) |
Prednisolone: 0.5‐0.8 mg/kg/d for 2 wk | Atopic dermatitis | Skin infection: 1 (4.0%) |
| Azathioprine | ||||
| Garritsen et al 23 |
n = 46 Mean age = 40.24 y Male = 22 (48%) |
Mean maximum dose = 121.56 mg/d | Atopic dermatitis | Infection leading to discontinuation: flu‐like symptoms: 1 (4.0%) |
| Caufield et al 17 |
n = 12 Mean age = 9.0 y Male = 4 (33%) |
1.25‐3.4 mg/kg/d | Atopic dermatitis | No infection reported |
| Berth‐Jones et al 18 |
n = 37 Mean age = 38 y Male = 25 (68%) |
2.5 mg/kg/d | Atopic dermatitis | URI: 5 (14.0%), folliculitis: 2 (5.0%), impetigo: 1 (3.0%), sore throat: 1 (3.0%) |
| Apremilast | ||||
| Crowley et al 22 |
n = 1184 Mean age = 45.9 y Male = 805 (68.0%) |
30 mg twice a day | Psoriasis | URI: 227 (19.2%), nasopharyngitis: 196 (16.6%), urinary infection: 2 (0%), serious infection: 17 (1.4%), serious opportunistic infection: 0 |
| Kavanaugh et al 28 |
n = 168 Mean age = 48.7 y Female = 83 (49.4%) |
20 mg daily | Psoriatic arthritis | URI: 10 (6.0%) |
| Kavanaugh et al 28 |
n = 168 Mean age = 51.4 y Female = 92 (54.8%) |
30 mg daily | Psoriatic arthritis | URI: 7 (4.2%) |
| Dommasch et al 15 |
n = 1623 Mean age = 51.37 y Male = 820 (50.5%) |
Unknown | Psoriasis | Rate of overall serious infections compared with methotrexate: hazard ratio, 0.50; 95% CI, 0.26‐0.94 |
| Simpson et al 20 | n = 82 | 30 mg twice daily | Atopic dermatitis | Nasopharyngitis: 8 (9.8%), URI: 8 (9.8%), cellulitis 0 |
| Simpson et al 20 | n = 86 | 40 mg twice daily | Atopic dermatitis | Nasopharyngitis: 14 (16.3%), URI: 6 (7.0%), cellulitis 7 (7.0%) |
| Samrao et al 21 |
n = 6 Mean age = 38 y Male: female ratio = 5:1 |
20 mg twice daily | Atopic dermatitis | URI: 2 (33.3%), other infection: 2 (33.3%) |
| Samrao et al 21 |
n = 10 Mean age = 45 y Male: female ratio = 5:5 |
30 mg twice daily | Atopic dermatitis | URI: 3 (30.0%), other infection: 3 (30.0%) |
| Acitretin | ||||
| Dommasch et al 15 |
n = 2726 Mean age = 52.31 y Male = 1582 (58%) |
Unknown | Psoriasis | Rate compared with methotrexate, hazard ratio (HR): overall serious infection: HR, 1.09; 95% CI, 0.83‐1.44, bacteremia/sepsis: HR, 0.93, 95% CI, 0.51‐1.70, cellulitis/soft‐tissue infection: HR, 1.76, 95% CI, 1.11–2.80, pneumonia: HR, 0.85, 95% (0.54‐1.35) |
Abbreviations: CI, confidence interval; HR, hazard ratio; URI, upper respiratory infection.
2.5. Biologic medications and Janus kinase inhibitor
Biologic medications are widely used for psoriasis and AD patients, with limited data regarding infection risk. Since biologics inhibit immune‐mediated pathways involving specific cytokines, there is at least theoretical risk of increased susceptibility to and severity of infection. A common reason for discontinuation of biologics is infection. 29 Among the targeted cytokines for these biologics, tumor necrosis factor alpha (TNF‐α) plays a crucial role in the immune response against intracellular pathogens and formation of granulomas, 30 and interleukin (IL)‐12 and IL‐23 are involved in cell‐mediated immunity by inducing interferon‐γ. 31 IL‐23 also induces T‐helper 17 cell differentiation and IL‐17 secretion, fundamental in providing immunity against bacteria, viruses, fungi, and parasites. 32 , 33 IL‐4 and IL‐13 play key roles in the immune response against helminth infections. 34
Five classes of biologic therapies are used for psoriasis or AD: TNF‐α inhibitors (Table 2), IL‐17 inhibitors, an IL‐12/23 inhibitor, IL‐23 inhibitors, an IL‐4/13 inhibitor, and a Janus kinase (JAK) inhibitor (Table 3).
TABLE 2.
Studies on infection risk of tumor necrosis factor alpha inhibitors for psoriasis
| Study, year/medication | Patient demographics | Medication, dosage | Indication | Outcome/ type of infection, n (%) |
|---|---|---|---|---|
| TNF‐α inhibitors | ||||
| Adalimumab | ||||
| Yiu et al 35 |
n = 3271 Mean age = 44.7 y Female = 1323 (40.4%) |
Unknown | Psoriasis | N (incidence rate per 1000 person‐years): all serious infection: 108 (13.78), lower respiratory infection: 31 (3.96), skin and soft tissue infection: 19 (2.42) |
| Menter et al 36 |
n = 814 Mean age = 44.1 y Male = 546 (67.1%) |
80 mg at week 0, followed by 40 mg every other week | Psoriasis | All infections: 235 (62.2%), serious infection: 5 (0.6%), URI: 59 (7.2%), opportunistic infection (excluding tuberculosis): 1, tuberculosis: 1 |
| Kalb et al 37 |
n = 2675 Mean age = 47.6 y Male = 1505 (56.3%) |
Unknown | Psoriasis | Incidence rate per 100 patient‐years: serious infection: 1.97 |
| Dommasch et al 15 |
n = 7181 Mean age = 46.10 y Male = 4061 (56.6%) |
Unknown | Psoriasis | Rate compared with methotrexate, HR: overall serious infection: HR, 1.08; 95% CI, 0.88‐1.33, bacteremia/sepsis: HR, 1.06; 95% CI, 0.66‐1.68, cellulitis/soft‐tissue infection: HR, 1.34; 95% CI, 0.95‐1.89, Meningitis/encephalitis: HR, 0.78; 95% CI, 0.10‐6.28, pneumonia: HR, 0.94; 95% CI, 0.68‐1.31, pyelonephritis: HR, 1.11; 95% CI, 0.27‐4.51, septic arthritis/osteomyelitis: HR, 0.78; 95% CI, 0.25‐2.19 |
| Mease et al 38 |
n = 106 Mean age = 47.4 y Female = 50 (47%) |
40 mg every 2 wk | Psoriatic arthritis | Nasopharyngitis: 10.0%, URI: 8.0%, serious infection (herpes simplex and streptococcal pyoderma): 1.0% |
| Reich et al 39 |
n = 248 Mean age = 43.2 y Male = 170 (68.5%) |
80 mg at week 0, then 40 mg at week 1, and every 2 wk through week 23 | Psoriasis | Nasopharyngitis: 34 (13.7%), URI: 10 (4.0%), all infections: 87 (35.1%), requiring treatment: 29 (11.7%), serious infection: 3 (1.2%) |
| Blauvelt et al 40 |
n = 334 Mean age = 42.9 y Male = 249 (74.6%) |
80 mg week 0, 40 mg week 1, then 40 mg every 2 wk through week 46 | Psoriasis | Nasopharyngitis: 74 (22.2%), URI: 42 (12.6%), all infections: 167 (50.2%), infections requiring treatment: 60 (18.0%), serious infection: 3 (0.9%) |
| Saurat et al 26 |
n = 108 Mean age = 42.9 y Male = 64.8% |
80 mg at week 0, then 40 mg every other week | Psoriasis | Serious infection: 0, nonserious infection: 51 (47.7%), nasopharyngitis: 30 (28.0%), viral infection: 0 |
| Etanercept | ||||
| Yiu et al 35 |
n = 1325 Mean age = 45.5 y Female = 565 (41.8%) |
Unknown | Psoriasis | N (incidence rate per 1000 person‐years): serious infection: 50 (14.2), lower respiratory infection: 10 (5.5), skin and soft tissue infection: 18 (5.5) |
| Mease et al 41 |
n = 30 Median age = 46.0 y Age range = 30‐70 y Male = 16 (53%) |
25 mg twice weekly | Psoriasis/psoriatic arthritis | URI: 8 (27%), pharyngitis: 8 (27%), sinusitis: 3 (10%), influenza syndrome: 0 |
| Kalb et al 37 |
n = 1854 Mean age = 48.7 y Male = 1038 (56.0%) |
Unknown | Psoriasis | Incidence rate per 100 patient‐years: serious infection: 1.47 |
| Langley et al (FIXTURE) 42 |
n = 326 Mean age = 43.8 y Male = 232 (71.2%) |
50 mg twice weekly for 12 wk, then once weekly | Psoriasis | N (incidence rate per 100 subject‐years): Infections and infestations: 170 (91.4), nasopharyngitis: 86 (35.7), URI 18 (6.4) |
| Van de Kerkhof et al 43 |
n = 323 Mean age = 43.8 y Male = 229 (70.9%) |
Unknown | Psoriasis | Exposure‐adjusted incidence rates per 100 subject‐years of all infections: 93.7 |
| Dommasch et al 15 |
n = 7102 Mean age = 45.45 Male = 3903 (55.0%) |
Unknown | Psoriasis | Rate compared with methotrexate, HR: overall serious infection: HR, 0.75; 95% CI, 0.61‐0.93, bacteremia/sepsis: HR, 0.51; 95% CI, 0.32‐0.82, cellulitis/soft‐tissue infection: HR, 1.16; 95% CI, 0.82‐1.65, pneumonia: HR, 0.94; 95% CI, 0.68‐1.31, pyelonephritis: HR, 0.68; 95% CI, 0.20‐2.34, septic arthritis/osteomyelitis: HR, 1.61; 95% CI, 0.36‐7.16 |
| Infliximab | ||||
| Yiu et al 44 |
n = 422 Mean age = 46.6 y Male = 159 (37.7%) |
Unknown | Psoriasis | Rate per 1000 person‐years of all serious infections: 47.82, lower respiratory infection: 11.69 |
| Gottlieb et al 45 |
n = 33 Age range = 21‐69 y |
3 groups: placebo or 5 mg/kg or 10 mg/kg at weeks 0, 2 and 6 | Psoriasis | All infections (excluding URI): 7 patients (21%) |
| Reich et al 46 |
n = 301 Mean age = 42.6 y Female = 94 (31%) |
5 mg/kg at weeks 0, 2, 6, and 14 | Psoriasis | All infections: 125 (42.0%), URI: 46 (15.0%), serious infection: 3 (1.0%) |
| Menter et al 47 |
n = 313 Mean age 43.4 y Male = 65.8% |
3 mg/kg at weeks 0, 2, and 6 | Psoriasis | Patients with ≥1 infection: 106 (33.9%), URI: 50 (16%) |
| Menter et al 47 |
n = 314 Mean age = 65.8 y Male = 65% |
5 mg/kg at weeks 0, 2, 6, and 14 | Psoriasis | Patients with ≥1 infection: 97 (30.9%), URI: 42 (13.4%) |
| Kalb et al 37 |
n = 1151 Mean age = 48.5 y Male = 655 (56.9%) |
Unknown | Psoriasis | Incidence rate of serious infection per 100 patient‐years: 2.49 |
| Dommasch et al 15 |
n = 408 Mean age = 50.20 y Male = 202 (49.5%) |
Unknown | Psoriasis | Rate compared with methotrexate, HR: overall serious infection: HR, 1.47; 95% CI, 0.75‐2.87, bacteremia/sepsis: HR, 1.30; 95% CI, 0.19‐8.63, cellulitis/soft‐tissue infection: HR, 1.76; 95% CI, 0.55‐5.63, pneumonia: HR, 0.80; 95% CI, 0.29‐2.24, pyelonephritis: HR, 0.68; 95% CI, 0.20‐2.34 |
| Certolizumab | ||||
| Blauvelt et al 48 |
n = 351 Mean age = 46.1 y Male = 238 (67.8%) |
200 mg every 2 wk | Psoriasis | [Incidence rate]: Infections and infestations: 108 (30.9) [121.6], serious infections: 0 |
| Blauvelt et al 48 |
n = 342 Mean age = 45.2 y Male = 210 (61.4%) |
400 mg every 2 wk | Psoriasis | [Incidence rate]: Infections and infestations: 124 (36.3) [146.6], serious infections: 2 (0.6) [1.9] |
Abbreviations: CI, confidence interval; HR, hazard ratio; URI, upper respiratory infection.
TABLE 3.
Studies on infection risk of interleukin‐17, 12/23, and 23 inhibitors, tofacitinib and dupilumab for psoriasis or atopic dermatitis
| Study, year | Patient demographicsand clinical characteristics | Medication, dosage | Indication | Outcome/ type of infection, n (%) |
|---|---|---|---|---|
| IL‐17 inhibitors | ||||
| Secukinumab | ||||
| Langley et al (ERASURE) 42 |
n = 245 Mean age = 44.9 y Male = 169 (69%) |
300 mg once weekly for 5 wk, then every 4 wk | Psoriasis | N (incidence rate per 100 subject‐years): infections and infestations: 193 (100.0), nasopharyngitis: 57 (20.9), URI: 32 (11.1), influenza‐like illness: 14 (4.7) |
| Langley et al (ERASURE) 42 |
n = 245 Mean age = 44.9 y Male = 168 (68.6%) |
150 mg once weekly for 5 wk, then every 4 wk | Psoriasis | N (incidence rate per 100 subject‐years): infections and infestations: 185 (95.4), nasopharyngitis: 69 (26.2), URI: 36 (12.7), influenza‐like illness: 17 (5.8) |
| Langley et al (FIXTURE) 42 |
n = 327 Mean age = 44.5 y Male = 224 (68.5%) |
300 mg once weekly for 5 wk, then every 4 wk | Psoriasis | N (incidence rate per 100 subject‐years): infections and infestations: 269 (105.4), nasopharyngitis: 122 (35.2), URI: 26 (6.6) |
| Langley et al (FIXTURE) 42 |
n = 327 Mean age = 45.4 y Male = 236 (72.2%) |
150 mg once weekly for 5 wk, then every 4 wk | Psoriasis | N (incidence rate per 100 subject‐years): infections and infestations: 240 (91.9), nasopharyngitis: 108 (31.4), URI: 26 (6.6) |
| Van de Kerkhof et al 43 | n = 3430 | 150 or 300 mg | Psoriasis |
Exposure‐adjusted incidence rates per 100 subject‐years of all infections 150 mg: 85.3; 300 mg: 91.1 |
| Reich et al 49 |
n = 514 Mean age = 45.3 y Male = 342 (67%) |
300 mg at weeks 0, 1, 2, 3, and 4, and then every 4 wk | Psoriasis | All infections: 331 (65.0%), infections requiring treatment: 147 (29.0%), serious infection: 5 (1.0%), Candida infection: 29 (6.0%), tinea infection: 23 (5.0%) nasopharyngitis: 125 (24.0%), URI: 92 (18.0%) |
| Ixekizumab | ||||
| Langley et al 50 | n = 5689 | 160 mg at week 0, followed by 80 mg every 4 or 2 wk | Psoriasis | Proportion of patients with any infection: 60.8%, mild: 25.4%, moderate: 32.4% and severe: 3% infections. N (%): of nasopharyngitis: 1302 (22.9%), URI: 769 (13.5%); the incidence risk (95% CI) of Candida infection: 0.9 (0.8, 1.1) |
| Armstrong et al 51 |
n = 5898 Mean age = 45.8 y Male = 4000 (67.8%) |
160 mg at week 0, followed by 80 mg every 4 or 2 wk | Psoriasis | N (%) [incidence rate per 100 patient‐years]: ≥1 infection: 3859 (65.4%) [22.7], nasopharyngitis: 1515 (25.7) [8.9], bronchitis: 398 (6.7%) [2.3], sinusitis: 369 (6.3%), 2 urinary infection: 333 (5.6) [2.0], influenza: 307 (5.2) [1.8], pharyngitis: 278 (4.7) [1.6], gastroenteritis: 237 (4.0) [1.4], patients with ≥1 serious infection/infestation: 223 (3.8) [1.3], cellulitis: 40 (0.7) [0.2], pneumonia: 25 (0.4) [0.1], appendicitis: 11(0.2) [0.1], erysipelas: 9 (0.2) [0.1] |
| Brodalumab | ||||
| Papp et al 52 |
n = 441 Mean age = 46 y Male = 323 (73%) |
140 mg or 210 mg every 2 wk | Psoriasis | Serious infectious episode: 4 (1.8%), suspected Candida infections: 18 (3.5%) |
| Lebwohl et al (AMAGINE‐2) 53 |
Total n = 1222 140 = mg group: n = 610 Mean age = 45 y Male = 413 (68%) 210‐mg group: n = 612 Mean age = 45 y Male = 421 (69%) |
140 mg or 210 mg every 2 wk | Psoriasis | Serious infections and infestations: 13 (1.0%), Candida infection: 71 (5.2%) |
| Lebwohl et al (AMAGINE‐3) 53 |
Total n = 1253 140‐mg group: n = 629 Mean age = 45 y Male = 437 (70%) 210‐mg group: n = 624 Mean age = 45 y Male = 431 (69%) |
140 or 210 mg every 2 wk | Psoriasis | Serious infections and infestations: 18 (1.3%), Candida infections: 80 (5.7%) |
| IL‐12/23 inhibitor (ustekinumab) | ||||
| Yiu et al 35 |
n = 994 Mean age = 45.9 y Female = 377 (37.9%) |
Unknown | Psoriasis | N (incidence rate per 1000 person‐years): all serious infections: 34 (15.07), lower respiratory infection: 12 (5.32), 8 (3.55), skin and soft tissue infection: 8 (3.55) |
| Kalb et al 37 |
n = 3474 Mean age = 47.2 y Male = 1999 (57.5%) |
Unknown | Psoriasis | Incidence rate of serious infections per 100 patient‐years: 0.83 |
| Gordon et al 54 |
n = 3219 Mean age = 45.6 y Male = 2206 (68.5%) |
45 or 90 mg | Psoriasis | Rate per 100 patient‐years during placebo‐controlled: rate of overall infection: 45 mg (145.7), 90 mg (132.2), and during controlled and uncontrolled period: 45 mg (113.7), 90 mg (111.2); rates of serious infections during placebo‐controlled period: 45 mg (0.49), 90 mg (1.97), and controlled and uncontrolled period: 45 mg (0.82), 90 mg (1.50) |
| Dommasch et al 15 |
n = 4085 Mean age = 46.50 y Male = 2302 (56.4%) |
Unknown | Psoriasis | Rate compared with methotrexate, hazard ratio (HR): overall serious infection: HR, 0.65; 95% CI, 0.47‐0.89, bacteremia/sepsis: HR, 0.83; 95% CI, 0.39‐1.73, cellulitis/soft‐tissue infection: HR, 0.87; 95% CI, 0.51‐1.48, pneumonia: HR, 0.53; 95% CI, 0.32‐0.88, pyelonephritis: HR, 1,32; 95% CI, 0.20‐8.78, septic arthritis/osteomyelitis: HR, 0.51; 95% CI, 0.08‐3.52 |
| Gordon et al (ULtIMMA‐1) 55 |
n = 100 Mean age = 46.5 y Male = 70 (70%) |
45 or 90 mg | Psoriasis | All infections: 20 (20.0%), serious infections: 3 (3.0%), active tuberculosis: 0, latent tuberculosis: 0 |
| Gordon et al (ULtIMMA‐2) 55 |
n = 99 Mean age = 48.6 y Male = 66 (67%) |
45 or 90 mg | Psoriasis | All infections: 20 (20.2%), serious infections: 1 (1.0%), active tuberculosis: 0, latent tuberculosis: 0 |
| Lebwohl et al (AMAGINE‐2) 53 |
n = 300 Mean age = 45 y Male = 205 (68%) |
45 mg for patients with a body weight ≤ 100 kg and 90 mg for patients >100 kg | Psoriasis | Serious infections and infestations: 2 (0.8%), candida infections: 10 (4.1%) |
| Lebwohl et al (AMAGINE‐3) 53 |
n = 313 Mean age = 45 y Male = 212 (68%) |
45 mg for patients with a body weight ≤ 100 kg and 90 mg for patients >100 kg | Psoriasis | Serious infections and infestations: 3 (1.2%), candida infections: 4 (1.6%) |
| IL‐23 inhibitors | ||||
| Guzselkumab | ||||
| Reich et al 39 |
n = 496 Mean age = 43.7 y Male = 349 (70.4%) |
100 mg at weeks 0, 4, then every 8 wk | Psoriasis | Nasopharyngitis: 51 (10.3%), URI: 25 (5.1%), all infections: 153 (31%), infections requiring treatment: 58 (11.7%), serious infections: 3 (0.6%) |
| Reich et al 39 |
n = 248 Mean age = 43.4 y Male = 173 (69.8%) |
Placebo to guselkumab 100 mg at weeks (0, 4 and 12 then guselkumab at weeks 16 and 20) | Psoriasis | Nasopharyngitis: 12 (6.2%), URI: 5 (2.1%), all infections: 153 (31%), infections requiring treatment: 41 (17.6%), serious infections: 1 (0.4%) |
| Blauvelt et al 40 |
n = 329 Mean age = 43.9 y Male = 240 (72.9%) |
100 mg at weeks 0, 4, then every 8 wk | Psoriasis | Nasopharyngitis: 83 (25.2%), URI: 46 (14.3%), all infections: 172 (62.3%), infections requiring treatment: 54 (16.4%), serious infections: 2 (0.6%) |
| Blauvelt et al 40 |
n = 174 Mean age = 44.9 y Male = 119 (68.4%) |
Placebo to guselkumab 100 mg at weeks (0, 4 and 12 then guselkumab at weeks 16 and 20) | Psoriasis | Nasopharyngitis: 34 (20.6%), URI: 17 (10.3%), all infections: 76 (46.1%), infections requiring treatment: 25 (15.2%), serious infections: 1 (0.6%) |
| Reich et al 49 |
n = 534 Mean age = 46.3 y Male = 365 (68%) |
100 mg at weeks 0, 4, then every 8 wk | Psoriasis | Overall infections: 313 (59.0%), infections requiring treatment: 118 (22.0%), serious infections: 6 (1.0%), candida infections: 12 (2.0%), tinea infections: 9 (2.0%) nasopharyngitis: 118 (22.0%), URI: 83 (16.0%) |
| Tildrakizumab | ||||
| Papp et al 56 |
n = 42 Mean years = 43.2 y Male = 31 (74%) |
5 mg at week 0, 4 and every 12 wk until week 52 | Psoriasis |
Weeks 0‐16: all infections: 0 Weeks 16‐52: all infections: 0 |
| Papp et al 56 |
n = 92 Mean age = 46.3 y Male = 60 (65%) |
25 mg at week 0, 4 and every 12 wk until week 52 | Psoriasis |
Weeks 0‐16: serious infections: 0, bacterial arthritis: 1 (1.0%) Weeks 16‐52: serious infections: 1 (1.0%), sinusitis 1 (1.0%) |
| Papp, 2015 56 | n = 89Mean age = 45.5Male = 76 (85%) | 100 mg at week 0, 4 andevery 12 weeks until week 52 | Psoriasis |
Weeks 0‐16: serious infections: 1 (1.0%)Weeks 16‐52: serious infections: 1 (1.0%), appendicitis: 1 (1.0%), epiglottitis: 1 (1.0%), sinusitis: 1 (1.0%) |
| Papp et al 56 |
n = 86 Mean age = 43.2 y Male = 65 (76%) |
200 mg at week 0, 4 and every 12 wk until week 52 | Psoriasis |
Weeks 0‐16: all infections: 0 Weeks 16‐52: serious infections: 1 (1.0%), postoperative wound infection: 1 (1.0%), bursitis: 1 (1.0%) |
| Risankizumab | ||||
| Gordon et al (ULtIMMA‐1) 55 |
Risankizumab: n = 304 Mean age = 48.3 y Male = 212 (70%) Placebo to risankizumab: n = 102 Mean age = 49.3 Male = 79 (77%) |
150 mg | Psoriasis |
Risankizumab group: all infections: 75 (24.7%), serious infection: 1 (0.3%), active tuberculosis: 0 Placebo to risankizumab group: all infections: 17 (16.7%), serious infections: 0, active tuberculosis: 0, latent tuberculosis: 0 |
| Gordon et al (ULtIMMA‐2) 55 |
Risankizumab: n = 294 Mean age = 46.2 y Male = 203 (69%) Placebo to risankizumab: n = 98 Mean age = 46.3 Male = 67 (68%) |
150 mg | Psoriasis |
Risankizumab group: all infections: 56 (19.0%), serious infections: 3 (1.0%), active tuberculosis: 0 Placebo to risankizumab group: all infections: 9 (9.2%), serious infections: 0, active tuberculosis: 0, latent tuberculosis: 0 |
| Janus kinase 1/3 inhibitor (tofacitinib) | ||||
| Mease et al 38 | n = 159 | 5 mg twice daily | Psoriatic arthritis | Nasopharyngitis: 7.0%, URI: 9.0%, serious infections: 4.0%, herpes zoster: 2.0% |
| Mease et al 38 | n = 157 | 10 mg twice daily | Psoriatic arthritis | Nasopharyngitis: 12.0%, URI: 11.0%, serious infections: 1.0%, herpes zoster: 2.0% |
| Papp et al 57 |
Total n = 745 OPT Pivotal 1: n = 363 Mean age = 46 (range = 18‐78) y Male = 261 (71.9%) OPT Pivotal 2: n = 382 Mean age = 47 (range = 19‐79) Male = 268 (70.2%) |
5 mg twice daily | Psoriasis | Serious infection: 3 (pneumonia, herpes zoster and erysipelas), herpes zoster: 6, herpes simplex: 2 |
| Papp et al 57 |
Total n = 741 OPT Pivotal 1: n = 360 Mean age = 46 (range = 18‐79) y Male = 261 (72.5%) OPT Pivotal 2: n = 381 Mean age = 44 (range = 18‐82) y Male = 257 (67.5%) |
10 mg twice daily | Psoriasis | Serious infection: 2 (appendicitis; pneumonia and pyelonephritis), herpes zoster: 6, herpes simplex: 3 |
| IL 4/13 inhibitor (dupilumab) | ||||
| Eichenfield et al 58 | n = 1095 | 300 mg weekly | Atopic dermatitis | N (number of patients per 100 patients‐years): all infections: 452 (126.49), infection leading to treatment discontinuation: 2 (0.38), serious infection: 7 (2.40) |
| Eichenfield et al 58 | n = 746 | 300 mg every 2 wk | Atopic dermatitis | N (number of patients per 100 patients‐years): all infections: 287 (133.59), infection leading to treatment discontinuation: 1 (0.37), serious infection: 7 (2.39) |
Abbreviations: CI, confidence interval; HR, hazard ratio; URI, upper respiratory infection.
2.6. TNF‐α inhibitors (adalimumab, etanercept, infliximab, certolizumab)
Anti‐TNF‐α therapies inhibit a crucial immunological pathway, therefore an immunosuppressive effect and increased infection risk are expected. There is an FDA‐required black box warning of infection susceptibility. 59 However, assessing infection risk is challenging because RCTs are often not adequately powered to detect rare events and ineligibility criteria may exclude up to 30% of real‐world patients. 60 Real‐world, postmarketing surveillance studies may be more helpful in evaluating infection rates. In a 10‐year cohort study of 422 infliximab‐treated psoriasis patients from the British Association of Dermatologists Biologic Interventions Register (BADBIR), there was increased infection risk compared to nonbiologic treated patients (adjusted HR, 1.95, 95% CI 1.01‐3.75) and methotrexate only (adjusted HR 3.49, 95% CI 1.14‐10.70). 44 Using real‐world data from the Psoriasis Longitudinal Assessment and Registry involving 11 466 psoriasis patients (n = 9154 biologics, n = 490 methotrexate or other nonbiologics [excluding cyclosporine], n = 1610 with other than biologics or methotrexate), cumulative incidence rates of serious infections were 0.83, 1.47, 1.97, and 2.49 per 100 patient‐years in ustekinumab, etanercept, adalimumab, and infliximab cohorts, respectively, and 1.28 and 1.05 per 100 patient‐years in methotrexate or other nonbiologics, and nonbiologics without methotrexate cohorts, respectively. 37 Cellulitis and pneumonia were the two most common serious infections. 37 In another BADBIR study of etanercept (n = 1352), adalimumab (n = 3271), and ustekinumab (n = 994)‐treated psoriasis patients, there were no increased risk of serious infections with etanercept (HR = 1.10, 95% CI = 0.75‐1.60), adalimumab (HR = 0.93, 95% CI = 0.69‐1.26), or ustekinumab (HR = 0.92, 95% CI = 0.60‐1.41) compared with nonbiologic systemic therapies or methotrexate‐only (etanercept: HR = 1.47, 95% CI = 0.95‐2.28; adalimumab: HR = 1.26, 95% CI = 0.86‐1.84; ustekinumab: HR = 1.22, 95% CI = 0.75‐1.99). 35 Nonetheless, a 7% increased risk of all infections with adalimumab compared with placebo was reported based on pivotal trials, with no increased risk for etanercept. 59 Certolizumab increased risks of all infections, URIs, and nasopharyngitis by 5%, 2% and 2%, respectively. 59 Additionally, anti‐TNF‐α therapy is associated with latent tuberculosis reactivation, even with chemoprophylaxis; infliximab is associated with increased risk of herpes zoster. 61 , 62 Therefore, based on available data, anti‐TNF‐α biologics should be held during active infection; in asymptomatic/healthy patients, safer and more effective alternatives should be considered. TNF‐α inhibitors have been hypothesized to treat SARS‐CoV‐2‐related cytokine storm. 63
2.7. IL‐17 inhibitors (secukinumab, ixekizumab, brodalumab)
Secukinumab selectively targets IL‐17A, a downstream product of Th17 cells, and does not interfere with other essential Th17 functions, including IL‐22 and TNF release; therefore, lower infection risk compared with anti‐TNF‐α therapies is expected. 64 Since anti‐IL‐17 therapies are relatively new medications, long‐term “real‐world” studies are sparse and estimation of infection risk is primarily based on RCTs. In a pooled analysis of 10 phase 2/3 studies assessing long‐term safety of secukinumab (150 or 300 mg) and etanercept, there were increased infection rates for all treatments compared to placebo during the first 12 weeks. 43 The risks of serious infections were 1.47 and 1.37 per 100 subject‐years in the secukinumab and etanercept groups, respectively. 43 No cases of tuberculosis reactivation were reported, and patients with latent tuberculosis were not excluded. 43 Similarly, in two phase 3 studies involving 3712 psoriasis patients randomized to treatment with brodalumab (n = 2475), ustekinumab or placebo, rates of serious infections were 1.0 (AMAGINE‐2) and 1.3 (AMAGINE‐3) per 100 patient‐year exposure to brodalumab, and Candida infections were more frequent with brodalumab vs ustekinumab or placebo. 53 Similarly, an 11% increased risk in overall infections with secukinumab was reported based on pivotal trials, with most attributable to yeast infections; URIs were increased slightly for secukinumab, but not for ixekizumab or brodalumab. 59 Since IL‐17 plays an important role in immunological response against Candida infections, there is a theoretical increased risk of yeast infections with anti‐IL17 therapies. 65 In a pooled analysis from 10 phase 2 and phase 3 clinical studies on 3430 psoriasis patients treated with secukinumab 300 mg (n = 11 410), 150 mg (n = 1395), and etanercept (n = 323), Candida infections were reported in 2.9%, 1.5%, and 1.2% of subjects, respectively. 66 All infections were mild, resolved spontaneously, or responded to standard treatment, without causing treatment discontinuation. 66 Overall, increased infection risk has been shown with IL‐17 inhibitors, but yeast infections may constitute a large proportion of that increase; URIs are particularly uncommon. Therefore, IL‐17 inhibitors may be safely prescribed and continued, unless the patient is symptomatic or positive for SARS‐CoV‐2.
2.8. IL‐12/23 inhibitors (ustekinumab)
Ustekinumab inhibits IL‐12 and IL‐23, with IL‐12 playing an important role in protection against viral infections. 59 , 67 , 68 However, no increased susceptibility to infection with ustekinumab has been reported. In a pooled analysis of four phase 2/3 studies of 3117 ustekinumab‐treated psoriasis patients, there were similar rates of all infections amongst placebo (121.0), ustekinumab 45‐mg (145.7), and ustekinumab 90‐mg (132.2) groups; also similar rates of serious infections between placebo (1.70) and 90‐mg (1.97) groups, and a lower rate in the 45‐mg group (0.49). 54 No cases of tuberculosis reactivation were reported. 54 In one observational cohort study of 107 707 systemically treated psoriasis patients, ustekinumab (HR: 0.65; 95% CI, 0.47‐0.89), apremilast (HR: 0.50; 95% CI, 0.26‐0.94) and etanercept (HR: 0.75; 95% CI, 0.61‐0.93) had decreased risks of overall serious infections compared with methotrexate. 15 Similar risks of infection between ustekinumab and placebo were reported by Lebwohl et al. 59 Thus, treatment with ustekinumab may be considered relatively safe during the COVID‐19 pandemic; however, switching to specific IL‐23 inhibitors may be prudent. Notably, ustekinumab may positively affect SARS‐CoV‐2‐related cytokine storm. 63
2.9. IL‐23 inhibitors (guselkumab, tildralkizumab, risankizumab)
Contrary to IL‐12/23 inhibitors, anti‐IL‐23 therapies do not target IL‐12, and IL‐12 plays a key role fighting viral infections. 59 , 69 Reduced risks of Salmonella, Candida, and Mycobacterium infections were seen in IL‐23p19‐targeted vs IL‐12/23p40‐targeted animal models. 56 , 70 , 71 , 72 Nonetheless, RCTs on IL‐23 inhibitors have shown conflicting results regarding infection risks. In a phase 3, double‐blinded, placebo‐controlled study on 837 psoriasis patients randomized to treatment with guselkumab, adalimumab, or placebo, overall, Candida, and serious infections, occurred at comparable rates across treatment groups. 40 In 798 risankizumab‐treated psoriasis patients, there was increased overall infection risk in two phase 3 studies. 55 The most common infections were URIs, urinary tract infections, and influenza. 55 Two cases of latent tuberculosis were reported in the risankizumab group; both patients tested negative at baseline. 55 Data assessing infection risk with tildrakizumab are sparse. Lebwohl et al reported an increase in nasopharyngitis (4%) with tildrakizumab compared with placebo. 59 Risk, however, is low and comparable to placebo. Therefore, based on available data, IL‐23 inhibitors may be continued/initiated, unless the patient is symptomatic or positive for SARS‐CoV‐2.
2.10. JAK inhibitor (tofacitinib)
Tofacitinib is a small molecule inhibitor of tyrosine kinases of the Janus family, preferentially JAK1 and JAK3, downregulating cytokines crucial for lymphocyte development; therefore, there is potential for increased risks of intracellular bacterial and viral infections. 73 It has been hypothesized, nonetheless, that fluctuations in plasma levels of JAK inhibitors throughout the day may preserve immunogenicity against infectious pathogens. 74 Tofacitinib carries an FDA‐required black box warning for serious infections. In one placebo‐controlled phase 3 trial of 422 patients with psoriatic arthritis, randomized to treatment with 5‐mg or 10‐mg tofacitinib, adalimumab, or placebo, nasopharyngitis (in 7%, 12% and 10%, respectively) and URIs (in 9%, 11%, and 8%, respectively) were the most common adverse events. 38 There were three cases of serious infections (influenza, appendicitis and pneumonia) and four cases of herpes zoster in the tofacitinib‐treated group. 38
Similarly, in 2 randomized, placebo‐controlled studies of 745 and 741 psoriasis patients treated with tofacitinib 5‐mg and 10‐mg, respectively, nasopharyngitis and URIs were the most common infections, and 5 serious infections (pneumonia, herpes zoster and erysipelas in the 5‐mg group; and appendicitis, pneumonia, and pyelonephritis in the 10‐mg group) were reported in tofacitinib‐treated patients. 57 Furthermore, herpes zoster was reported in 12 tofacitinib‐treated patients vs none in the placebo groups. 57 Thus, tofacitinib has an association with increased infection risk in psoriasis/psoriatic arthritis patients. Tofacitinib‐treated patients may be more susceptible to COVID‐19, strict protective measures are recommended to minimize viral exposure.
2.11. Dupilumab
Dupilumab targets IL‐4 and IL‐13, elements of the type 2 immune response. 58 As type 1 and type 2 immune responses crossregulate each other, suppression of type 1 immunity can potentially facilitate uncontrolled or persistent viral and bacterial infections. 75 Nonetheless, dupilumab has been associated with a reduced infection rate in AD patients. A pooled analysis of seven RCTs on dupilumab‐treated AD adults showed a decreased risk of serious infections, skin infections, and herpes infections (eczema herpeticum or herpes zoster) in the dupilumab groups compared with placebo. Furthermore, by also treating asthma, dupilumab may theoretically decrease risk for COVID‐19‐infected patients for severe respiratory disease. 58 Therefore, current evidence suggests continuing and initiating dupilumab treatment in AD patients during the COVID‐19 pandemic.
3. CONCLUSIONS
It is difficult to make definitive conclusions about susceptibility to SARS‐CoV‐2 infection in psoriasis or AD patients on systemic treatments, solely based on general infection risk data. Furthermore, the majority of studies included patients with mean age of approximately 40 years; therefore, these recommendations may not be applicable to older individuals, who on average have higher COVID‐19 associated mortality. There is also a potential role for some of these medications as treatments of COVID‐19 but this remains largely unknown. In conclusion, in patients with active infection, systemic conventional medications, the JAK inhibitor tofacitinib, and biologics for psoriasis should be temporarily held until there is more data. Otherwise, conventional systemic immunosuppressive medications (corticosteroids, methotrexate, cyclosporine, and azathioprine) are associated with increased infection risk and therefore warrant strict measures to minimize exposure. Tofacitinib and TNF‐α inhibitors may also increase infection risk and safer alternatives may be considered. IL‐17/12/23 inhibitors seem to be among the safer medications (IL‐17, IL‐12/23 > IL‐23), but exact infection risks have not been fully characterized. Finally, apremilast, dupilumab, and acitretin are not associated with increased infection risks and appear to have favorable safety profiles. We suggest the following algorithms for treatment of psoriasis and AD during the COVID‐19 pandemic (Figures 1 and 2).
FIGURE 1.
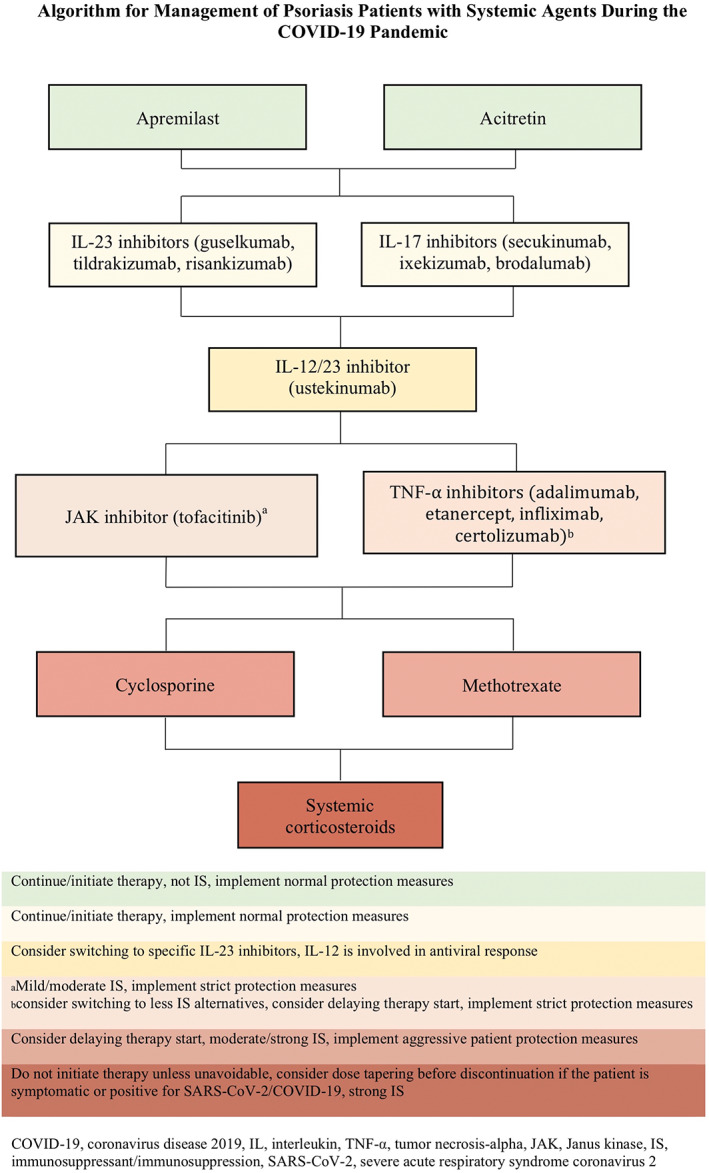
Proposed treatment algorithm of systemically treated psoriasis patients during the COVID‐19 pandemic. In case the patient is positive or symptomatic for SARS‐CoV‐2/COVID‐19, all immunomodulating medications must be discontinued. COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
FIGURE 2.
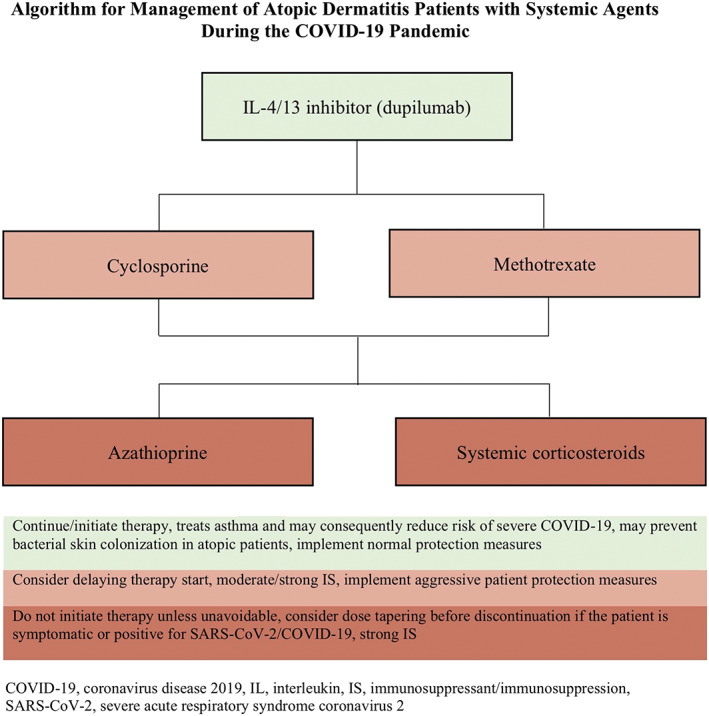
Proposed treatment algorithm of systemically treated atopic dermatitis patients during the COVID‐19 pandemic. In case the patient is positive or symptomatic for SARS‐CoV‐2/COVID‐19, all immunomodulating medications must be discontinued. COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
ACKNOWLEDGMENTS
The authors wish to thank Mark Lebwohl for his expert critical review of this manuscript. This work is not under consideration at any other journal and has not been previously presented.
Ricardo JW, Lipner SR. Considerations for safety in the use of systemic medications for psoriasis and atopic dermatitis during the COVID‐19 pandemic. Dermatologic Therapy. 2020;33:e13687. 10.1111/dth.13687
REFERENCES
- 1. Johns Hopkins University & Medicine CRC . Coronavirus COVID‐19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. https://coronavirus.jhu.edu/map.html.
- 2. Khafaie MA, Rahim F. Cross‐country comparison of case fatality rates of COVID‐19/SARS‐COV‐2. Osong Public Health Res Perspect. 2020;11(2):74‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population‐based study. J Invest Dermatol. 2015;135(1):56‐66. [DOI] [PubMed] [Google Scholar]
- 4. Helmick CG, Lee‐Han H, Hirsch SC, Baird TL, Bartlett CL. Prevalence of psoriasis among adults in the U.S.: 2003‐2006 and 2009‐2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47(1):37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guttman‐Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68‐73. [DOI] [PubMed] [Google Scholar]
- 6. Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the international psoriasis council. J Am Acad Dermatol. 2020;82(1):117‐122. [DOI] [PubMed] [Google Scholar]
- 7. Megna M, Napolitano M, Patruno C, et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb). 2017;7(1):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aljebab F, Choonara I, Conroy S. Systematic review of the toxicity of long‐course oral corticosteroids in children. PLoS One. 2017;12(1):e0170259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta‐analysis. Crit Care. 2019;23(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davila‐Seijo P, Dauden E, Descalzo MA, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM Registry. J Invest Dermatol. 2017;137(2):313‐321. [DOI] [PubMed] [Google Scholar]
- 11. Goujon C, Viguier M, Staumont‐Salle D, et al. Methotrexate versus cyclosporine in adults with moderate‐to‐severe atopic dermatitis: a phase III randomized noninferiority trial. J Allergy Clin Immunol Pract. 2018;6(2):562‐569 e3. [DOI] [PubMed] [Google Scholar]
- 12. Schneeweiss MC, Perez‐Chada L, Merola JF. Comparative safety of systemic immuno‐modulatory medications in adults with atopic dermatitis. J Am Acad Dermatol. 2019;S0190‐9622(19):30877‐1. 10.1016/j.jaad.2019.05.073. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5):1250‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilkington T, Brogden RN. Acitretin. A review of its pharmacology and therapeutic use. Drugs. 1992;43(4):597‐627. [DOI] [PubMed] [Google Scholar]
- 15. Dommasch ED, Kim SC, Lee MP, Gagne JJ. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caselli E, Galvan M, Santoni F, et al. Retinoic acid analogues inhibit human herpesvirus 8 replication. Antivir Ther. 2008;13(2):199‐209. [PubMed] [Google Scholar]
- 17. Caufield M, Tom WL. Oral azathioprine for recalcitrant pediatric atopic dermatitis: clinical response and thiopurine monitoring. J Am Acad Dermatol. 2013;68(1):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berth‐Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double‐blind, placebo‐controlled, crossover trial. Br J Dermatol. 2002;147(2):324‐330. [DOI] [PubMed] [Google Scholar]
- 19. Fala L. Otezla (apremilast), an Oral PDE‐4 inhibitor, receives FDA approval for the treatment of patients with active psoriatic arthritis and plaque psoriasis. Am Health Drug Benefits. 2015;8(Spec Feature:105‐110. [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139(5):1063‐1072. [DOI] [PubMed] [Google Scholar]
- 21. Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148(8):890‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crowley J, Thaci D, Joly P, et al. Long‐term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77(2):310‐317 e1. [DOI] [PubMed] [Google Scholar]
- 23. Garritsen FM, Roekevisch E, van der Schaft J, Deinum J, Spuls PI, de Bruin‐Weller MS. Ten years experience with oral immunosuppressive treatment in adult patients with atopic dermatitis in two academic centres. J Eur Acad Dermatol Venereol. 2015;29(10):1905‐1912. [DOI] [PubMed] [Google Scholar]
- 24. Schmitt J, Schakel K, Folster‐Holst R, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator‐initiated double‐blind placebo‐controlled multicentre trial. Br J Dermatol. 2010;162(3):661‐668. [DOI] [PubMed] [Google Scholar]
- 25. Baranauskaite A, Raffayova H, Kungurov NV, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate‐naive patients: the RESPOND study. Ann Rheum Dis. 2012;71(4):541‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558‐566. [DOI] [PubMed] [Google Scholar]
- 27. Aljebab F, Choonara I, Conroy S. Systematic review of the toxicity of short‐course oral corticosteroids in children. Arch Dis Child. 2016;101(4):365‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kavanaugh A, Mease PJ, Gomez‐Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo‐controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632‐2640. [DOI] [PubMed] [Google Scholar]
- 30. Zganiacz A, Santosuosso M, Wang J, et al. TNF‐alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113(3):401‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL‐12 and IL‐23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139‐156. [DOI] [PubMed] [Google Scholar]
- 32. Matsuzaki G, Umemura M. Interleukin‐17 family cytokines in protective immunity against infections: role of hematopoietic cell‐derived and non‐hematopoietic cell‐derived interleukin‐17s. Microbiol Immunol. 2018;62(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 33. Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL‐17 cytokines? Curr Opin Immunol. 2010;22(4):467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bao K, Reinhardt RL. The differential expression of IL‐4 and IL‐13 and its impact on type‐2 immunity. Cytokine. 2015;75(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yiu ZZN, Smith CH, Ashcroft DM, et al. Risk of serious infection in patients with psoriasis receiving biologic therapies: a prospective cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2018;138(3):534‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106‐115. [DOI] [PubMed] [Google Scholar]
- 37. Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151(9):961‐969. [DOI] [PubMed] [Google Scholar]
- 38. Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537‐1550. [DOI] [PubMed] [Google Scholar]
- 39. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418‐431. [DOI] [PubMed] [Google Scholar]
- 40. Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405‐417. [DOI] [PubMed] [Google Scholar]
- 41. Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356(9227):385‐390. [DOI] [PubMed] [Google Scholar]
- 42. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326‐338. [DOI] [PubMed] [Google Scholar]
- 43. van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long‐term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83‐98 e4. [DOI] [PubMed] [Google Scholar]
- 44. Yiu ZZN, Ashcroft DM, Evans I, et al. Infliximab is associated with an increased risk of serious infection in patients with psoriasis in the U.K. and Republic of Ireland: results from the British Association of Dermatologists Biologic Interventions Register (BADBIR). Br J Dermatol. 2019;180(2):329‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque‐type psoriasis. J Am Acad Dermatol. 2003;48(6):829‐835. [DOI] [PubMed] [Google Scholar]
- 46. Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet. 2005;366(9494):1367‐1374. [DOI] [PubMed] [Google Scholar]
- 47. Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate‐to‐severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31 e1‐31 e15. [DOI] [PubMed] [Google Scholar]
- 48. Blauvelt A, Reich K, Lebwohl M, et al. Certolizumab pegol for the treatment of patients with moderate‐to‐severe chronic plaque psoriasis: pooled analysis of week 16 data from three randomized controlled trials. J Eur Acad Dermatol Venereol. 2019;33(3):546‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate‐to‐severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831‐839. [DOI] [PubMed] [Google Scholar]
- 50. Langley RG, Kimball AB, Nak H, et al. Long‐term safety profile of ixekizumab in patients with moderate‐to‐severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol. 2019;33(2):333‐339. [DOI] [PubMed] [Google Scholar]
- 51. Armstrong A, Paul C, Puig L, et al. Safety of ixekizumab treatment for up to 5 years in adult patients with moderate‐to‐severe psoriasis: results from greater than 17,000 patient‐years of exposure. Dermatol Ther (Heidelb). 2020;10(1):133‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double‐blind, placebo‐controlled study of brodalumab in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol. 2016;175(2):273‐286. [DOI] [PubMed] [Google Scholar]
- 53. Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318‐1328. [DOI] [PubMed] [Google Scholar]
- 54. Gordon KB, Papp KA, Langley RG, et al. Long‐term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66(5):742‐751. [DOI] [PubMed] [Google Scholar]
- 55. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet. 2018;392(10148):650‐661. [DOI] [PubMed] [Google Scholar]
- 56. Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK‐3222), an anti‐interleukin‐23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo‐controlled trial. Br J Dermatol. 2015;173(4):930‐939. [DOI] [PubMed] [Google Scholar]
- 57. Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo‐controlled, phase III trials. Br J Dermatol. 2015;173(4):949‐961. [DOI] [PubMed] [Google Scholar]
- 58. Eichenfield LF, Bieber T, Beck LA, et al. Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol. 2019;20(3):443‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? J Am Acad Dermatol. 2020;82:1217‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garcia‐Doval I, Carretero G, Vanaclocha F, et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch Dermatol. 2012;148(4):463‐470. [DOI] [PubMed] [Google Scholar]
- 61. Ergun T, Seckin D, Baskan Bulbul E, et al. The risk of tuberculosis in patients with psoriasis treated with anti‐tumor necrosis factor agents. Int J Dermatol. 2015;54(5):594‐599. [DOI] [PubMed] [Google Scholar]
- 62. Adelzadeh L, Jourabchi N, Wu JJ. The risk of herpes zoster during biological therapy for psoriasis and other inflammatory conditions. J Eur Acad Dermatol Venereol. 2014;28(7):846‐852. [DOI] [PubMed] [Google Scholar]
- 63. Monteleone G, Sarzi‐Puttini PC, Ardizzone SJTLR. Preventing COVID‐19‐induced pneumonia with anticytokine therapy. Lancet. 2020;2(5):E255‐E256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deodhar A, Mease PJ, McInnes IB, et al. Long‐term safety of secukinumab in patients with moderate‐to‐severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post‐marketing surveillance data. Arthritis Res Ther. 2019;21(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin‐17 inhibitors and their practical management. Br J Dermatol. 2017;177(1):47‐62. [DOI] [PubMed] [Google Scholar]
- 66. Conrad C, Reich K, Blauvelt A, et al. Secukinumab‐treated subjects experience low rates of Candida and recurrent Candida infections: a pooled analysis from 10 phase 2 and 3 clinical studies in psoriasis: 3377. 2016;74:5. [Google Scholar]
- 67. Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL‐12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8(1):40‐52. [DOI] [PubMed] [Google Scholar]
- 68. Torti DC, Feldman SR. Interleukin‐12, interleukin‐23, and psoriasis: current prospects. J Am Acad Dermatol. 2007;57(6):1059‐1068. [DOI] [PubMed] [Google Scholar]
- 69. Komastu T, Ireland DD, Reiss CS. IL‐12 and viral infections. Cytokine Growth Factor Rev. 1998;9(3–4):277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chackerian AA, Chen SJ, Brodie SJ, et al. Neutralization or absence of the interleukin‐23 pathway does not compromise immunity to mycobacterial infection. Infect Immun. 2006;74(11):6092‐6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL‐23 and IL‐17A, but not IL‐12 and IL‐22, are required for optimal skin host defense against Candida albicans . J Immunol. 2010;185(9):5453‐5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schulz SM, Kohler G, Schutze N, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL‐12 is associated with IL‐23‐dependent IL‐22, but not IL‐17. J Immunol. 2008;181(11):7891‐7901. [DOI] [PubMed] [Google Scholar]
- 73. Welsch K, Holstein J, Laurence A, Ghoreschi K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol. 2017;47(7):1096‐1107. [DOI] [PubMed] [Google Scholar]
- 74. Peterson D, Damsky W, King B. The use of Janus kinase inhibitors in the time of SARS‐CoV‐2. J Am Acad Dermatol. 2020;82:e223‐e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271‐282. [DOI] [PubMed] [Google Scholar]


