Abstract
Background
Data on the development of Covid‐19 among people with intellectual disabilities (IDs) are scarce and it is uncertain to what extent general population data applies to people with ID. To give an indication of possible implications, this study investigated excess mortality patterns during a previous influenza epidemic.
Methods
Using Dutch population and mortality registers, a historical cohort study was designed to compare mortality during the 2017–2018 influenza epidemic with mortality in the same period in the three previous years. People with ID were identified by entitlements to residential ID‐care services as retrieved from a national database.
Results
Data covered the entire adult Dutch population (12.6 million; GenPop), of which 91 064 individuals were identified with an ID. During the influenza epidemic, mortality among people with ID increased almost three times as much than in the GenPop (15.2% vs. 5.4%), and more among male individuals with ID (+19.5%) than among female individuals with ID (+10.6%), as compared with baseline. In both cohorts, comparable increases in mortality within older age groups and due to respiratory causes were seen. Particularly in the ID‐cohort, excess deaths also occurred in younger age groups, due to endocrine diseases and ID‐specific causes.
Conclusions
During the 2017–2018 influenza epidemic, excess mortality among people with ID was three times higher than in the general Dutch population, appeared more often at young age and with a broader range of underlying causes. These findings suggest that a pandemic may disproportionally affect people with ID while population data may not immediately raise warnings. Early detection of diverging patterns and faster implementation of tailored strategies therefore require collection of good quality data.
Keywords: epidemics, mortality, population data, virus infections
Introduction
People with intellectual disabilities (IDs) have poorer overall health than other people in the general population, and are often in close contact to others when receiving care and support in daily activities, and when living in group homes (Krahn et al. 2006; Balogh et al. 2016). Health problems include overweight, diabetes, cardiovascular and respiratory problems (De Winter et al. 2012; McCarron et al. 2013; Hsieh et al. 2014; Hosking et al. 2016; Flygare Wallen et al. 2018). It is suspected that these circumstances put people with ID at increased risk of getting infected by a virus, such as the novel severe acute respiratory syndrome corona virus‐2 (SARS‐CoV‐2), and poorer prognosis once the disease develops, resulting in an increased mortality risk (Sayers et al. 2013; Zhou et al. 2020; Fang et al. 2020).
Although the new corona virus disease (Covid‐19) has reached pandemic status, there are little reliable epidemiological statistics or literature available to inform policy makers and care providers on specific risks and consequences of virus outbreaks in the ID population as compared with the general population (Campbell et al. 2009; Sayers et al. 2013; Lai et al. 2020; Tummers et al. 2020). Potential implications, however, could be explored by investigating historical data. Mortality statistics are in these situations among the most valuable statistics to provide insight at developments at the population level.
The most recent example of a virus outbreak with a sharp increase of excess mortality was in the 2017–2018 influenza season in The Netherlands (Fig. 1; Reukers et al., 2018). Through a variety of reasons, including an ineffective influenza vaccine and a very cold winter, excess mortality during that season summed up to an exceptional number of 9400 cases in the entire population (Reukers et al. 2018). Although the disease (mostly influenza type‐B) and its development were different from Covid‐19, and no significant measures were taken to control its spread at the time, learning from the differences in excess mortality patterns among people with and without ID may provide insights on potential implications for people with ID in pandemics such as the Covid‐19 outbreak.
Figure 1.
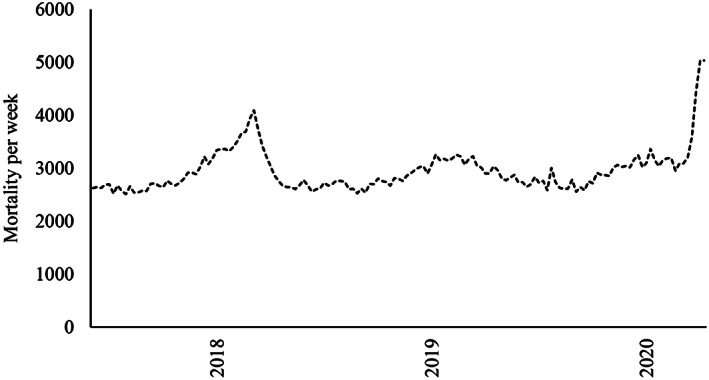
Weekly mortality in The Netherlands from 2017 to 2018 influenza epidemic to 2020 Covid‐19 pandemic (until week‐19 2020).
Method
Setting and design
This historical cohort study used data from the Dutch national mortality register to investigate mortality patterns before and during the 2017–2018 influenza season. Information on all Dutch adults alive on 1 January 2015 (start of follow‐up) was retrieved from the Dutch population register. The population register was linked with the Chronic care database to identify people entitled to residential ID care and group home facilities. An official ID diagnosis is required for such entitlement. All people identified with ID were enrolled in the ID‐cohort, and the remaining Dutch population served as general population comparator cohort (GenPop).
Following reports from the National Institute for Public Health and the Environment, the influenza epidemic was identified to stretch from week 40 (2017) until week 20 (2018) (Reukers et al. 2018). Here, we use ‘epidemic’ to refer to the influenza season as studied because it only concerns the Dutch setting, and use ‘pandemic’ in relation to Covid‐19, being its formal status (World Health Organisation, 2004). The same seasonal periods between 2015 and 2017 served as baseline period (Fig. 2). This excluded the 20‐weeks spring/summer season of each year during which mortality rates are usually slightly lower.
Figure 2.
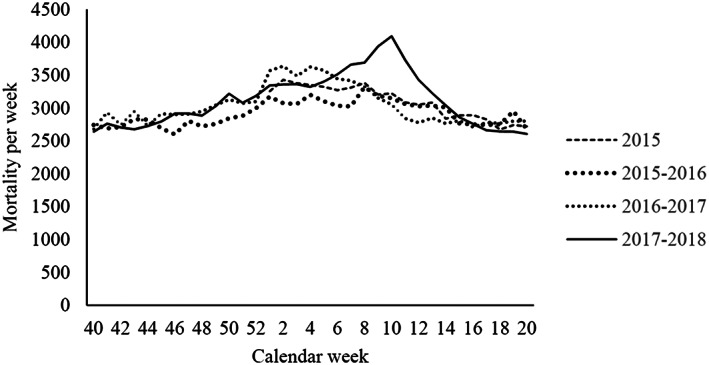
Weekly mortality during study period, by season.
This study used non‐public microdata at the individual level from Statistics Netherlands, the Dutch national statistics office, which under certain conditions are accessible for statistical and scientific research. The need for formal ethical assessment for studies with anonymous population data has earlier been waived by the Radboud University Medical Center Institutional Ethics Committee (2017‐3921).
Outcome variables
Mortality was the primary outcome of this study, consisting of the date and underlying cause of death, specified according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD‐10; World Health Organisation, 2020). Date of death was used to determine whether death occurred in the 2017–2018 influenza season or before.
Statistical analysis
Demographic variables for both cohorts were presented as frequencies (with percentages) or means (with standard deviation). Age at death was calculated by subtracting date of death from date of birth. Person years (PYs) were calculated for the baseline seasons (first 2.5 seasons) and the 2017–2018 epidemic season (last 34 weeks of follow‐up). Crude mortality rates per 10 000PYs were calculated for overall mortality and stratified per sex and age group. Age was grouped into 18–44, 45–64, and 65 years and older to avoid generating subgroups containing less than 10 individuals. Separate calculations were made for cause‐specific mortality per ICD‐10 chapter. Differences in mortality rates between baseline and the epidemic season were given as excess mortality rate per 10 000PYs and by relative changes. Differences to baseline were considered statistically significant if P values were smaller than 0.05. Analyses were conducted using spss (version 25.0).
Results
Data covered the entire Dutch adult population alive at 1 January 2015 of which 91 064 individuals with ID were enrolled in the ID‐cohort and 12 677 678 individuals into the GenPop‐cohort (ID prevalence 0.71%). The ID‐cohort consisted of more male individuals (56.0 vs. 48.9%) and was younger than the GenPop‐cohort (mean age 40.3 vs. 48.3; Table 1). A total of 3526 deaths in the ID‐cohort and 356 834 deaths in the GenPop‐cohort occurred during the observed seasonal periods (ID prevalence among deaths 0.99%; Table 1). Fig. 2 presents the weekly mortality by season.
Table 1.
Demographics at enrolment and total number of analysed cases of mortality
| Characteristic | ID | GenPop | ||
| Population (N = 91 064) | Mortality (N = 3526) | Population (N = 12 677 768) | Mortality (N = 356 834) | |
| Sex, N (%) | ||||
| Males | 50 983 (56.0%) | 1856 (52.6%) | 6 196 789 (48.9%) | 170 611 (47.8%) |
| Females | 40 081 (44.0%) | 1670 (47.4%) | 6 480 979 (51.1%) | 186 223 (52.2%) |
| Age, M (SD) | 40.3 (16.4) | 63.9 (14.5) | 48.3 (17.8) | 78.9 (12.8) |
| Age groups, N (%) | ||||
| 18–44 years † | 54 251 (59.6%) | 438 (12.4%) | 5 519 390 (43.5%) | 8644 (2.4%) |
| 45–64 years | 29 103 (32.0%) | 1742 (49.4%) | 4 562 499 (36.0%) | 58 616 (16.4%) |
| 65 years and older | 7710 (8.5%) | 1346 (38.2%) | 2 595 879 (20.5%) | 289 574 (81.2%) |
ID, intellectual disability.
Age groups refer to age at enrolment.
During the 2017–2018 influenza epidemic, mortality increased by 15.2% in the ID‐cohort and by 5.4% in the GenPop‐cohort as compared with baseline (Table 2). In the ID‐cohort, excess mortality was higher among males (excess 29.7 per 10 000PY, +19.5%) than among females (excess 18.9 per 10 000PY, +10.6%). Most excess deaths were seen in the age group of 65 years and older (excess 86.4 per 10 000PY, +10.4%), and the relative increase was largest in the age group 45–64 years of age (excess 84.3 per 10 000PY, +34.4%). In the GenPop‐cohort, deaths among male individuals (excess 6.5 per 10 000PY, +5.5%) and female individuals (excess 6.7 per 10 000PY, +5.4%) increased at a comparable rate. Per age group in the GenPop, increase in mortality was largest among individuals of 65 years and older (excess 54.4 per 10 000PY, +10.7%), with the largest relative increase appearing in the 45–64 age group (excess 7.4 per 10 000PY, +13.8%).
Table 2.
Baseline seasonal mortality and during 2017–2018 influenza season, crude rates per 10 000PYs
| Characteristic | ID (N = 91 064) | GenPop (N = 12 677 678) | ||||||
| Baseline seasons | Influenza season | Baseline seasons | Influenza season | |||||
| Excess mortality | Difference (%) | Excess mortality | Difference (%) | |||||
| Total all‐cause mortality | 164.2 | 189.2 | 25.0 | 15.2 | 121.6 | 128.3 | 6.7 | 5.4 |
| Sex | ||||||||
| Males | 152.3 | 182.0 | 29.7 | 19.5 | 118.9 | 125.5 | 6.5 | 5.5 |
| Females | 179.3 | 198.2 | 18.9 | 10.6 | 124.2 | 131.0 | 6.7 | 5.4 |
| Age groups † | ||||||||
| 18‐44 years | 34.5 | 35.9 | 1.4 | 4.0 | 6.5 | 7.3 | 0.8 | 12.3 |
| 45–64 years | 245.3 | 329.7 | 84.3 | 34.4 | 53.7 | 61.1 | 7.4 | 13.8 |
| 65 years and older | 834.6 | 921.0 | 86.4 | 10.4 | 507.2 | 561.6 | 54.4 | 10.7 |
| Cause‐specific mortality | ||||||||
| I. Infectious (A00‐B99) | 6.8 | 4.8 | −2.0 | −29.6 | 2.8 | 2.9 | 0.1 | 5.3 |
| II. Neoplasms (C00‐D48) | 24.0 | 26.1 | 2.1 | 8.9 | 36.6 | 37.5 | 0.9 | 2.5 |
| III. Blood, and blood‐forming (D50‐D89) | 0.4 | 0.2 | No reliable estimate | 0.4 | 0.4 | – | – | |
| IV. Endocrine (E00‐E90) | 5.0 | 8.6 | 3.6 | 74.4 | 2.9 | 2.9 | – | – |
| V. Mental and behavioural (F00‐F99) | 13.7 | 18.0 | 4.3 | 31.7 | 9.8 | 11.1 | 1.3 | 13.0 |
| VI. Nervous system (G00‐G99) | 13.5 | 13.6 | 0.1 | 0.4 | 6.6 | 7.4 | 0.8 | 11.2 |
| VII. Circulatory system (I00‐I99) | 31.3 | 35.6 | 4.3 | 13.7 | 32.6 | 32.7 | 0.1 | 0.3 |
| VIII. Respiratory system (J00‐J99) | 25.0 | 29.8 | 4.8 | 19.4 | 11.6 | 13.4 | 1.8 | 15.7 |
| IX. Digestive system (K00‐K93) | 7.0 | 7.2 | 0.2 | 2.9 | 3.7 | 3.8 | 0.1 | 3.8 |
| X. Muscoskeletal system (M00‐M99) | 1.4 | 0.5 | No reliable estimate | 0.9 | 1.0 | 0.1 | 8.0 | |
| X1. Genitourinary system (N00‐N99) | 4.7 | 5.3 | 0.6 | 12.9 | 2.7 | 2.7 | – | – |
| XII. Congenital and chromosomal (Q00‐Q99) | 21.6 | 30.0 | 8.4 | 39.1 | <0.1 | <0.1 | No reliable estimate | |
| XIII. External causes (V01‐Y98) | 6.0 | 5.1 | −0.8 | −14.2 | 6.0 | 6.7 | 0.7 | 11.7 |
| XIV. Other and not specified (all H/L/O/P/R‐codes) | 4.1 | 4.4 | 0.3 | 8 | 4.9 | 5.3 | 0.4 | 7.8 |
Most common specific causes characterizing for each ICD‐10 chapter. I: A41‐Sepsis; II: C34‐Lung cancer; C18‐Colon cancer; C50‐Breast cancer; III: D61‐Anemia; IV: E10‐E14‐Diabetes mellitus; V: F03‐Dementia unspecified; F79‐Mental retardation; VI: G30‐Alzheimer's disease; G40‐Epilepsy; VII: I50‐Heart failure; I21‐Acute myocardial infarction; I64‐Stroke VIII: J18‐Pneumonia; J44‐Chronic obstructive pulmonary disease; IX: K92‐Gastrointestinal hemorrhage; XI: N39‐Urinary tract infection; XII: Q90‐Down syndrome; XIII: W19‐Fall unspecified; XIV: R99‐Other ill‐defined and unknown causes. Estimates of change have not been calculated for categories containing less than 10 deaths or rates <0.1. P < .05 reflected in bold.
ID, intellectual disability.
Age groups refer to age at enrolment.
Most deaths in the ID‐cohort at baseline and during the influenza epidemic were due to diseases of the circulatory system (31.3 resp. 35.6 per 10 000PY). Highest contribution to excess deaths in the ID‐cohort was caused by an increase in deaths from congenital and chromosomal abnormalities (e.g. Down's syndrome; excess 8.4 per 10 000PY, +39.1%). Other causes contributing to excess deaths were mental and behavioural disorders (e.g. dementia or mental retardation; excess 4.3 per 10 000PY, +31.7%), and specific causes from respiratory problems (e.g. pneumonia; excess 4.8 per 10 000PY, +19.4%), and diseases of the endocrine system (e.g. diabetes; excess 3.6 per 10 000PY, +74.4%). Infections and external causes of death occurred less frequent than expected during the influenza epidemic, although differences to baseline were not statistically significant (Table 2).
In the GenPop‐cohort, neoplasms were the most common cause of death at baseline and during the influenza epidemic (36.6 resp. 37.5 per 10 000PY). Excess deaths in the GenPop‐cohort were most often caused by diseases of the respiratory system (excess 1.8 per 10 000PY, +15.7%) or from mental and behavioural disorders (excess 1.3 per 10 000PY, +13.0%). Excess mortality from other causes in the GenPop‐cohort was lower than 1 per 10 000PY. The full comparison between baseline and influenza season deaths is specified in Table 2 for both the ID and GenPop‐cohort.
Discussion
This study investigated mortality patterns before and during the 2017–2018 influenza epidemic in The Netherlands and found that during this epidemic, mortality among people with ID increased three times as much than in the general population. Moreover, patterns of excess mortality across sex, age groups and underlying causes of death were different for people with and without ID. These potential risk factors for the ID population could not be learned from general population statistics.
Mortality rates being higher in ID populations than in the general population is a common finding in literature (Ouellette‐Kuntz et al. 2015; Hosking et al. 2016; Ng et al. 2017). What this study adds is the knowledge that despite higher mortality rates at baseline, the excess mortality during an epidemic was also more pronounced than in the general population.
Next to observing three times the increase in mortality than in the general population, different patterns in sex and age groups were seen as well in the ID‐cohort. Women with ID had higher mortality rates than men, but excess mortality during the epidemic was higher among men with ID than among women with ID. Sex differences are currently also being reported as factor in severity and mortality of Covid‐19, possibly due to different immune responses between male individuals and female individuals (Cai 2020; Klein et al. 2016). And although age groups of 65 years and older were most affected during the epidemic, the 45–65 year age group in the ID population showed an almost similar absolute increase in mortality rate. This aligns with previous reports about early onset of frailty in people with ID and is an important finding when preventive measures during an epidemic focus on protecting the elderly, it may be wrongly assumed that people with ID below the age of 65 years of age are less at risk (Evenhuis et al. 2012).
Respiratory diseases, including pneumonia, were the prime suspect for having caused the excess mortality during the 2017–2018 influenza epidemic. For the general population, this was true for a large extent as the largest absolute and relative increases were seen in this category. In the ID‐cohort, however, excess mortality was also reported to be caused by more generic causes such as the ID itself and dementia. Most likely this reflects a more vulnerable overall health status leading to death during the epidemic. Striking was the large relative increase of deaths caused by endocrine and metabolic diseases in the ID‐cohort while no change in deaths from this cause was observed in the general population. It is suggested that diabetes among people with ID is more prevalent and has more impact on physical health, in particular in response to viruses (Casqueiro et al. 2012; McVilly et al. 2014). It is important, however, to keep this excess co‐morbidity risk particularly in mind for people with ID during future pandemics, including Covid‐19. The reason for why both generic and certain specific causes of death increase more than in the general population during a virus outbreak requires further investigation with data containing more information on the precise circumstances of death.
Findings from this study have other implications as well for preparing and responding to an epidemic or pandemic outbreak in the context of people with ID. Protective measures targeted at the general population should not immediately be assumed to be sufficient for the ID population. People with ID could be disproportionately at risk for exposure to a virus, have a worse prognosis when infected and are more threatened in their wellbeing and longevity when care is disrupted for any reason that can occur during an epidemic (Campbell et al. 2009). Policy makers and routine daily caregivers should, in such circumstances, be able to rely on good quality data to take appropriate measures. As this study showed, national population data may not show the full picture, requiring additional collection of specific information about people with ID. Including ID as variable in national data sets, prospective registration in dedicated ID‐related databases, and accurate reporting of causes of death could help to detect patterns as revealed in this study at an earlier stage, to provide better understanding of underlying causes and to allow faster implementation of protective measures and tailored strategies.
Other strengths of this study include national coverage and the possibility to precisely analyse the epidemic period and the same weeks in previous years, which excluded potential seasonal effects. Moreover, the ID‐cohort appeared to be a credible representation of people with moderate to severe ID living in group homes. A limitation, however, was the unavailability of information on ID diagnoses or severity. Nor was any information available about diagnosed co‐morbidities, such as diabetes or dementia. Furthermore, analysis of the causes of death relied on the quality of reporting, that allowed causes like mental retardation (ICD‐10: F79) and Down's syndrome (ICD‐10: Q90) being reported frequently while these are rarely a sufficient explanation for the true causal pathway leading to death (Dunwoodie Stirton and Heslop 2018). As these ID‐related causes of death were almost exclusively found in the ID‐cohort, it did, however, confirm our successful classification of ID cases. Finally, even by using population data, the risk of reporting small numbers was present, requiring some results to be more aggregated than desirable to avoid any privacy risks.
Conclusion
During the 2017–2018 influenza epidemic, excess mortality among people with ID was three times higher than in the general Dutch population, appeared more often at young age and with a broader range of underlying causes. These findings suggest that a pandemic may disproportionally affect people with ID while population data may not immediately raise warnings. Early detection of diverging patterns and faster implementation of tailored strategies therefore require collection of good quality data.
Source of funding
This study was funded by the Dutch Ministry of Health, Welfare, and Sport (Grant 325418). The funding source had no role in the study design, data collection, data analysis, data interpretation or writing of this report.
Data availability
Aggregated data from the databases used in this study are publicly available on a dedicated website of Statistics Netherlands (http://statline.cbs.nl). The non‐public microdata used to link databases are, under certain conditions, accessible for statistical and scientific research (fees apply). Procedures can be found at www.cbs.nl, for further information: microdata@cbs.nl
Conflict of interest
Authors declare to have no conflicts of interest.
Acknowledgments
We thank the National Institute for Public Health and the Environment (RIVM) for their earlier analyses and reports on this influenza epidemic.
Cuypers, M. , Schalk, B. W. M. , Koks‐Leensen, M. C. J. , Nägele, M. E. , Bakker‐van Gijssel, E. J. , Naaldenberg, J. , and Leusink, G. L. (2020) Mortality of people with intellectual disabilities during the 2017/2018 influenza epidemic in the Netherlands: potential implications for the COVID‐19 pandemic. Journal of Intellectual Disability Research, 64: 482–488. 10.1111/jir.12739.
Footnotes
Procedures can be found at: https://www.cbs.nl/en‐gb/our‐services/customised‐services‐microdata/microdata‐conducting‐your‐own‐research, or for further information: microdata@cbs.nl
References
- Balogh R., McMorris C. A., Lunsky Y., Ouellette‐Kuntz H., Bourne L., Colantonio A. et al (2016) Organising healthcare services for persons with an intellectual disability. Cochrane Database of Systematic Reviews, 1‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. (2020) Sex difference and smoking predisposition in patients with COVID‐19. The Lancet Respiratory Medicine 8, e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell V. A., Gilyard J. A., Sinclair L., Sternberg T. & Kailes J. I. (2009) Preparing for and responding to pandemic influenza: implications for people with disabilities. American Journal of Public Health 99, S294–S300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casqueiro J., Casqueiro J. & Alves C. (2012) Infections in patients with diabetes mellitus: a review of pathogenesis. Indian journal of endocrinology and metabolism 16, S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter C. F., Bastiaanse L. P., Hilgenkamp T. I. M., Evenhuis H. M. & Echteld M. A. (2012) Overweight and obesity in older people with intellectual disability. Research in Developmental Disabilities 33, 398–405. [DOI] [PubMed] [Google Scholar]
- Dunwoodie Stirton F. & Heslop P. (2018) Medical certificates of cause of death for people with intellectual disabilities: a systematic literature review. Journal of Applied Research in Intellectual Disabilities 31, 659–668. [DOI] [PubMed] [Google Scholar]
- Evenhuis H. M., Hermans H., Hilgenkamp T. I., Bastiaanse L. P. & Echteld M. A. (2012) Frailty and disability in older adults with intellectual disabilities: results from the healthy ageing and intellectual disability study. Journal of the American Geriatrics Society 60, 934–938. [DOI] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G. & Roth M. (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? The Lancet. Respiratory Medicine 8, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flygare Wallen E., Ljunggren G., Carlsson A. C., Pettersson D. & Wändell P. (2018) High prevalence of diabetes mellitus, hypertension and obesity among persons with a recorded diagnosis of intellectual disability or autism spectrum disorder. Journal of Intellectual Disability Research 62, 269–280. [DOI] [PubMed] [Google Scholar]
- Hosking F. J., Carey I. M., Shah S. M., Harris T., DeWilde S., Beighton C. et al (2016) Mortality among adults with intellectual disability in England: comparisons with the general population. American Journal of Public Health 106, 1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K., Rimmer J. H. & Heller T. (2014) Obesity and associated factors in adults with intellectual disability. Journal of Intellectual Disability Research 58, 851–863. [DOI] [PubMed] [Google Scholar]
- Klein S. L. & Flanagan K. L. (2016) Sex differences in immune responses. Nature Reviews Immunology 16, 626–638. [DOI] [PubMed] [Google Scholar]
- Krahn G. L., Hammond L. & Turner A. (2006) A cascade of disparities: health and health care access for people with intellectual disabilities. Mental Retardation and Developmental Disabilities Research Reviews 12, 70–82. [DOI] [PubMed] [Google Scholar]
- Lai C. C., Shih T. P., Ko W. C., Tang H. J. & Hsueh P. R. (2020) Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. International Journal of Antimicrobial Agents 55, 105924, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron M., Swinburne J., Burke E., McGlinchey E., Carroll R. & McCallion P. (2013) Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS‐TILDA). Research in Developmental Disabilities 34, 521–527. [DOI] [PubMed] [Google Scholar]
- McVilly K., McGillivray J., Curtis A., Lehmann J., Morrish L. & Speight J. (2014) Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabetic Medicine 31, 897–904. [DOI] [PubMed] [Google Scholar]
- Ng N., Wallén E. F. & Ahlström G. (2017) Mortality patterns and risk among older men and women with intellectual disability: a Swedish national retrospective cohort study. BMC Geriatrics 17, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette‐Kuntz H., Shooshtari S., Balogh R. & Martens P. (2015) Understanding information about mortality among people with intellectual and developmental disabilities in Canada. Journal of Applied Research in Intellectual Disabilities 28, 423–435. [DOI] [PubMed] [Google Scholar]
- Reukers D. M. F., van Asten L., Brandsema P. S., Dijkstra F., Donker G. A., & Dam‐Deisz, W. D. C. , et al. (2018). Annual report surveillance of influenza and other respiratory infections: winter 2017/2018.
- Sayers G., Igoe D., Carr M., Cosgrave M., Duffy M., Crowley B. et al (2013) High morbidity and mortality associated with an outbreak of influenza A (H3N2) in a psycho‐geriatric facility. Epidemiology and Infection 141, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers J., Catal C., Tobi H., Tekinerdogan B. & Leusink G. (2020) Coronaviruses and people with intellectual disability: an exploratory data analysis. Journal of Intellectual Disability Research, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2004) ICD‐10: International Statistical Classification of Diseases and Related Health Problems: Tenth revision. World Health Organization. [Google Scholar]
- World Health Organisation (2020) WHO Director‐General's Opening Remarks at the Media Briefing on COVID‐19‐11 March 2020. Switzerland, Geneva. [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet, pp. 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Aggregated data from the databases used in this study are publicly available on a dedicated website of Statistics Netherlands (http://statline.cbs.nl). The non‐public microdata used to link databases are, under certain conditions, accessible for statistical and scientific research (fees apply). Procedures can be found at www.cbs.nl, for further information: microdata@cbs.nl


